Abstract
Background
In muscle-invasive bladder cancer (MIBC), neoadjuvant chemotherapy (NAC) combined with radical cystectomy (RC) is critical in reducing disease recurrence, with GC (gemcitabine and cisplatin) being one of the most commonly used NACs. Different GC schedules have been used, but the best neoadjuvant regimen is still unknown. The clinical outcomes of 3 and 4 cycles of neoadjuvant GC are compared in this systematic review and meta-analysis to determine which is best for patients with MIBC.
Methods
We searched PubMed, Embase, Web of Science, Cochrane Library, CBM, CNKI, WAN FANG DATA, and meeting abstracts to identify relevant studies up to March 2023. Studies that compared 3 and 4 cycles of neoadjuvant GC for MIBC were included. The primary outcomes were pCR, pDS, OS, and CSS. The secondary outcome was recurrence and SAEs.
Results
A total of 3 studies, with 1091 patients, were included in the final analysis. Patients that received 4 cycles of GC had a higher pCR (OR = 0.66; 95% CI, 0.50–0.87; p = 0.003) and pDS (OR = 0.63; 95% CI, 0.48–0.84; p = 0.002) than those who received 3 cycles. Regarding recurrence rate (OR = 1.23; 95% CI, 0.91–1.65; p = 0.18), there were no appreciable differences between the 3 and 4 cycles of GC. Survival parameters such as OS (HR, 1.35; 95% CI, 0.86–2.12; p = 0.19) and CSS (HR, 1.06; 95% CI, 0.82–1.38; p = 0.20) were similar. Only one trial reported on the outcomes of SAEs. And there were no statistically significant differences in thrombocytopenia, infection rate, neutropenic fever, anemia, or decreased renal function between patients. The neutropenia of patients was statistically different (OR = 0.72; 95% CI, 0.52–0.99; p = 0.04).
Conclusion
The 4-cycle GC regimen was superior to the 3-cycle regimen in only the pCR and pDS results. Survival and recurrence rates were similar between the two regimens. In both treatment regimes, the toxicity profile was manageable. However, due to the inherent drawbacks of retrospective research, this should be regarded with caution.
Similar content being viewed by others
Introduction
Bladder cancer is the 10th most common cancer worldwide, with 213,000 deaths and 573,000 new cases annually, and a quarter of these newly diagnosed bladder cancer cases had invaded the muscle tissue at the time of diagnosis [1].
Although radical cystectomy (RC) is the gold standard for the treatment of muscle-invasive bladder cancer (MIBC), half of the patients still experience distant metastasis after surgery [2]. Since the mid-1980s, multiple clinical studies have shown that bladder cancer has a good response to platinum-based combination chemotherapy. Randomized clinical trials (RCTs) and meta-analyses have shown that neoadjuvant chemotherapy (NAC) in MIBC can increase the overall survival rate (OS) of MIBC patients by 5–6% compared to direct RC [3,4,5]. Currently, patients with cT2-4aN0M0 (stage II or IIIA) are recommended to receive platinum-based combination neoadjuvant chemotherapy [6].
It was once thought that GC regimens (gemcitabine and cisplatin) and conventional MVAC regimens (methotrexate, vinblastine, doxorubicin, and cisplatin) have similar treatment response rates and survival rates, and GC is superior to MVAC in terms of safety and tolerability [7]. However, the VESPER trial recently came to a different conclusion: 6 cycles of dose-dense MVAC were more effective than 4 cycles of GC in terms of progression-free survival (PFS) and OS. However, dd-MVAC caused more severe asthenia and gastrointestinal side effects than GC [8, 9]. Because of their good therapeutic effects (Table 1), dd-MVAC and GC have become more widely used in clinical treatment. Both protocols were also written into the National Comprehensive Cancer Network guidelines in 2020 [10].
Currently, the GC regimen used in clinical practice has both a 21-day and a 28-day treatment cycle, as well as 3 or 4 cycles. The specific drug cycle, dose intensity, and total dose of different GC regimens are not the same. There are few studies that compare and analyze the clinical outcomes of different GC-neoadjuvant chemotherapy regimens. Therefore, the optimal GC neoadjuvant chemotherapy regimen has yet to be determined, and the optimal number of GC-neoadjuvant chemotherapy cycles remains uncertain. This systematic review and meta-analysis aims to compare the clinical results and toxicity among patients who received 3 or 4 cycles of neoadjuvant GC to guide clinical practice.
Materials and methods
Literature search and data extraction
This systematic review has been registered on PROSPERO (CRD42023409693). We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and the recommendations of the Cochrane Collaboration (http://www.prisma-statement.org/) for reporting preferences during the conduct of this meta-analysis.
We searched PubMed, Cochrane Library, Web of Science, Embase, the Chinese Biomedical Database (CMB), the Chinese National Knowledge Infrastructure (CNKI), and WAN FANG DATA up to March 2023 for pertinent studies that contrasted 3 and 4 cycles of the neoadjuvant GC for MIBC. The terms “bladder cancer,“ “bladder carcinoma,“ “neoadjuvant chemotherapy,“ “gemcitabine,“ and “cisplatin,“ as well as pertinent variations of these terms, are pertinent Medical Subject Headings. ((gemcitabine) OR (cisplatin)) AND ((bladder cancer) OR (bladder carcinoma)) AND ((neoadjuvant chemotherapy)) were used to create the search algorithm.
