Abstract
Background
Platelet (PLT) count at diagnosis plays an important role in cancer development and progression in solid tumors. However, it remains controversial whether PLT count at diagnosis influences therapeutic outcome in patients with non-acute promyelocytic leukemia (APL) acute myeloid leukemia (AML).
Methods
This study analyzed the relationship between PLT count at diagnosis and genetic mutations in a cohort of 330 newly diagnosed non-APL AML patients. The impact of PLT count on complete remission, minimal residual disease status and relapse-free survival (RFS) were evaluated after chemotherapy or allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Results
Our studies showed that patients with DNMT3A mutations have a higher PLT count at diagnosis, while patients with CEBPA biallelic mutations or t(8;21)(q22; q22) translocation had lower PLT count at diagnosis. Furthermore, non-APL AML patients with high platelet count (> 65 × 109/L) at diagnosis had worse response to induction chemotherapy and RFS than those with low PLT count. In addition, allo-HSCT could not absolutely attenuated the negative impact of high PLT count on the survival of non-APL AML patients.
Conclusion
PLT count at diagnosis has a predictive value for therapeutic outcome for non-APL AML patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML) is one of the most common adult hematopoietic malignancies with poor prognosis [1, 2]. 70% of AML patients achieve complete morphologic remission after standard “3 + 7” induction treatment, most patients with complete remission (CR) relapse and progress to refractory leukemia after consolidation therapy [3, 4]. The persistence of minimal residual disease (MRD) is a risk factor for leukemic recurrence in AML patients after chemotherapy [5, 6]. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) was necessary to decrease the probability of relapse for AML patients [7, 8]. Therefore, it was important to identify the adverse outcome-related risk factors at diagnosis in AML.
Cytogenetics, molecular abnormalities and epigenetic alterations have been acknowledged as the most important prognostic factors in AML patients [1, 2, 4, 9]. In addition, clinical characteristics contribute to chemotherapeutic response and survival in these patients; for instance, high white blood cell count (WBC) is associated with more probability of early mortality and occurrence of central nervous system leukemia [10, 11]. Clinically, the majority of AML patients have thrombocytopenia, leukocytosis and anemia at diagnosis, and only a small number of patients have normal or high platelet (PLT) count [12, 13]. Studies showed that PLTs increased the resistance of colon and ovarian cancer cell lines to 5-fluorouracil and paclitaxel [14, 15]. Increasing evidence suggested that PLTs played a predominant role in colon and breast cancer cells metastasis to lung and brain [16, 17]. Thrombocytosis was considered as an adverse-risk factor in lung, gastric, colon, breast and kidney cancers [18,19,20,21]. Reportedly, platelet microparticles (PMPs) in AML was important in leukemic development and contributed to chemotherapeutic resistance [22, 23]. A clinical trial showed that AML patients with a medium PLT count of 50–120 × 109/L had longer overall survival (OS) and disease-free survival (DFS) than the other patients [24]. Other studies showed that low PLT count were associated with better survival in intermediate-risk AML patients [24, 25]. However, the influence of high PLT count on therapeutic outcome remains obscure in AML patients.
In this study, we investigated the relationship between clinic characteristics and PLT count, analyzed the impact of PLT count on therapeutic outcome and MRD status, followed up their survival after chemotherapy or allo-HSCT in newly diagnosed non-APL AML patients.
Patients and methods
Patients
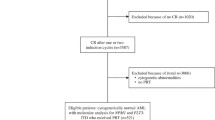
This retrospective study enrolled 330 newly diagnosed de novo non-APL AML patients aged 16–65 years in Nanfang Hospital (Guangzhou, China) from June 2018 to December 2020. The inclusion criteria were as following: (1) age 16–65 years, (2) de novo AML, (3) standard “3 + 7” induction regime and (4) at least four courses of chemotherapy with regular follow-up. The exclusion criteria were as following: (1) preceding hematological disorders, (2) therapy-related AML or (3) other carcinomas. Patients were diagnosed according to the French-American-British (FAB) and 2016 revision of the World Health Organization classification of myeloid neoplasms [26]. Molecular mutational abnormalities were detected by next-generation sequencing (NGS). The designed panel included: CEBPA, FLT3, KIT, NPM1, ASXL1, RUNX1, BCOR, EZH2, SF3B1, SRSF2, U2AF1, ZRSR2, DNMT3A, GATA2, IDH1, IDH2, NRAS, MLL, KRAS, PHF6, TET2, TP53, WT1, STAG2, SETBP1, ETV6, JAK2, CALR, MPL, SH2B3, and CSF3R (Supplement. Table S1). Risk groups were classified according to the 2022 European Leukemia Net (ELN) guideline [2]. The study protocol was reviewed and approved by ethics committee, and patient flow diagram was shown in Fig. 1.
