Abstract
Background
The recurrence site that influences post-recurrence survival (PRS) in patients with non-small cell lung cancer (NSCLC) undergoing surgery and the preoperative predictors of recurrence remain unclear.
Methods
Cohorts 1 and 2 had 4520 (who underwent complete resection for p-stage 0-IIIA NSCLC) and 727 (who experienced recurrence after surgery) patients, respectively. The initial sites of recurrence were the lungs (309 cases), thoracic lymph nodes (225 cases), pleura (112 cases), bone (110 cases), central nervous system (86 cases), adrenal gland (25 cases), abdomen (60 cases), cervical and axillary lymph nodes (38 cases), chest wall (13 cases), skin (5 cases), and eye and tongue (3 cases). For cohort 2 analysis, the initial recurrence site that resulted in poor PRS was analyzed by multivariable analysis using a Cox proportional hazard model. For cohort 1 analysis, the preoperative predictors of recurrence patterns with poor PRS were analyzed by multivariable analysis using a logistic regression model.
Results
In cohort 2 analysis, recurrence in the central nervous system (hazard ratio [HR], 1.70; p < 0.001), bone (HR, 1.75; p < 0.001), abdomen (HR, 2.39; p < 0.001), and pleura (HR, 1.69; p < 0.001) were independent poor prognostic recurrent sites for PRS and they were high-risk sites (HRS). Intrathoracic lymph nodes, cervical and axillary lymph nodes, lungs, chest wall, adrenal gland, eye and tongue, and skin were low-risk sites (LRS) that did not affect PRS. Patients with multiple LRS without HRS recurrence had a worse prognosis than those with a single LRS without HRS recurrence (5-year PRS 20.2% vs. 37.7%, p < 0.001) and were comparable to those with HRS recurrence (p = 1.000). In cohort 1 analysis, preoperative predictors for HRS and multiple LRS recurrences were positron emission tomography (PET) maximum standardized uptake value (maxSUV) ≥ 3.2 (HR, 5.09; p < 0.001), clinical nodal metastasis (HR, 2.00; p < 0.001), tumor size ≥ 2.4 cm (HR, 1.96; p < 0.001) and carcinoembryonic antigen (CEA) ≥ 5 ng/ml (HR, 1.41; p = 0.004). The cumulative incidence rates of HRS and multiple LRS recurrences within 5 years were 55.9%, 40.9%, 26.3%, 11.1%, and 3.5% (p < 0.001) in patients with 4, 3, 2, 1 and 0 of the above risks, respectively.
Conclusions
HRS and multiple LRS were vital recurrences associated with poor PRS. Preoperative PET maxSUV, clinical nodal metastasis, tumor size, and CEA level predicted the incidence of vital recurrence.
Similar content being viewed by others
Background
Lung cancer is the leading cause of death worldwide [1]. Among patients with non-small cell lung cancer (NSCLC) who undergo surgery, 30–55% show recurrence [2, 3]. The standard treatment for patients with NSCLC recurrence is similar to that for advanced-stage lung cancer, with a poor post-recurrence survival (PRS); median PRS of 17.6–30 months [3,4,5,6], and a 5-year PRS of 18.8–31.9% [3,4,5,6,7]. The local and distant recurrences have been reported in approximately 24–38% and 40–78% of cases, respectively [3, 4, 6].
Male sex, older age, smoking history, low-performance status, short-term recurrence, histological presence of symptoms, and poor differentiation have been reported to be poor prognostic factors for PRS [3,4,5,6, 8]. However, there is still no consensus regarding the relationship between PRS and the site of recurrence. There is also no consensus on the risk of recurrence at specific sites. Previous studies have reported that extrathoracic or distant recurrence is associated with poor prognosis [9, 10]. Other studies have reported that patients with local recurrence had a survival advantage over those with distant recurrence [7, 8, 11]; however, in some studies, distant recurrence did not affect PRS [6, 12, 13].
Analyzing recurrence sites that result in poor prognosis is necessary for personalized postoperative surveillance. Moreover, predicting these vital recurrences is important in selecting patients for neoadjuvant/adjuvant therapy. In recent years, several clinical trials for preoperative treatment of clinical stage IB-III NSCLC have been conducted, including CheckMate-816, AEGEAN, CheckMate-77T, KEYNOTE-671, IMpower030, and NeoADAURA, and evidence is gradually being established [14]. Preoperative prediction of vital recurrence is important in selecting patients who should receive neoadjuvant or adjuvant therapy.