The titles and overviews of the retrieved articles were independently reviewed by two reviewers based on their inclusion criteria. A third reviewer settled any disagreements within the two reviewers on the data extracted. To get any missing data, the authors were contacted. The missing data was not included if they didn’t respond to our persistent inquiries. We chose the most recent article that contained the most recent information when the very same study was given more than once.
As a meta-analysis, ethical approval from an institutional review board or ethics committee was not required for this study.
Inclusion criteria and study eligibility
The eligibility of each study was determined based on the PICOS framework (par-ticipants, interventions, comparators, outcomes, and study design) to determine their suitability for inclusion [15]. Participants: patients with confirmed MIBC by biopsy who have undergone systemic neoadjuvant GC therapy; Interventions: MIBC patients who have received three cycles of systemic GC; Comparison group: MIBC patients with similar characteristics who have received four cycles of systemic GC; Outcomes: Comparison of oncological outcomes, including pathologic downstaging (pDS), pathologic complete response (pCR), overall survival (OS), cancer-specific survival (CSS), recurrence, and severe adverse events (SAE); Study design: There were no restrictions on study design, and randomized controlled trials and non-randomized observational studies were included in the analysis.
The primary outcomes were pCR, pDS, OS, and CSS. The secondary outcome were recurrence and SAEs. After surgery, a pathological investigation was used to determine both pCR and pDS. The TNM classification for pT0N0 or ypT0N0 in the final pathology was used to determine pCR. A lower pathologic stage than the preoperative clinical phase, or down staging to a non-muscle-invasive illness, was used to define pDS. The interval between the date of surgery and cancer-specific mortality or death from any cause, respectively, was used to determine CSS and OS. According to the National Cancer Institute’s (NCI) Common Terminology Criteria for Adverse Events, SAEs are those with a rating of ≥ 3.
Quality assessment
The quality assessment was independently conducted by two reviewers using the Newcastle-Ottawa Scale [16]. The Newcastle-Ottawa scale has three main assessment categories: exposure, comparability, and selection. Studies can receive up to nine points. A final rating of six points or higher denotes high quality.
Statistical analysis
The pCR, pDS, SAEs, and recurrence are represented as two dichotomous variables. In order to determine their 95% confidence intervals (CIs) and the odds ratios (ORs), the frequency of the events was subtracted. The OS and CSS outcomes were represented by hazard ratios (HRs) and their 95% confidence intervals (CIs). The results of studies with survival curve results were gathered from the Kaplan-Meier curve using published methods [17].
Between-study heterogeneity was assessed using chi-square and I2 tests. A Cochran Q statistic p-value < 0.05 or an I2 statistic > 50% was used to indicate statistically significant heterogeneity between trials [18].
According to the degree of heterogeneity, fixed-effect models or random-effects models were used to calculate summary statistics. For the initial analysis, a fixed-effects model was used, and a random-effects model was used for confirmatory analysis if significant heterogeneity was present [19]. When at least ten studies were involved in a particular outcome, a funnel plot was recommended for small-study effects assessment, according to version 6.3 of the Cochrane Handbook for Systematic Reviews of Interventions. However, only three studies met the inclusion criteria for this review. Review Manager 5.3 was used for meta-analysis.
Results
Literature search and characteristics
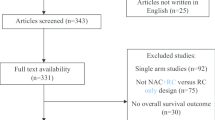
A flow chart of study selection according to PRISMA guidelines is presented in Fig. 1. The preliminary database search yielded 4982 studies (984 in PubMed, 3749 in OV-ID-EMBASE, and 249 in the Cochran library). After deleting duplicates, 2338 of these studies were left for review. Following a review of the titles and abstracts, 13 articles were disqualified. Using predetermined inclusion criteria, ten studies were examined. Two studies [20, 21] that used MVAC were disregarded. Additionally, two conference abstracts [22, 23] were further excluded. Three studies [24,25,26], with a total of 1091 patients, were included in the final analysis. Characteristics and interventions for them are presented in Table 2. All studies were retrospective case-control studies. Patients who had received neoadjuvant GC and had been diagnosed with MIBC were enrolled in all trials.
Quality assessment
The Newcastle-Ottawa scale was used to assess the quality of the nonrandomized studies, as shown in Table 3. Three studies received a 7-point rating, indicating that they were of high quality.
Pathologic complete response rate
For pCR, 3 studies with 1,065 patients were eligible for this analysis. The pCR was observed in 26.8% (136/508) of the 3 cycles of GC and in 40.1% (227/557) of the 4 cycles of GC (Table 1). An analysis was conducted, as shown in Fig. 2, and the pCR rate was higher in the 4 cycles of the GC group (OR = 0.66; 95% CI, 0.50–0.87; p = 0.003), and heterogeneity was not found across studies (I2 statistic, 51%; Cochran Q statistic, p = 0.13).
Pathologic down staging rate
For pDS, 2 studies of 839 patients were eligible for analysis. In the 3 and 4 cycle regimens, the pDS rates were 44.8% (156/348) and 58.0% (285/491), respectively (Table 2). According to Fig. 3, the analysis was carried out. The two regimens had significantly different pDS rates (OR = 0.63; 95% CI, 0.48–0.84; p = 0.002), and there was no study heterogeneity (I2 statistic, 2%; Cochran Q statistic, p = 0.31) between them.