Treatments
First, patients were treated with standard induction chemotherapy based on the “3 + 7” regimen, including idarubicin (IDA 10 mg/m2, days 1–3) or daunorubicin (DNR 60 mg/m2, days 1–3) and cytarabine (Ara-C 100 mg/m2, days 1–7). Bone marrow (BM) aspiration was performed after 14–21 days post-induction chemotherapy to evaluate the treatment response. Patients with CR continued to receive two cycles of consolidation chemotherapy based on high-dose cytarabine (HD-Ara-C 2 g/m2, twice, days 1–3). Patients without CR received the second cycle of induction chemotherapy based on high-dose Ara-C (Ara-C 2 g/m2 plus cladribine 5 mg/m2, days 1–5 and G-CSF 300 ug days 0–5). Routine blood tests were performed to provide necessary supportive treatment. Tyrosine kinase inhibitors or FLT3 inhibitors were added to the induction and consolidation treatments in Philadelphia or FLT3-internal tandem duplication (ITD)-positive AML patients. After CR, all patients received two cycles of cytarabine-based consolidation chemotherapy. For adverse-risk patients, allo-HSCT was administrated after two cycles of consolidation chemotherapy, except for those without HLA-matched donors or refusing haploidentical transplantation. For favorable and intermediate-risk patients, MRD status after two cycles of consolidation chemotherapy was a critical indicator to determine subsequent treatment strategies. Patients with negative MRD (MRD-) continue with two cycles of consolidation chemotherapy and those with positive MRD (MRD+) underwent allo-HSCT.
Allo-HSCT
As described previously [27], there were two alternative myeloablative conditioning regimens in our center, including busulfan (Bu 3.2 mg/kg/day, -7 to -4 days) + cyclophosphamide (Cy 60 mg/kg/day, -3 to -2 days) and Bu (3.2 mg/kg/day, -6 to -3 days) + fludarabine (Flu 30 mg/m2, -6 to -2 days). Graft-versus-host disease (GVHD) prophylaxis was regularly administered, such as cyclosporine A (CsA) plus methotrexate (MTX) in HLA-matched sibling donor transplant, CsA + MTX + antithymocyte globulin and/or mycophenolate in HLA-matched unrelated donor and haploidentical transplants. CsA was gradually withdrawn after 30 days post-transplantation, and donor lymphocyte infusion was applied after 60 days post-transplantation in patients without GVHD. Effective regiments were used to keep GVHD under control, such as methylprednisolone, CsA, CD25 monoclonal antibody or combined with other immunosuppressive agents for acute GVHD, corticosteroids and CsA for chronic GVHD.
Definition of clinical end points
Treatment response was assessed by routine blood tests and BM morphology according to standardization response criteria. CR was defined as < 5% BM leukemic blasts with normal maturation of all cell lineages [28]. In addition, recovery of neutrophils (≥ 1500/µl) and PLTs (≥ 100,000/µl) in peripheral blood was mandatory, with no evidence of circulating blasts and/or extramedullary leukemia. Relapse was defined as the re-occurrence of 5% leukemic blasts in BM, re-appearance of circulating blasts or development of extramedullary leukemia [28]. MRD was performed by multiparametric flow cytometry to detect abnormal leukemia populations with leukemia-associated immunophenotypes in total CD45 + cells in patients with CR before each cycle of consolidation therapy. MRD- was defined as the detection of < 0.1% abnormal cells, and MRD + was defined as the detection of ≥ 0.1% abnormal cells. Patients achieved CR were followed up for 2 years to calculate their relapse-free survival (RFS). RFS was measured from the date of first CR (CR1) until death, the first relapse, or the last follow-up in continuous CR.