This study aimed to identify vital recurrence resulting in poor PRS in patients with postoperative recurrence of NSCLC using a multicenter database and to analyze the preoperative predictors of vital recurrence.
Methods
Ethics statement
The study adhered to the tenets of the Declaration of Helsinki. The institutional review boards of the participating institutions approved this retrospective review of a prospective database and waived the requirement for informed consent for each patient (Kanagawa Cancer Center, approval 24EKI54; Tokyo Medical University Hospital, approval SH2969; Hiroshima University Hospital, approval E-1216).
Patients and study design
In this study, 4520 patients who underwent complete resection for pathological stage 0-IIIA NSCLC at Kanagawa Cancer Center, Hiroshima University Hospital, and Tokyo Medical University between January 2010 and December 2020 were included in cohort 1 (Fig. 1). This study excluded patients who received neoadjuvant therapy and who have unavailable information on positron emission tomography (PET) maximum standardized uptake value (maxSUV) or carcinoembryonic antigen (CEA) level (Fig. 1). Among the 4520 patients, 727 experienced recurrences after surgery and were included in cohort 2 (Fig. 1). The present study had the following two parts: First, the recurrence patterns that resulted in poor PRS, namely vital recurrence, were examined in cohort 2. Second, the preoperative predictors of vital recurrence were analyzed in cohort 1.
Categorization of the initial recurrence sites and word definitions
The initial recurrence site was defined as recurrent organs that could be identified by diagnostic imaging performed before treatment for recurrence. The initial metastatic organs were classified into the following ten recurrence site categories: (1) thoracic lymph node recurrence, including mediastinal and hilar lymph node recurrence; (2) cervical and axillary lymph node recurrence, including sub/supraclavicular lymph node; (3) lung recurrence, including ipsilateral or contralateral lung recurrence; (4) pleural recurrence, including pleural dissemination; (5) chest wall recurrence; (6) bone recurrence; (7) central nervous system (CNS) recurrence, including brain metastasis and meningeal dissemination; (8) adrenal recurrence; (9) abdominal organ recurrence, including liver, pancreas, intestine, and intraperitoneal lymph node recurrence; (10) skin recurrence; and (11) eye and tongue recurrence.
PRS was defined as the duration from the first evidence of relapse to the time of all-cause death, censoring patients without an event during the last observation period. Overall survival (OS) was defined as the period from the date of surgery to the date of all-cause death, wherein patients were censored without any events in the last observation period.
Statistical analysis
Continuous variables were compared using the Mann–Whitney U test and categorical variables were compared using Fisher’s exact test. OS and PRS were analyzed using the Kaplan–Meier method and compared between the groups using log-rank tests. Cut-off values for the computed tomography (CT) tumor size, Brinkmann index, and PET maxSUV were determined using receiver operating characteristic curve analysis in cohort 1.
In cohort 2, univariable and multivariable analyses of PRS were performed using the Cox proportional hazard model to analyze the following variables: age (≥ 65 years), sex, histology, smoking history, CT tumor size, surgical procedure, pathological stage, lymphatic invasion, vessel invasion, pleural invasion, nodal metastasis, and each recurrence site. All variables with a p-value of < 0.10 in the univariable analysis were analyzed in the multivariable analysis.
In cohort 1, multivariable analysis was performed to examine the predictors of vital recurrence using logistic regression analysis of the preoperative variables: age (≥ 65 years), sex, Brinkmann index, CEA, laterality, tumor location, CT tumor size, PET maxSUV, clinical nodal metastasis, and surgical procedure. The cumulative incidence of vital recurrence according to risk was analyzed using Gray’s test.
Statistical significance was set at p-value of < 0.05. Statistical analyses were performed using EZR on R commander version 1.30 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
The median observation period, median PRS, and 5-year PRS rate of cohort 2 were 32 (19–54) months, 26 months, and 24.5%, respectively. The median age of the patients was 72 years (range, 65–77 years), and 512 (70.4%) patients were males (Supplementary Table). There were 309 (42.5%) patients with lung recurrence, 225 (30.9%) patients with intrathoracic lymph node recurrence, 112 (15.4%) patients with pleural recurrence, 110 (15.1%) patients with bone recurrence, 86 (11.8%) patients with CNS recurrence, 25 (3.4%) patients with adrenal recurrence, 60 (8.3%) patients with abdominal organ recurrence, 38 (5.2%) patients with cervical and axillary lymph nodes metastasis, 13 (1.8%) patients with chest wall recurrence, 5 (0.7%) patients with skin recurrence, and 3 (0.4%) patients with eye and tongue recurrence (Supplementary Table).