Overall survival
OS results between the two treatment regimens are shown in Fig. 4. The OS analysis included three trials, and the findings showed that there was no statistically significant difference in OS across the arms (HR, 1.35; 95% CI, 0.86–2.12; p = 0.19). The studies were statistically different from one another (I2 statistic, 65%; Cochran Q statistic, p = 0.06).
Cancer-specific survival
Three studies reported CSS outcomes shown in Fig. 5. There was no significant difference between 3 cycles and 4 cycles of GC (HR, 1.06; 95% CI, 0.82–1.38; p = 0.20). Heterogeneity was not present (I2 statistic, 38%; Cochran Q statistic, p = 0.20).
Recurrence
The recurrence analysis shown in Fig. 6 could be performed on two studies with 925 patients. In 3 cycles, 31.8% of recurrences (131/411) and in 4 cycles, 28.4% of recurrences (146/514) occurred. The rate of recurrence did not differ substantially between the two treatment plans (OR = 1.23; 95% CI, 0.91–1.65; p = 0.18), and there was no heterogeneity among results from the trials (I2 statistic, 0%; Cochran Q statistic, p = 0.49).
Sever adverse events
Only one trial reported on the outcomes of SAEs. We conducted six analyses of SAEs, including neutropenia, thrombocytopenia, infection, neutropenic fever, anaemia, and decreased renal function, as shown in Fig. 7. The neutropenia of patients was statistically different (OR = 0.72; 95% CI, 0.52–0.99; p = 0.04) (Fig. 7(A)). There were no statistically significant differences in thrombocytopenia between patients (OR = 1.00; 95% CI, 0.68–1.47; p = 1.00) (Fig. 7(B)). The infection rate among patients did not differ statistically. (OR = 1.32; 95% CI, 0.67–2.57; p = 0.42; Fig. 7(C)). The rate of neutropenic fever among patients was not statistically different (OR = 1.00; 95% CI, 0.43–2.30; p = 1.00) (Fig. 7(D)). There was no difference in anemia (OR = 1.57; 95% CI, 0.94–2.62; p = 0.08) (Fig. 7(E)). Decreased renal function showed no differences (OR = 0.90; 95% CI, 0.36–2.23; p = 0.82) (Fig. 7(F)).
Discussion
Since the Southwest Oncology Group reported positive results with NAC for MIBC using MVAC in 2003 [27], the exploration of NAC regimens has gradually diversified. With the advancement of Phase II and III clinical trials for MIBC NAC, the GC regimen and the MVAC regimen have gradually emerged from many regimens because of their advantages of both less toxic side effects and good tumor treatment outcomes, becoming the most commonly used NAC regimen for MIBC [10, 28]. For radically resectable MIBC, neoadjuvant chemotherapy combined with radical cystectomy and pelvic lymphadenectomy is currently the gold standard of treatment. In NAC for MIBC, there was no significant difference in response rate or survival between GC and MVAC, the two most common preoperative chemotherapy regimens. According to a large, multicenter trial report of 935 patients, the incidence of pT0N0 disease was 23.9% in patients receiving GC regimen chemotherapy compared with 24.5% in patients receiving MVAC regimen chemotherapy (p = 0.2) [29]. GC, however, has a higher safety and tolerability profile compared with MVAC. The VESPER trial study showed that dose-dense MVAC caused more severe fatigue and gastrointestinal side effects than GC in perioperative chemotherapy. Despite its limitations, the study showed that 6 cycles of dose-dense MVAC were more effective in terms of PFS and OS than 4 cycles of GC [9]. Therefore, GC regimens are among the most widely used regimens in clinical practice.
In some studies, optimal NAC regimens were defined as 3 cycles of cisplatin-based regimens, whereas suboptimal NAC was defined as 1–2 cycles of cisplatin-based or non-cisplatin-based regimens. According to a retrospective multicenter study, optimal NAC had better RFS and OS compared with suboptimal or no NAC after propensity score matching [30]. Another study showed a higher rate of complete pathological response in the optimal NAC group (30.8%) than in the suboptimal group (15.6%) (p = 0.03) [31]. Similar conclusions were reached by Zhegalik AG et al [32]. One of the highest downstaging (pT2N0) rates to date (57%) has been reported in MIBC patients in a study of 6 cycles of a neoadjuvant GC regimen; however, 39% of these patients had to down- titrate because of toxic side effects. Only 67% of patients completed the planned six cycles [33]. Thus, NAC has significant differences between clinical trial design and actual clinical practice. Although long courses of NAC therapy have a more pronounced downstaging effect, the delay of surgery and chemotherapy-related toxicity may still negatively impact survival, so the optimal cycle of preoperative GC should be considered comprehensively.