Statistical analysis
All clinical data were analyzed using SPSS (SPSS, Chicago, IL), Prism9 (GraphPad Software, La Jolla, CA). Median values and ranges were used for continuous variables and percentages for categorical variables. Groups were compared using the Pearson chi-square test or Fisher’s exact test for categorical variables and Mann-Whitney U tests for continuous variables. The discriminatory power of PLT count value to predict CR was assessed by estimating the area under the receiver operating characteristic (ROC) curve (AUC). The optimal cut-off value was determined by maximizing sensitivity and specificity and their 95% confidence intervals (CIs). Cox proportional hazards regression models were used to determine the influence of PLT count on RFS in AML patients after chemotherapy or allo-HSCT, and the results are expressed as hazard ratio (HR) with 95% CI. All statistical tests were 2-sided, and a P value of < 0.05 was considered statistically significant.
Results
Clinical characteristics of AML patients
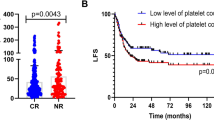
A cohort of 330 patients were included in the study. PLT counts ranged from 6 to 314, with a median of 42 × 109/L. The distribution of PLT counts did not suggest apparent grouping (Fig. 2a). Based on CR after the first cycle of induction chemotherapy, the cut-off value of PLT count for therapeutic outcome in AML patients according to the ROC curve analysis was 65 × 109/L (Fig. 2b). Therefore, the patients were divided into low PLT count group (≤ 65 × 109/L, n = 216, 65.5%) and high PLT count group (> 65 × 109/L, n = 114, 34.5%). The clinical characteristics of these patients were shown in Table 1. Patients with high PLT count had higher hemoglobin levels and more megakaryocytes (MKs) in the BM (P = 0.006 and 0.001). Cytogenetics and molecular abnormalities were compared between both groups. As shown in Table 2, the high PLT count group had more patients with DNMT3A mutation, and the low PLT count group had more patients with t(8;21)(q22;q22) translocation and CEBPA biallelic mutation. In addition, we found that PLT counts in patients with t(8;21)(q22;q22) were lower than in those with normal karyotype (27 × 109/L vs. 68 × 109/L, P = 0.000, Fig. 2c). Patients with CEBPA biallelic mutations had lower PLT count (31 × 109/L, P = 0.013, Fig. 2d), and those with DNMT3A mutations had higher PLT count (105 × 109/L, P = 0.000, Fig. 2e), as compared with CEBPA and DNMT3A wild-type at diagnosis (68 × 109/L and 60 × 109/L).
(a) Distribution of PLT counts in 330 newly diagnosed AML patients. (b) ROC curve analysis for initial PLT count > 65 × 109/L. (c) Comparison of PLT counts between patients with t(8;21) (n = 34) and those with normal karyotypes (n = 217). (d) Comparison of PLT counts between patients with mutated CEBPA biallelic mutation (n = 29) and wild-type mutation (n = 301). (e) Comparison of PLT counts between patients with mutated DNMT3A (n = 33) and wild-type DNMT3A (n = 297). *P < 0.05, **P < 0.01,***P < 0.001
PLT: platelet; AML: acute myeloid leukemia; ROC: Receiver operating characteristic
Impact of platelet count on induction chemotherapy response in AML
After the first course of induction chemotherapy, 85.6% (185/216) of the patients in low PLT group achieved CR while 57.9% (66/114) in high PLT group (P = 0.000). Among risk groups based on 2022 ELN classification, there were significant difference in CR rate between low and high PLT group in intermediate-risk group (75.9% vs. 47.6%, P = 0.004) and adverse-risk group (81.0% vs. 49.0%, P < 0.001) except favorable-risk group (93.9% vs. 95.7%, P = 0.750).
Patients without CR received the second cycle of induction chemotherapy (n = 79); 95.4% (206/216) of patients in the low PLT group achieved CR as compared to 81.6% (93/114) in the high PLT group (P = 0.000, Fig. 3a). After two cycles of induction chemotherapy, patients in the low PLT group had a higher CR rate than the high PLT group in intermediate-risk group (92.6% vs. 69.0%, P = 0.003, Fig. 3a). However, the CR rates were not significantly different between the low and high PLT groups in favorable-risk group (99.0% vs. 100%, P = 0.628, Fig. 3a) and adverse-risk group (92.1% vs. 81.6%, P = 0.098, Fig. 3a).