In univariable and multivariable analyses of cohort 2, CNS recurrence (hazard ratio [HR], 1.70; 95% confidence interval [CI], 1.25–2.33; p < 0.001), bone recurrence (HR, 1.75; 95% CI, 1.31–2.35; p < 0.001), abdominal organ recurrence (HR, 2.39; 95% CI, 1.68–3.41; p < 0.001), and pleural recurrence (HR, 1.69; 95% CI, 1.25–2.27; p < 0.001) were poor prognostic factors for PRS, along with older age (≥ 65 years), smoking history, and non-adenocarcinoma histology (Table 1). These four recurrence sites were defined as high-risk sites (HRS), and the other sites (lung, intrathoracic lymph node, cervical and axillary lymph nodes, adrenal gland, chest wall, skin, and eye and tongue) were defined as low-risk sites (LRS).
The PRS of patients with single and multiple HRS recurrences was comparable (p = 0.434, Fig. 2a). The PRS of patients with both HRS and LRS recurrence tended to show worse prognosis than that of patient with HRS recurrence without LRS recurrence, but not statistically significant (p = 0.085, Fig. 2b). The PRS of multiple LRS recurrent patients was significantly worse than that of single LRS recurrent patients among patients without HRS recurrence (p < 0.001, Fig. 2c). The PRS of patients with HRS recurrence was significantly worse than that of patients with single LRS recurrence (p < 0.001, Fig. 3a); however, they were comparable to those of patients with multiple LRS (p = 1.000, Fig. 3a).
The post-recurrence survival (PRS) of patients with a single high-risk site (HRS) and multiple HRS recurrences were comparable (p = 0.434, Fig. 2a). The PRS of patients with both HRS and low-risk site (LRS) recurrence tended to show worse prognosis than that of patient with HRS recurrence without LRS recurrence, but not statistically significant (p = 0.085, Fig. 2b). The PRS of multiple LRS recurrent patients were significantly worse than that of single LRS recurrent patients among the patients without HRS recurrence (p < 0.001, Fig. 2c)
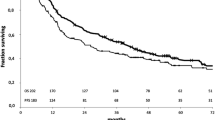
The post-recurrence survival (PRS) of a high-risk site (HRS) recurrent patients was significantly worse than that of a single low-risk site (LRS) recurrent patients (p < 0.001, Fig. 3a) and were comparable to that of multiple LRS recurrent patients (p = 1.000, Fig. 3a). The overall survival (OS) of patients with HRS and multiple LRS recurrences was significantly worse than that of patients with a single LRS recurrence (p < 0.001, Fig. 3b)
The characteristics of patients with HRS and multiple LRS recurrences were compared with those of patients with single LRS recurrences (Table 2). Larger tumor size, lymphatic invasion, blood vessel invasion, pleural invasion, and nodal metastasis were more common in the HRS and multiple LRS recurrence groups. Postoperative interval until a relapse was significantly shorter in the HRS and multiple LRS recurrence groups than in the single LRS recurrence group (12-month vs. 17-month, p < 0.001). The OS of patients with HRS and multiple LRS recurrences was significantly worse than that of patients with a single LRS recurrence (p < 0.001, Fig. 3b).
The median observation period for cohort 1 was 50 (25–67) months. The median patient age was 70 years (range, 64–76 years), and 2550 (56.4%) patients were males (Supplementary Table). In the multivariable analysis in cohort 1, preoperative predictors of HRS or multiple LRS recurrence were CEA ≥ 5 ng/ml (odds ratio [OR], 1.41; 95% CI:1.12–1.77; p = 0.004), PET maxSUV ≥ 3.2 (OR, 5.09; 95% CI:3.66–7.08; p < 0.001), CT tumor size ≥ 2.4 cm (OR, 1.96; 95% CI:1.50–2.56; p < 0.001),and clinical nodal metastasis (OR, 2.00; 95% CI:1.53–2.60; p < 0.001) (Table 3). The cumulative incidences of HRS and multiple LRS recurrences at 5 postoperative years were 55.9%, 40.9%, 26.3%, 11.1%, and 3.5% in patients with 4, 3, 2, 1, and 0 of the above risks, respectively (p < 0.001; Fig. 4).