In the randomized Phase 3 studies comparing GC and MVAC regimens, the GC regimen was set as a 28-day regimen, and cisplatin dosing in the MVAC regimen was consistent with the GC regimen, which reduced between-group differences and improved comparability between the GC and MVAC regimens. The GC regimen showed significantly fewer side effects than the MVAC regimen, and since then, GC has replaced MVAC as the standard NAC regimen for MIBC. In addition, 3-cycle and 4-cycle GC regimens have also been used in the clinical treatment of advanced urothelial carcinoma [7]. Currently, the NCCN guidelines recommend a 4-cycle GC chemotherapy regimen as the NAC regimen for MIBC [34], while the AUA and EAU guidelines lack specific recommendations regarding the optimal number of cycles of GC chemotherapy due to limited evidence [6, 35]. Because of this, 3–4 cycles of the GC regimen continue to be used in clinical practice, but few studies have reported differences in clinical treatment outcomes between 3 and 4 cycles of chemotherapy [36]. Although GC is one of the most commonly used neoadjuvant regimens, knowledge of its optimal number of neoadjuvant GC cycles remains limited in the academic community.
Recently, two multicenter retrospective studies have reported differences in clinical treatment outcomes between three cycles versus four cycles of neoadjuvant chemotherapy in MIBC patients, but the results are conflicting. Patel et al. [21] reported the clinical outcome of patients receiving cisplatin-based neoadjuvant chemotherapy (GC, MVAC, gemcitabine, carboplatin, and other non-cisplatin-based combinations). Some patients changed their original treatment plan because they could not tolerate the toxic side effects caused by chemotherapy, and about 30% of patients who originally planned to receive 4 cycles of NAC completed only 3 cycles of chemotherapy. Patients who completed 3 cycles of cisplatin-based neoadjuvant chemotherapy showed similar pathological responses and short-term survival compared with those who completed 4 cycles of chemotherapy. However, the study by D’Andrea et al. [20], showed that patients receiving 4 cycles of NAC (GC, MVAC, and dose-dense MVAC) had a higher pCR rate (28% vs. 21%, p = 0.02) and OS (HR 0.68; 95% CI 0.49, 0.94; p = 0.02) than patients receiving 3 cycles of NAC (GC, MVAC, and dose-dense MVAC).
In retrospective analyses, the pCR rate with NAC for MIBC ranged from 20–30% [36,37,38]. However, none of these reports have investigated and analyzed different NAC cycle numbers. A single-center retrospective study found that patients who received 4 cycles with dose reduction or 3 cycles without dose reduction had a higher rate of pathological response than patients who received fewer cycles of chemotherapy [39]. Unfortunately, this study did not directly compare the differences between 3 and 4 cycles of chemotherapy and did not correlate the number of cycles administered with survival for further studies and analyses. Our study showed that patients receiving the 4-cycle GC regimen had a higher rate of pathological response than those receiving the 3-cycle GC regimen. The proportion of pT0N0 was greater in patients receiving the 4-cycle GC regimen, which we speculate may be due to the higher cisplatin dose intensity and cumulative dose in the 4-song cycle regimen. Similarly, a recently published meta-analysis indicated that the dd-MVAC regimen had a more pronounced pathological downstaging effect because its total cisplatin dose and dose intensity were higher than those of the GC regimen [40].
Normally, the gold standard for evaluating the effectiveness of a therapy is often a pathological examination, and pCR is associated with a long-term survival benefit. Patients who achieve a pCR, which is defined as ypT0 ypN0 or ypT0/is ypN0, have improved survival [41]. In present study, there was no further corresponding improvement in CSS or OS in the patient group treated with 4 cycles of neoadjuvant GC, despite the positive downstaging efficacy. We speculate that the following reasons may explain the discrepancies among the available studies: (1) The OS and CSS outcomes of bladder cancer NAC patients are influenced by the subsequent treatment, as the disparities in OS and CSS between 3 and 4 rounds of chemotherapy can be hidden by RC. The study found that more than 20% of cT0 patients who received bladder-sparing therapy suffered recurrence or died during follow-up [42]. GC can eliminate micrometastases, reduce tumor volume, and lower invasiveness, thereby increasing the success rate of surgical removal [43, 44]. However, both 3-cycle and 4-cycle chemotherapy patients received RC treatment after GC. The scope of surgical resection of RC in MIBC patients was unaffected by the cycles of GC, and the extent of surgical removal is closely associated with patients’ post-operative recovery and long-term survival [45]. The surgical scope for both 3-cycle and 4-cycle chemotherapy patients is the same, significantly reducing the possibility of the primary lesion being the source for tumor recurrence and metastasis and eliminating the differences in OS and CSS indicators between 3-cycle and 4-cycle chemotherapy patients. (2) 3-cycle GC may already be effective in eliminating existing micrometastases, and 4-cycle GC might not enhance this efficacy. Although the effect of NAC treatment on micrometastases is positive, its reduction effect is not the same for tumors with different stages [46]. The study found that because cT3 tumors are larger and more infiltrative, with a higher rate of nodal and distant dissemination, the relative ratio of micro metastatic disease is higher in cT3 tumors than in cT2, whereas the relative ratio of untreatable micro metastatic nodal dissemination peaks in cT4a tumors [47]. As a result, the effect of NAC on OS would be greatest in cT3 tumors [37]. Based on this, we can infer that when tumor staging is relatively low, the reduction effect of 3-cycle and 4-cycle GC treatments is similar, and the effect on CSS and OS outcomes is not much different for these patients.