(a) The proportion of CR after 1–2 cycles of induction chemotherapy between low and high PLT groups in the whole cohort of AML, favorable-risk AML (FR)-AML, intermediate-risk AML (IR-AML) and adverse-risk AML (AR-AML) patients. (b) The proportion of MRD-negative CR (CR/MRD-) after induction chemotherapy between low and high PLT groups in the whole cohort of AML, FR-AML, IR-AML and AR-AML patients. (c) The proportion of CR/MRD- after the first cycle of consolidation of chemotherapy between low and high PLT groups in the whole AML and different subgroups. (d) The proportion of CR/MRD- after two cycles of consolidation of chemotherapy between low and high PLT groups in the whole cohort and different subgroups. *P < 0.05, **P < 0.01,***P < 0.001
CR: complete remission; PLT: platelet; AML: acute myeloid leukemia; FR: favorable-risk; IR: intermediate-risk; AR: adverse-risk; MRD: minimal residual disease
Impact of platelet count on MRD status in AML
After induction chemotherapy, more patients in the low PLT group (75/206) achieved CR/MRD- compared with patients in the high PLT group (17/93) (36.4% vs. 18.3%, P = 0.002, Fig. 3b). In the intermediate-risk, there were more patients with CR/MRD- in the low PLT group than in the high PLT group (38.0% vs. 13.8%, P = 0.022, Fig. 3b). There was no difference in the proportion of CR/MRD- between patients with low and high PLT count in favorable-risk group (43.9% vs. 34.8%, P = 0.427, Fig. 3b) and adverse-risk groups (22.4% vs. 12.2%, P = 0.194, Fig. 3b). After the first cycle of consolidation chemotherapy, the low PLT group had more patients with CR/MRD- than the high PLT group in the whole cohort (62.5% vs. 37.5%, P = 0.000, Fig. 3c). This finding was also observed in the intermediate-risk group (64.0% vs. 37.9%, P = 0.025, Fig. 3c) but not in favorable-risk (81.6% vs. 65.2%, P = 0.085, Fig. 3c) and adverse-risk groups (56.9% vs. 39.0%, P = 0.081, Fig. 3c). After two cycles of consolidation chemotherapy, there were no significant differences in the proportion of CR/MRD- between the low and high PLT groups in the whole cohort (83.3% vs. 74.2%, P = 0.070, Fig. 3d), including favorable-risk (90.6% vs. 86.4%, P = 0.551, Fig. 3d), intermediate-risk (77.1% vs. 67.9%, P = 0.378, Fig. 3d) and adverse-risk groups (75.9% vs. 71.8%, P = 0.653, Fig. 3d).
Impact of platelet count on relapse-free survival in AML patients treated with chemotherapy
After 1–2 cycles of induction chemotherapy, 299 patients achieved CR. Among them, 40.8% (122/299) of patients received chemotherapy alone; here, patients with low PLT count had better 2-year RFS than those with high PLT count (89.9% vs. 58.1%, P = 0.000, Fig. 4a). In the subgroup analysis, a better RFS was also observed in favorable-risk group (98.2% vs. 66.7%, P = 0.000, Fig. 4b) and intermediate-risk group (90.0% vs. 46.7%, P = 0.017, Fig. 4c), but not in adverse-risk group (53.8% vs. 60.0%, P = 0.787, Fig. 4d).