Discussion
This study demonstrated that HRS (CNS, bone, abdominal organ, and pleural) recurrence or multiple LRS (lung, intrathoracic lymph node, cervical and axillary lymph node, adrenal gland, chest wall, eye and tongue, and skin) recurrences were vital recurrences that were associated with poor PRS. Preoperative predictors for vital recurrence were CEA ≥ 5 ng/ml, PET maxSUV ≥ 3.2, CT tumor size ≥ 2.4 cm, and clinical nodal metastasis. Patients with all four predictors had vital recurrence at 55.9% within 5 years after surgery.
The median PRS and 5-year PRS rates were 26 months and 24.5%, respectively, which were similar to those in previous reports [3,4,5,6]. In the previous study, the frequency of recurrence to intrathoracic lymph nodes, lung, bone, pleura, brain, adrenal gland, abdominal organ, cervical lymph node, and chest wall was 22–42% [4, 6], 37–42% [4,5,6], 12–18% [4,5,6], 7–16% [4, 6], 11–18% [4,5,6], 3–6% [4,5,6], 7–9% [4,5,6], 9% [4], and 2% [4], respectively, and those frequencies were comparable to present study. There are a limited number of reports analyzing prognosis according to initial recurrence sites. A previous study reported that poor PRS has been observed in patients with lung [15], brain [16], bone [5, 6, 16, 17], and liver recurrence [6, 18]. However, since these studies were based on small sample size, there is no consensus on the association between PRS and recurrence site. In the present study of 727 recurrent patients, the largest study using a multicenter database revealed that HRS or multiple LRS recurrences were associated with a significantly poor PRS. In contrast, PRS was significantly better in patients with single LRS recurrence.
The specific reason why PRS differs in recurrence site is as follows: first, HRS including bone, brain, and pleural recurrence may decrease the patient's quality of life or lower performance status, making treatment after recurrence more difficult [19,20,21,22,23]. Second, recurrences in the liver and CNS have been reported to be less responsive to chemotherapy [22]. As shown in Table 2, a more aggressive tumor was observed in the HRS or multiple LRS recurrence groups than in the single LRS recurrence group, which may result in poor PRS.
Recently, it has been reported that local therapy prolongs PRS in patients with 3–5 or fewer oligo-recurrent foci [3, 24,25,26]. The present study suggests that local therapy may improve the prognosis of patients with a single LRS recurrence because cancer cells with a less aggressive nature are localized. Previously, Hishida et al. reported that oligo-distant recurrence (single site) has no difference in prognosis compared with oligo-locoregional recurrences (1–3 sites) [24]. Torok et al. reported oligo-distant recurrence (1–3 sites) had a significantly better PRS than diffuse distant recurrence (> 3 sites or pleural dissemination). Moreover, adrenalectomy for patients with isolated adrenal metastasis from NSCLC showed a favorable prognosis [27]. Further investigation is necessary to determine the efficacy of local therapy for the oligo-distant recurrence of a single LRS.
Patients with HRS or multiple LRS recurrences had poor PRS and OS, suggesting that these patients had systemic cancer at the time of surgery. These patients had difficulty in controlling cancer through local therapy such as surgery and radiation therapy alone, and combination therapy with systemic therapy, such as immune checkpoint inhibitors, tyrosine kinase inhibitors, and chemotherapy was necessary during the perioperative period. In recent years, nivolumab plus platinum-based chemotherapy has demonstrated longer event-free survival for clinical stage IB-IIIA NSCLC without epidermal growth factor receptor gene mutation (EGFR) and anaplastic lymphoma kinase (ALK) translocation in CheckMate-816. The HR for death or distant metastases was 0.53 (95% CI, 0.36–0.77) and the subgroup analysis showed that greater benefit was observed in a population with a poor prognosis [14].
There are few studies on predictors of recurrence in specific organs and even fewer studies on preoperative predictors. A previous study reported that tumor grade, metastatic lymph node ratio ≥ 30% (LNR), non-squamous cell carcinoma histology, bronchial invasion, perineural invasion, and adjuvant chemotherapy were associated with brain recurrence [7, 28, 29]. Motono et al. analyzed seven postoperative patients with pleural dissemination and reported that young age and poor differentiation are risk factors for pleural dissemination [30]. Previous studies have reported that older age [8], adenocarcinoma histology [8, 31], and higher stage [8, 32] are associated with distant recurrence. Wu et al. scored the risk of distal recurrence as smoking history, additional primary malignancy, non-anatomic resections, adenocarcinoma histology, pleural invasion, and angiolymphatic invasion and reported that intermediate- or high-risk groups had a higher frequency of distal recurrence [33]. The strength of this study is the analysis of preoperative CEA values and the PET maxSUV in all patients. Furthermore, the present study is the first to show that CEA values and PET maxSUV, along with CT tumor size and clinical nodal metastasis, are preoperative predictors of HRS and multiple LRS recurrences leading to poor PRS.