The sensitivity of tumors to cisplatin is the most critical factor determining the therapeutic effect for cancer patients; cisplatin sensitivity is far more important than the final cumulative dose and dose intensity of cisplatin [48, 49]. Some patients are insensitive to cisplatin [50, 51], and even higher doses of GC combination chemotherapy cannot completely eliminate drug-resistant micrometastases. To eradicate these micrometastases, the combination of GC with other antitumor drugs can be considered to improve the efficacy of neoadjuvant chemotherapy. For example, this can be accomplished by combining immunotherapy with immune checkpoint inhibitors (ICIs) [52], targeted therapies such as inhibitors of fibroblast growth factor receptor (FGFR) [53], or antibody-drug conjugates (ADCs) [54]. Recently, it was suggested that a less stringent follow-up regimen could be applied to patients with pCR after RC [55]. However, pT0 did not mean a patient was free of bladder cancer [56]. Previous studies have shown that pT0 patients have lower disease-specific mortality; nonetheless, there are still pT0 patients who die from bladder cancer [57]. As a result, this should be considered during treatment planning, and patients should be closely monitored during their survivorship.
Four cycles of GC chemotherapy were associated with a higher incidence of grade 3/4 adverse events, particularly hematologic toxicity. According to our findings, there were no statistically significant differences in thrombocytopenia, infection rate, neutropenic fever, anemia, or decreased renal function between 3 and 4 cycles of GC. Four cycles of GC chemotherapy were found to be related to a higher incidence of neutropenia. This finding indicates the use of granulocyte colony stimulating factor (G-CSF) prophylaxis as part of the 4-cycle GC regimen should probably be considered routine. However, only one of the included studies mentioned the adverse events; inadequate data may have hampered this assessment. We reviewed relevant studies regarding adverse effects of neoadjuvant GC regimens; a total of 5 studies showed toxicity data, of which 3 were retrospective studies [11, 58, 59], 1 RCT [60], and 1 prospective study [61]. Anemia, leukopenia, thrombocytopenia, vomiting, and diarrhea are among the most frequently reported side effects in the literature (Table 4). Grade 3/4 anemia has been reported in 0–32% of patients. The frequency of vomiting in patients has been reported to be 2.6–40% in the literature. Differences in the frequency of adverse reactions were explained by the different patient characteristics, dosage forms, and number of chemotherapy cycles used in the different studies.
According to our data, patients receiving 4 courses of the GC regimen with cisplatin dose intensification had significantly higher rates of pCR and pDS compared with patients receiving 3 courses of the GC regimen. OS and CSS were similar, and toxic side effects were manageable with both treatment regimens. Therefore, we suggest using a 4-cycle NAC plan for the GC regimen. Patients who cannot tolerate chemotherapy can stop receiving NAC after three cycles of the GC regimen without affecting the treatment’s effectiveness.
Our study has several limitations. First, only three studies met the criteria, and all were retrospective. Lack of studies, particularly prospective studies, would result in low levels of evidence. Second, some of the results in our study remained significantly heterogeneous, possibly due to different protocol doses and inclusion population bias. Third, the median follow-up time is relatively short, and a longer follow-up may help to estimate long-term survival data more precisely. In future work, further studies are warranted to determine the optimal number of cycles of neoadjuvant GC chemotherapy for MIBC patients.
Conclusions
In our meta-analysis, 4-cycle GC was superior to 3-cycle GC with regards to pCR and pDS, suggesting that 4-cycle GC is more effective than 3-cycle GC for MIBC, from pathology perspectives. The two regimens had similar survival rates, recurrence rates, and adverse effects.
However, this finding should be interpreted with caution because of the inherent limitations of retrospective studies. Large-scale RCTs and long-term follow-up studies are warranted to validate these outcomes.
Data availability
All data analyzed during this study is included in this published article.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Patel VG, Oh WK, Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin. 2020;70(5):404–23. https://doi.org/10.3322/caac.21631.
Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet. 2003;361(9373):1927–34. https://doi.org/10.1016/s0140-6736(03)13580-5.
Yin M, Joshi M, Meijer RP, Glantz M, Holder S, Harvey HA, et al. Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis. Oncologist. 2016;21(6):708–15. https://doi.org/10.1634/theoncologist.2015-0440.
Griffiths G, Hall R, Sylvester R, Raghavan D, Parmar MK. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29(16):2171–7. https://doi.org/10.1200/jco.2010.32.3139.
Witjes JA, Bruins HM, Cathomas R, Compérat EM, Cowan NC, Gakis G, et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur Urol. 2021;79(1):82–104. https://doi.org/10.1016/j.eururo.2020.03.055.
von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068–77. https://doi.org/10.1200/jco.2000.18.17.3068.
Joyce DD, Wymer KM, Sharma V, Moriarty JP, Borah BJ, Geynisman DM, et al. Comparative cost-effectiveness of neoadjuvant chemotherapy regimens for muscle-invasive bladder cancer: Results according to VESPER data. Cancer. 2022;128(24):4194–202. https://doi.org/10.1002/cncr.34502.