(a-d) For whole AML patients treated with chemotherapy only, the 2-year RFS was compared between low- and high-PLT groups; (a) The 2-year RFS between low- and high-PLT groups in AML; (b) In favorable-risk AML, the 2-year RFS between low- and high-PLT groups; (c) In intermediate-risk AML, the 2-year RFS between low- and high-PLT groups; (d) In adverse-risk AML, the 2-year RFS between low- and high-PLT groups in AML; (e-h) For whole AML patients treated with allo-HSCT, the 2-year RFS was compared between low- and high-PLT groups; (e) The 2-year RFS between low- and high-PLT groups in AML; (f) In favorable-risk AML, the 2-year RFS between low- and high-PLT groups; (g) In intermediate-risk AML, the 2-year RFS between low- and high-PLT groups; (h) In adverse-risk AML, the 2-year RFS between low- and high-PLT groups in AML
Impact of platelet count on relapse-free survival in AML patients treated with allo-HSCT
There were 177 (59.2%, 177/299) patients with CR received allo-HSCT. Clinic and transplant characteristics of these patients were shown in Table 3. The patients with low PLT count had better 2-year RFS than those with high PLT count (82.7% vs. 60.0%, P = 0.001, Fig. 4e). In the subgroup analyses, patients with low PLT count had better 2-year RFS than those with high PLT count in favorable-risk (86.0% vs. 40.0%, P = 0.010, Fig. 4f) and intermediate-risk group (92.3% vs. 64.3%, P = 0.013, Fig. 4g). However, there weren’t different 2-year RFS between patients with low and high PLT count in adverse-risk group (71.7% vs. 61.3%, P = 0.393, Fig. 4h).
Multivariate analysis of relapse-free survival
The multivariate analyses of RFS were presented in Table 4. Cox proportional hazards regression analysis revealed that low PLT count (≤ 65 × 109/L, HR 0.334, 95% CI 0.197–0.565, P = 0.001), WBC < 20 × 109/L (HR 0.321, 95% CI 0.187–0.550, P = 0.001) and ELN favorable risk (HR 0.408, 95% CI 0.207–0.804, P = 0.010) were found to be significantly associated with an increasing RFS in the whole AML group. For patients in favorable-risk group, low PLT count (≤ 65 × 109/L) was an independent protective factor for RFS (HR 0.068, 95% CI 0.020–0.237, P = 0.001). In addition, low platelet count (≤ 65 × 109/L, HR 0.084, 95% CI 0.021–0.342, P = 0.001) had a beneficial association with RFS in intermediate-risk group.
Discussion
AML is a hematological malignancy with significant clinical heterogeneity. Cytogenetic abnormalities and molecular mutations are critical indicators for the prognostic stratification of AML patients, which can help formulate an optimal therapy strategy [29,30,31]. In addition, clinical characteristics at diagnosis also contribute to chemotherapeutic response and survival in AML patients [10]. In this study, our data showed that higher PLT count was associated with worse response to induction chemotherapy, fewer proportion of CR/MRD- and shorter RFS in AML patients. We further noted that these effects were more profound in intermediate-risk patients than favorable and adverse-risk AML patients. These findings were consistent with other investigations that lower PLT count predicted better survival in intermediate-risk group [24, 32]. Moreover, hyperleukocytosis defined as WBC > 100 × 109/L at diagnosis was demonstrated to relate with increasing early mortality in AML patients [10, 33], which prognostic significance in RFS is not widely recognized [34, 35]. Our data showed that WBC < 20 × 109/L was a beneficial factor for RFS in AML patients. It was also reported that WBC ≥ 20 × 109/L was correlated with decreasing EFS in newly diagnosed cytogenetically normal AML patients [36].
Some research showed that AML patients with medium PLT count at diagnosis in the range of 50–120 × 109/L had longer OS and DFS than the other patients [24]. Others reported that pretreatment PLT count > 130 × 109/L was an unfavorable prognostic factor for chemotherapy response and prognosis in AML patients [32]. Although cut-off value of PLT count was various in different studies, these clinical data demonstrated that higher PLT count harbored a negative impact on survival of AML patients. However, the relationship between PLT count and therapeutic outcome in different risk groups was unclear. About 50% AML patients are classified as intermediate-risk group based on ELN risk classification, which 4-year OS was no more than 50% [37]. Therefore, it is critical to identify novel risk factors for patients in intermediate-risk group who fail to induction therapy. Our study demonstrated that PLT count was considered as a valuable indicator to predict therapeutic response and RFS of AML patients in intermediate-risk group.