Patients with CEA ≥ 5 ng/ml, PET maxSUV ≥ 3.2, CT tumor size ≥ 2.4 cm, and clinical nodal metastasis recur in HRS or multiple LRS at 55.9% within 5 years after surgery; therefore, these patients should receive aggressive neoadjuvant/adjuvant therapy. On the other hand, neoadjuvant therapy may not be necessary in patients with any of these predictors, as vital metastasis occurs in as low as 3.5% of cases. These preoperative predictors are important for personalized neoadjuvant/adjuvant therapy in resectable clinical stage IB-III NSCLC. Further studies are necessary to compare the effectiveness of neoadjuvant therapy and adjuvant therapy for patients who are likely to experience recurrence at HRS or multiple LRS in a large-scale clinical trial.
This study demonstrated the importance of comprehensive surveillance of all recurrence sites at the time of recurrence to predict survival after recurrence. Moreover, recognition of HRS/LRS and the risk of recurrence are useful for postoperative surveillance. Patients with symptomatic recurrence have been reported to have a poorer prognosis than those with asymptomatic recurrence detected during surveillance [34]. Detection of a single LRS before developing into multiple LRSs may result in a better PRS. Although the ASCO guidelines do not recommend routine follow-up with PET-CT or head magnetic resonance imaging (MRI) (evidence quality: low; strength of recommendation: moderate) [35], intentional follow-up, including head MRI and PET-CT, is considered necessary for patients with one or more predictors of vital recurrence. Future clinical trials of personalized surveillance based on CEA level, PET maxSUV, tumor size and clinical nodal status are warranted.
This study has several limitations. First, this was a retrospective study and selection bias may have been possible. No common surveillance protocol has been established at the three institutions in this study, both postoperatively and at recurrence. Second, we did not investigate the effects of postoperative adjuvant therapy and post-recurrence therapy. Third, this study did not examine the number of recurrent foci. Further studies on the association between the number of recurrent foci and PRS are necessary. Fourth, information on EGFR mutations, ALK translocation, and programmed cell death 1- ligand 1 status was not available in this study. The relationship between these statuses and recurrence sites needs to be further verified. Fifth, this study included a small number of patients with skin (n = 5) and eye and tongue (n = 3) recurrences, and further studies based on a large number are necessary to analyze the prognosis of patients with these recurrence sites.
Conclusion
The PRS of patients with HRS and multiple LRS metastases was significantly poorer than that of patients with a single LRS. The preoperative predictors for these vital recurrences were CEA elevation (≥ 5 ng/mL), high PET maxSUV (≥ 3.2), CT tumor size (≥ 2.4 cm), and clinical nodal metastasis, and 55.9% of patients with these four factors experienced recurrence within 5 years after surgery. Aggressive neoadjuvant/adjuvant therapy should be administered to these patients. Intentional follow-up is necessary for patients with one or more predictors of vital recurrence.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from corresponding author on reasonable request.
Abbreviations
- ALK:
-
Anaplastic lymphoma kinase
- CEA:
-
Carcinoembryonic antigen
- CI:
-
Confidence interval
- CNS:
-
Central nerve system
- CT:
-
Computed tomography
- EGFR:
-
Epidermal growth factor receptor gene mutation
- HR:
-
Hazard ratio
- HRS:
-
High-risk site
- LRS:
-
Low-risk site
- MRI:
-
Magnetic resonance imaging
- maxSUV:
-
Maximum standardized uptake value
- NSCLC:
-
Non-small cell lung cancer
- PET:
-
Positron emission tomography
- PRS:
-
Post-recurrence survival
- QOL:
-
Quality of life
- TKI:
-
Tyrosine kinase inhibitor
References
Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;32:605–44.
Uramoto H, Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res. 2014;3(4):242–9. https://doi.org/10.3978/j.issn.2218-6751.2013.12.05.PMID:25806307;PMCID:PMC4367696.