Pfister C, Gravis G, Fléchon A, Soulié M, Guy L, Laguerre B, et al. Randomized Phase III Trial of Dose-dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin, or Gemcitabine and Cisplatin as Perioperative Chemotherapy for Patients with Muscle-invasive Bladder Cancer. Analysis of the GETUG/AFU V05 VESPER Trial Secondary Endpoints: Chemotherapy Toxicity and Pathological Responses. Eur Urol. 2021;79(2):214–21. https://doi.org/10.1016/j.eururo.2020.08.024.
Flaig TW, Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2020;18(3):329–54. https://doi.org/10.6004/jnccn.2020.0011.
van de Putte EE, Mertens LS, Meijer RP, van der Heijden MS, Bex A, van der Poel HG, et al. Neoadjuvant induction dose-dense MVAC for muscle invasive bladder cancer: efficacy and safety compared with classic MVAC and gemcitabine/cisplatin. World J Urol. 2016;34(2):157–62. https://doi.org/10.1007/s00345-015-1636-y.
Zargar H, Shah JB, van Rhijn BW, Daneshmand S, Bivalacqua TJ, Spiess PE, et al. Neoadjuvant Dose Dense MVAC versus Gemcitabine and Cisplatin in Patients with cT3-4aN0M0 Bladder Cancer Treated with Radical Cystectomy. J Urol. 2018;199(6):1452–8. https://doi.org/10.1016/j.juro.2017.12.062.
Peyton CC, Tang D, Reich RR, Azizi M, Chipollini J, Pow-Sang JM, et al. Downstaging and Survival Outcomes Associated With Neoadjuvant Chemotherapy Regimens Among Patients Treated With Cystectomy for Muscle-Invasive Bladder Cancer. JAMA Oncol. 2018;4(11):1535–42. https://doi.org/10.1001/jamaoncol.2018.3542.
Ruplin AT, Spengler AMZ, Montgomery RB, Wright JL. Downstaging of Muscle-Invasive Bladder Cancer Using Neoadjuvant Gemcitabine and Cisplatin or Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin as Single Regimens or as Switch Therapy Modalities. Clin Genitourin Cancer. 2020;18(5):e557–e62. https://doi.org/10.1016/j.clgc.2020.02.010.
Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16. https://doi.org/10.1186/1472-6947-7-16.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. https://doi.org/10.1007/s10654-010-9491-z.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. https://doi.org/10.1186/1745-6215-8-16.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–60. https://doi.org/10.1136/bmj.327.7414.557.
Melsen WG, Bootsma MC, Rovers MM, Bonten MJ. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. 2014;20(2):123–9. https://doi.org/10.1111/1469-0691.12494.
D’Andrea D, Black PC, Zargar H, Dinney CP, Soria F, Cookson MS, et al. Identifying the Optimal Number of Neoadjuvant Chemotherapy Cycles in Patients with Muscle Invasive Bladder Cancer. J Urol. 2022;207(1):70–6. https://doi.org/10.1097/ju.0000000000002190.
Patel HD, Patel SH, Blanco-Martinez E, Kuzbel J, Chen VS, Druck A, et al. Four versus 3 Cycles of Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: Implications for Pathological Response and Survival. J Urol. 2022;207(1):77–85. https://doi.org/10.1097/ju.0000000000002189.
Major CK, Williams MB, Fleming MT. Three versus four cycles of neoadjuvant gemcitabine cisplatin for muscle invasive bladder cancer (MIBC). Journal of Clinical Oncology. 2021;39(15_suppl):e16524–e. https://doi.org/10.1200/JCO.2021.39.15_suppl.e16524.
Blanco-Martinez E, Patel HD, Patel SH, Kuzbel J, Chen VS, Druck A, et al. MP41-11 Deviations in cycles of neoadjuvant chemotherapy for muscle-invasive bladder cancer and implications for pathologic response and survival. Journal of Urology. 2021;206(Supplement 3):e764–e5. https://doi.org/10.1097/JU.0000000000002062.11.
Holmsten K, Omland LH, Als AB, Agerbæk M, Dohn LH, Lindberg H, et al. Implications for Efficacy and Safety of Total Dose and Dose-Intensity of Neoadjuvant Gemcitabine-Cisplatin in Muscle-Invasive Bladder Cancer: Three-Week Versus Four-Week Regimen. Bladder Cancer. 2022;8(1):71–80. https://doi.org/10.3233/blc-211556.
Aydin AM, Cheriyan SK, Reich R, Hajiran A, Peyton CC, Zemp L, et al. Comparative analysis of three vs. four cycles of neoadjuvant gemcitabine and cisplatin for muscle invasive bladder cancer. Urol Oncol. 2022;40(10):453.e19–.e26. https://doi.org/10.1016/j.urolonc.2022.05.023.
Ferro M, de Cobelli O, Musi G, Lucarelli G, Terracciano D, Pacella D, et al. Three vs. Four Cycles of Neoadjuvant Chemotherapy for Localized Muscle Invasive Bladder Cancer Undergoing Radical Cystectomy: A Retrospective Multi-Institutional Analysis. Front Oncol. 2021;11:651745. https://doi.org/10.3389/fonc.2021.651745.
Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859–66. https://doi.org/10.1056/NEJMoa022148.
von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602–8. https://doi.org/10.1200/jco.2005.07.757.