Sonoda D, Matsuura Y, Kondo Y, Ichinose J, Nakao M, Ninomiya H, Ishikawa Y, Nishio M, Okumura S, Satoh Y, Mun M. Characteristics of surgically resected non-small cell lung cancer patients with post-recurrence cure. Thorac Cancer. 2020 Nov;11(11):3280–3288. https://doi.org/10.1111/1759-7714.13669. Epub 2020 Sep 22. PMID: 32961037; PMCID: PMC7605994.
Takenaka T, Yano T, Yamazaki K, Okamoto T, Hamatake M, Shimokawa M, Mori M; Kyushu University Lung Surgery Study Group Japan. Survival after recurrence following surgical resected non-small cell lung cancer: A multicenter, prospective cohort study. JTCVS Open. 2022 Apr 4;10:370–381. https://doi.org/10.1016/j.xjon.2022.03.004. PMID: 36004269; PMCID: PMC9390543.
Isaka T, Ito H, Nakayama H, Yokose T, Saito H, Masuda M. Impact of the initial site of recurrence on prognosis after curative surgery for primary lung cancer. Eur J Cardiothorac Surg. 2022;61(4):778–86. https://doi.org/10.1093/ejcts/ezab442. (PMID: 34686875).
Shimada Y, Saji H, Yoshida K, Kakihana M, Honda H, Nomura M, Usuda J, Kajiwara N, Ohira T, Ikeda N. Prognostic factors and the significance of treatment after recurrence in completely resected stage I non-small cell lung cancer. Chest. 2013;143(6):1626–34. https://doi.org/10.1378/chest.12-1717. (PMID: 23348916).
Consonni D, Pierobon M, Gail MH, Rubagotti M, Rotunno M, Goldstein A, Goldin L, Lubin J, Wacholder S, Caporaso NE, Bertazzi PA, Tucker MA, Pesatori AC, Landi MT. Lung cancer prognosis before and after recurrence in a population-based setting. J Natl Cancer Inst. 2015 Mar 23;107(6):djv059. https://doi.org/10.1093/jnci/djv059. PMID: 25802059; PMCID: PMC4838060.
Jeong WG, Choi H, Chae KJ, Kim J. Prognosis and recurrence patterns in patients with early stage lung cancer: a multi-state model approach. Transl Lung Cancer Res. 2022;11(7):1279–91. https://doi.org/10.21037/tlcr-22-148.PMID:35958321;PMCID:PMC9359942.
Endo C, Sakurada A, Notsuda H, Noda M, Hoshikawa Y, Okada Y, et al. Results of long-term follow-up of patients with completely resected non-small cell lung cancer. Ann Thorac Surg. 2012;93:1061–8.
Ujiie H, Kadota K, Chaft JE, Buitrago D, Sima CS. Lee MC et al Solid Predominant Histologic Subtype in Resected Stage I Lung Adenocarcinoma Is an Independent Predictor of Early, Extrathoracic, Multisite Recurrence and of Poor Postrecurrence Survival. J Clin Oncol. 2015;33:2877–84.
Fedor D, Johnson WR, Singhal S. Local recurrence following lung cancer surgery: incidence, risk factors, and outcomes. Surg Oncol. 2013;22:156–61.
Sugimura H, Nichols FC, Yang P, Allen MS, Cassivi SD, Deschamps C, et al. Survival after recurrent nonsmall-cell lung cancer after complete pulmonary resection. Ann Thorac Surg. 2007;83:409–17.
Matsuguma H, Nakahara R, Wakamatsu I, Kishikawa T, Sugiyama T, Nakamura Y, et al. Definitive Local Therapy for Oligo-recurrence in Patients With Completely Resected Non-small Cell Lung Cancer. Am J Clin Oncol. 2020;43:210–7.
Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson SJ, Kerr K, Wang C, Ciuleanu TE, Saylors GB, Tanaka F, Ito H, Chen KN, Liberman M, Vokes EE, Taube JM, Dorange C, Cai J, Fiore J, Jarkowski A, Balli D, Sausen M, Pandya D, Calvet CY, Girard N; CheckMate 816 Investigators. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med. 2022 May 26;386(21):1973–1985. https://doi.org/10.1056/NEJMoa2202170. Epub 2022 Apr 11. PMID: 35403841; PMCID: PMC9844511.
Yoshino I, Yohena T, Kitajima M, Ushijima C, Nishioka K, Ichinose Y, et al. Survival of non-small cell lung cancer patients with postoperative recurrence at distant organs. Ann Thorac Cardiovasc Surg. 2001;7:204–9.
Wang C, Wu Y, Shao J, Liu D, Li W. Clinicopathological variables influencing overall survival, recurrence and post-recurrence survival in resected stage I non-small-cell lung cancer. BMC Cancer. 2020;20:150.
Kubouchi Y, Kidokoro Y, Ohno T, Yurugi Y, Wakahara M. Haruki T et al Prognostic Factors for Post Recurrence Survival in Resected Pathological Stage I Non-small Cell Lung Cancer. Yonago Acta Med. 2018;60:213–9.
Nakagawa T, Okumura N, Ohata K, Igai H, Matsuoka T, Kameyama K. Postrecurrence survival in patients with stage I non-small cell lung cancer. Eur J Cardiothorac Surg. 2008;34:499–504.
Zhang L, Gong Z. Clinical Characteristics and Prognostic Factors in Bone Metastases from Lung Cancer. Med Sci Monit. 2017;24(23):4087–94. https://doi.org/10.12659/msm.902971.PMID:28835603;PMCID:PMC5580519.
Peters S, Bexelius C, Munk V, Leighl N. The impact of brain metastasis on quality of life, resource utilization and survival in patients with non-small-cell lung cancer. Cancer Treat Rev. 2016;45:139–62. https://doi.org/10.1016/j.ctrv.2016.03.009. (Epub 2016 Mar 15 PMID: 27019457).
Davies HE, Lee YC. Management of malignant pleural effusions: questions that need answers. Curr Opin Pulm Med. 2013;19(4):374–9. https://doi.org/10.1097/MCP.0b013e3283615b67. (PMID: 23673450).
Nakazawa K, Kurishima K, Tamura T, Kagohashi K, Ishikawa H, Satoh H, Hizawa N. Specific organ metastases and survival in small cell lung cancer. Oncol Lett. 2012 Oct;4(4):617–620. https://doi.org/10.3892/ol.2012.792. Epub 2012 Jul 9. PMID: 23205072; PMCID: PMC3506697.
Sridhar S, Paz-Ares L, Liu H, Shen K, Morehouse C, Rizvi N, Segal NH, Jin X, Zheng Y, Narwal R, Gupta A, Dennis PA, Ye J, Mukhopadhyay P, Higgs BW, Ranade K. Prognostic Significance of Liver Metastasis in Durvalumab-Treated Lung Cancer Patients. Clin Lung Cancer. 2019;20(6):e601–8. https://doi.org/10.1016/j.cllc.2019.06.020. (Epub 2019 Jun 26 PMID: 31327642).
Hishida T, Yoshida J, Aokage K, Nagai K, Tsuboi M. Postoperative oligo-recurrence of non-small-cell lung cancer: clinical features and survival†. Eur J Cardiothorac Surg. 2016;49(3):847–53. https://doi.org/10.1093/ejcts/ezv249. (Epub 2015 Jul 22 PMID: 26201958).
Matsuguma H, Nakahara R, Wakamatsu I, Kishikawa T, Sugiyama T, Nakamura Y, Kasai T, Kamiyama Y, Hoshi N, Inoue K, Katano S, Yokoi K. Definitive Local Therapy for Oligo-recurrence in Patients With Completely Resected Non-small Cell Lung Cancer. Am J Clin Oncol. 2020;43(3):210–7. https://doi.org/10.1097/COC.0000000000000656. (PMID: 31850917).
Gomez DR, Blumenschein GR Jr, Lee JJ, Hernandez M, Ye R, Camidge DR, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17:1672–82.
Raz DJ, Lanuti M, Gaissert HC, Wright CD, Mathisen DJ, Wain JC. Outcomes of patients with isolated adrenal metastasis from non-small cell lung carcinoma. Ann Thorac Surg. 2011 Nov;92(5):1788–92; discussion 1793. https://doi.org/10.1016/j.athoracsur.2011.05.116. Epub 2011 Sep 22. PMID: 21944257.
Ding X, Dai H, Hui Z, Ji W, Liang J, Lv J, Zhou Z, Yin W, He J, Wang L. Risk factors of brain metastases in completely resected pathological stage IIIA-N2 non-small cell lung cancer. Radiat Oncol. 2012;30(7):119. https://doi.org/10.1186/1748-717X-7-119. (PMID:22846375;PMCID:PMC3430600).