Zargar H, Espiritu PN, Fairey AS, Mertens LS, Dinney CP, Mir MC, et al. Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2015;67(2):241–9. https://doi.org/10.1016/j.eururo.2014.09.007.
Hinata N, Hussein AA, George S, Trump DL, Levine EG, Omar K, et al. Impact of suboptimal neoadjuvant chemotherapy on peri-operative outcomes and survival after robot-assisted radical cystectomy: a multicentre multinational study. BJU Int. 2017;119(4):605–11. https://doi.org/10.1111/bju.13678.
Boeri L, Soligo M, Frank I, Boorjian SA, Thompson RH, Tollefson M, et al. Clinical predictors and survival outcome of patients receiving suboptimal neoadjuvant chemotherapy and radical cystectomy for muscle-invasive bladder cancer: a single-center experience. World J Urol. 2019;37(11):2409–18. https://doi.org/10.1007/s00345-019-02689-8.
Zhegalik AG, Polyakov SL, Rolevich AI, Volkov AN, Minich AA, Vasilevich VJ, et al. Long-term results of a single-center prospective randomized trial assessing efficacy of a shortened course of adjuvant chemotherapy after radical cystectomy in patients with locally advanced bladder cancer. Cent European J Urol. 2020;73(1):26–32. https://doi.org/10.5173/ceju.2020.0032.
Iyer G, Balar AV, Milowsky MI, Bochner BH, Dalbagni G, Donat SM, et al. Multicenter Prospective Phase II Trial of Neoadjuvant Dose-Dense Gemcitabine Plus Cisplatin in Patients With Muscle-Invasive Bladder Cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2018;36(19):1949–56. https://doi.org/10.1200/JCO.2017.75.0158.
Flaig TW, Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network: JNCCN. 2020;18(3):329–54. https://doi.org/10.6004/jnccn.2020.0011.
Chang SS, Bochner BH, Chou R, Dreicer R, Kamat AM, Lerner SP, et al. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. J Urol. 2017;198(3):552–9. https://doi.org/10.1016/j.juro.2017.04.086.
Galsky MD, Pal SK, Chowdhury S, Harshman LC, Crabb SJ, Wong YN, et al. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer. 2015;121(15):2586–93. https://doi.org/10.1002/cncr.29387.
Rosenblatt R, Sherif A, Rintala E, Wahlqvist R, Ullén A, Nilsson S, et al. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur Urol. 2012;61(6):1229–38. https://doi.org/10.1016/j.eururo.2011.12.010.
Pignot G, Loriot Y, Kamat AM, Shariat SF, Plimack ER. Effect of Immunotherapy on Local Treatment of Genitourinary Malignancies. Eur Urol Oncol. 2019;2(4):355–64. https://doi.org/10.1016/j.euo.2019.01.002.
Gandhi NM, Baras A, Munari E, Faraj S, Reis LO, Liu JJ, et al. Gemcitabine and cisplatin neoadjuvant chemotherapy for muscle-invasive urothelial carcinoma: Predicting response and assessing outcomes. Urol Oncol. 2015;33(5):204.e1–7. https://doi.org/10.1016/j.urolonc.2015.02.011.
Chung DY, Kang DH, Kim JW, Ha JS, Kim DK, Cho KS. Comparison of Oncologic Outcomes of Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin (ddMVAC) with Gemcitabine and Cisplatin (GC) as Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: Systematic Review and Meta-Analysis. Cancers (Basel). 2021;13(11). https://doi.org/10.3390/cancers13112770.
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet (London, England). 2014;384(9938):164–72. https://doi.org/10.1016/S0140-6736(13)62422-8.
Hellenthal NJ, Ramirez ML, Evans CP, Devere White RW. The role of surveillance in the treatment of patients with muscle-invasive bladder cancer after chemotherapy. BJU Int. 2010;105(4):485–8. https://doi.org/10.1111/j.1464-410X.2009.08905.x.
Heinrich S, Lang H. Neoadjuvant Therapy of Pancreatic Cancer: Definitions and Benefits. Int J Mol Sci. 2017;18(8). https://doi.org/10.3390/ijms18081622.
Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):29–42. https://doi.org/10.1016/s1470-2045(20)30555-6.
Su KY, Lee WL. Fourier Transform Infrared Spectroscopy as a Cancer Screening and Diagnostic Tool: A Review and Prospects. Cancers (Basel). 2020;12(1). https://doi.org/10.3390/cancers12010115.
Jiang DM, Jiang H, Chung PWM, Zlotta AR, Fleshner NE, Bristow RG, et al. Neoadjuvant Chemotherapy Before Bladder-Sparing Chemoradiotherapy in Patients With Nonmetastatic Muscle-Invasive Bladder Cancer. Clin Genitourin Cancer. 2019;17(1):38–45. https://doi.org/10.1016/j.clgc.2018.09.021.
Vazina A, Dugi D, Shariat SF, Evans J, Link R, Lerner SP. Stage specific lymph node metastasis mapping in radical cystectomy specimens. J Urol. 2004;171(5):1830–4. https://doi.org/10.1097/01.ju.0000121604.58067.95.
de Vries G, Rosas-Plaza X, van Vugt M, Gietema JA, de Jong S. Testicular cancer: Determinants of cisplatin sensitivity and novel therapeutic opportunities. Cancer Treat Rev. 2020;88:102054. https://doi.org/10.1016/j.ctrv.2020.102054.