Sun S, Men Y, Kang J, Sun X, Yuan M, Yang X, Bao Y, Wang J, Deng L, Wang W, Zhai Y, Liu W, Zhang T, Wang X, Bi N, Lv J, Liang J, Feng Q, Chen D, Xiao Z, Zhou Z, Wang L, Hui Z. A Nomogram for Predicting Brain Metastasis in IIIA-N2 Non-Small Cell Lung Cancer After Complete Resection: A Competing Risk Analysis. Front Oncol. 2021;13(11):781340. https://doi.org/10.3389/fonc.2021.781340. (PMID:34966684;PMCID:PMC8710765).
Motono N, Iwai S, Yoshihito I, Usuda K, Yamada S, Uramoto H. Predictive factors related to pleural dissemination in non-small cell lung cancer. J Thorac Dis. 2020;12(10):5647–56.
Hung JJ, Jeng WJ, Hsu WH, Chou TY, Huang BS, Wu YC. Predictors of death, local recurrence, and distant metastasis in completely resected pathological stage-I non-small-cell lung cancer. J Thorac Oncol. 2012 Jul;7(7):1115–23. https://doi.org/10.1097/JTO.0b013e31824cbad8. PMID: 22592210. Hung JJ, Jeng WJ, Hsu WH, Chou TY, Huang BS, Wu YC. Predictors of death, local recurrence, and distant metastasis in completely resected pathological stage-I non-small-cell lung cancer. J Thorac Oncol. 2012 Jul;7(7):1115–23. https://doi.org/10.1097/JTO.0b013e31824cbad8. PMID: 22592210.
Tian Y, He Y, Li X, Liu X. Novel nomograms to predict lymph node metastasis and distant metastasis in resected patients with early-stage non-small cell lung cancer. Ann Palliat Med. 2021;10(3):2548–66. https://doi.org/10.21037/apm-20-1756. (Epub 2021 Mar 1 PMID: 33691451).
Wu CF, Fu JY, Yeh CJ, Liu YH, Hsieh MJ, Wu YC, Wu CY, Tsai YH, Chou WC. Recurrence Risk Factors Analysis for Stage I Non-small Cell Lung Cancer. Medicine (Baltimore). 2015;94(32):e1337. https://doi.org/10.1097/MD.0000000000001337. (Erratum.In:Medicine(Baltimore).2015Sep;94(39):1.PMID:26266381;PMCID:PMC4616676).
Torok JA, Gu L, Tandberg DJ, Wang X, Harpole DH Jr, Kelsey CR, Salama JK. Patterns of Distant Metastases After Surgical Management of Non-Small-cell Lung Cancer. Clin Lung Cancer. 2017;18(1):e57–70. https://doi.org/10.1016/j.cllc.2016.06.011. (Epub 2016 Jul 5 PMID: 27477488).
Schneider BJ, Ismaila N, Aerts J, Chiles C, Daly ME, Detterbeck FC, Hearn JWD, Katz SI, Leighl NB, Levy B, Meyers B, Murgu S, Nekhlyudov L, Santos ES, Singh N, Tashbar J, Yankelevitz D, Altorki N. Lung Cancer Surveillance After Definitive Curative-Intent Therapy: ASCO Guideline. J Clin Oncol. 2020;38(7):753–66. https://doi.org/10.1200/JCO.19.02748. (Epub 2019 Dec 12 PMID: 31829901).
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Study design: TI, HA and HI. Sample collection: TI, HA, KM, JM, NK, NS, YK, YM, MO, NI and HI. Data analysis: TI, HA and HI. Preparation of the manuscript: TI, HA and HI. Reviewed and commented on the manuscript: TI, HA, YK, YM, NI, MO and HI. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethic approval and consent to participate
The present study conformed to the Declaration of Helsinki on Human Research Ethics standards. The institutional review board of the participating institutions has approved this retrospective review of the multicenter database and waived the requirement for informed consent for each patient (Kanagawa Cancer Center, approval 24EKI54 (Approved on June 14, 2021); Tokyo Medical University Hospital, approval SH2969; Hiroshima University Hospital, approval E-1216).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table.
Patient characteristics of cohort 1 and cohort 2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Isaka, T., Adachi, H., Murakami, K. et al. Preoperative predictors for recurrence sites associated with poor post-recurrence survival after surgery of non-small cell lung cancer: a multicenter study. BMC Cancer 23, 1064 (2023). https://doi.org/10.1186/s12885-023-11582-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11582-y