Országhová Z, Kalavska K, Mego M, Chovanec M. Overcoming Chemotherapy Resistance in Germ Cell Tumors. Biomedicines. 2022;10(5). https://doi.org/10.3390/biomedicines10050972.
Chen J, Wang L, Tang Y, Gong G, Liu L, Chen M, et al. Maspin enhances cisplatin chemosensitivity in bladder cancer T24 and 5637 cells and correlates with prognosis of muscle-invasive bladder cancer patients receiving cisplatin based neoadjuvant chemotherapy. J Exp Clin Cancer Res. 2016;35:2. https://doi.org/10.1186/s13046-015-0282-y.
Xing J, Chen W, Chen K, Zhu S, Lin F, Qi Y, et al. TFAP2C Knockdown Sensitizes Bladder Cancer Cells to Cisplatin Treatment via Regulation of EGFR and NF-κB. Cancers (Basel). 2022;14(19). https://doi.org/10.3390/cancers14194809.
Funt SA, Lattanzi M, Whiting K, Al-Ahmadie H, Quinlan C, Teo MY, et al. Neoadjuvant Atezolizumab With Gemcitabine and Cisplatin in Patients With Muscle-Invasive Bladder Cancer: A Multicenter, Single-Arm, Phase II Trial. J Clin Oncol. 2022;40(12):1312–22. https://doi.org/10.1200/jco.21.01485.
Loriot Y, Necchi A, Park SH, Garcia-Donas J, Huddart R, Burgess E, et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2019;381(4):338–48. https://doi.org/10.1056/NEJMoa1817323.
Rosenberg JE, O’Donnell PH, Balar AV, McGregor BA, Heath EI, Yu EY, et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J Clin Oncol. 2019;37(29):2592–600. https://doi.org/10.1200/jco.19.01140.
Ravi P, Pond GR, Diamantopoulos LN, Su C, Alva A, Jain RK, et al. Optimal pathological response after neoadjuvant chemotherapy for muscle-invasive bladder cancer: results from a global, multicentre collaboration. BJU Int. 2021;128(5):607–14. https://doi.org/10.1111/bju.15434.
Parker WP, Ho PL, Boorjian SA, Melquist JJ, Thapa P, Holzbeierlein JM, et al. The importance of clinical stage among patients with a complete pathologic response at radical cystectomy after neoadjuvant chemotherapy. World J Urol. 2016;34(11):1561–6. https://doi.org/10.1007/s00345-016-1801-y.
Kassouf W, Spiess PE, Brown GA, Munsell MF, Grossman HB, Siefker-Radtke A, et al. P0 stage at radical cystectomy for bladder cancer is associated with improved outcome independent of traditional clinical risk factors. Eur Urol. 2007;52(3):769–74. https://doi.org/10.1016/j.eururo.2007.03.086.
Matsubara N, Mukai H, Naito Y, Nezu M, Itoh K. Comparison between neoadjuvant and adjuvant gemcitabine plus cisplatin chemotherapy for muscle-invasive bladder cancer. Asia Pac J Clin Oncol. 2013;9(4):310–7. https://doi.org/10.1111/ajco.12017.
Kaneko G, Kikuchi E, Matsumoto K, Obata J, Nakamura S, Miyajima A, et al. Neoadjuvant gemcitabine plus cisplatin for muscle-invasive bladder cancer. Jpn J Clin Oncol. 2011;41(7):908–14. https://doi.org/10.1093/jjco/hyr068.
Khaled HM, Shafik HE, Zabhloul MS, Ghoneim M, Saber RA, Manie M, et al. Gemcitabine and cisplatin as neoadjuvant chemotherapy for invasive transitional and squamous cell carcinoma of the bladder: effect on survival and bladder preservation. Clin Genitourin Cancer. 2014;12(5):e233–40. https://doi.org/10.1016/j.clgc.2014.04.002.
Herchenhorn D, Dienstmann R, Peixoto FA, de Campos FS, Santos VO, Moreira DM, et al. Phase II trial of neoadjuvant gemcitabine and cisplatin in patients with resectable bladder carcinoma. Int Braz J Urol. 2007;33(5):630–8; discussion 8. https://doi.org/10.1590/s1677-55382007000500004.
Acknowledgements
We are grateful to all researchers for their enrolled studies.
Funding
This research was supported by the National Natural Science Foundation of China (grant no. 82060459) and the Gansu Health Industry Research Program (grant no. GSWSKY2021-014).
Author information
Authors and Affiliations
Contributions
Lanpeng Lu and Chaohu Chen contributed equally to this work. Lanpeng Lu and Chaohu Chen drafted the manuscript. Hui Cheng revised the manuscript. The hypothesis was developed by Zhiping Wang, who also helped to draft the manuscript’s final draft. The final manuscript was authorized by all authors after they had read the draft.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest have been disclosed by the authors.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lu, L., Chen, C., Cheng, H. et al. Comparison of 3 and 4 cycles of neoadjuvant gemcitabine and cisplatin for muscle-invasive bladder cancer: a systematic review and meta-analysis. BMC Cancer 23, 1066 (2023). https://doi.org/10.1186/s12885-023-11572-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11572-0