Platelets have been reported to play a pivotal role in cell proliferation, metastasis, drug resistance in lung and ovarian cancers [38, 39]. Higher PLT count confers poor prognosis in many cancer types, including colon, lung, ovary, and stomach [19, 20, 40, 41]. A variety of substances stored and secreted by platelets had effect on proliferation of leukemic cells, such as platelet-derived growth factor, vascular endothelial growth factor, transforming growth factor-β and serotonin [42,43,44,45,46]. It was shown that PMPs could be internalized by AML cells, which transferred microRNAs in relation to chemotherapy resistance from platelets to leukemic cells via PMPs internalization [22, 47,48,49]. Our study showed that PLT count were negatively associated with chemosensitivity of AML patients in intermediate-risk group, but the mechanisms need to be further explored.
Studies showed that cytogenetic or molecular abnormalities had an influence on proliferation and differentiation of MKs as well as platelets production in AML patients [32, 50]. The thrombopoietin (TPO)/myeloproliferative leukemia virus oncogene (MPL) pathway plays a critical role in both normal and malignant hematopoiesis and megakaryopoiesis [51, 52]. Furthermore, TPO/MPL pathway are involved in the interaction of human leukemic stem cells (LSCs) with hematopoietic microenvironment [53, 54]. Upregulation of the TPO/MPL signaling pathway protects the human LSCs from chemotherapy, which results in chemoresistance and recurrence [54]. It was reported that TPO/MPL signaling was up-regulated in DNMT3A mutated AML patients with high PLT count and poor prognosis [55]. Our data showed that high PLT count at diagnosis was found in DNMT3A mutated AML, which was more probability detected in M5 subtype [56, 57]. Interestingly, it was reported that high expression of MPL on blasts in AML with t(8;21) led to severe thrombocytopenia by scavenging TPO [25, 58], which was consistent with our results.
There were limited numbers of patients in our single-center retrospective study, it wasn’t found that normal or elevated platelet counts were frequently detected in AML patents with some genetic mutations or cytogenetic abnormalities as RUNX1 and chromosome 3q abnormalities, which were reported in other studies [59, 60]. Otherwise, the mechanism of influence on therapeutic outcome by platelets wasn’t explored, investigations was needed in our further study.
In conclusion, our study demonstrated that higher platelet count at diagnosis was related to worse therapeutic outcome and shorter RFS in AML patients, especially in intermediate-risk AML patients. Further mechanistic investigations are needed to provide novel potential targets for AML patients.
Data Availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Papaemmanuil E, et al. Genomic classification and prognosis in Acute Myeloid Leukemia. N Engl J Med. 2016;374(23):2209–21.
Döhner H, Wei AH. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN 2022. 140(12): p. 1345–1377.
Tefferi A, Letendre L. Going beyond 7 + 3 regimens in the treatment of adult acute Myeloid Leukemia. J Clin Oncol. 2012;30(20):2425–8.
Short NJ, Rytting ME, Cortes JE. Acute Myeloid Leukaemia Lancet. 2018;392(10147):593–606.
Chen X, et al. Relation of clinical response and minimal residual Disease and their prognostic impact on outcome in acute Myeloid Leukemia. J Clin Oncol. 2015;33(11):1258–64.
Paiva B, Vidriales MB. Impact of measurable residual Disease by decentralized flow cytometry: a PETHEMA real-world study in 1076 patients with acute Myeloid Leukemia. 2021. 35(8): p. 2358–70.
Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127(1):62–70.
Schmid C, et al. Outcome of patients with distinct molecular genotypes and cytogenetically normal AML after allogeneic transplantation. Blood. 2015;126(17):2062–9.
Döhner H, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–47.
Greenwood MJ, et al. Leukocyte count as a predictor of death during remission induction in acute Myeloid Leukemia. Leuk Lymphoma. 2006;47(7):1245–52.
Kelaidi C, Adès L, Fenaux P. Treatment of acute promyelocytic Leukemia with high white cell blood counts. Mediterr J Hematol Infect Dis. 2011;3(1):e2011038.
Qian X, Wen-jun L. Platelet changes in acute Leukemia. Cell Biochem Biophys. 2013;67(3):1473–9.
Hu X, et al. Kinetics of normal hematopoietic stem and progenitor cells in a Notch1-induced Leukemia model. Blood. 2009;114(18):3783–92.
Haemmerle M, et al. The platelet lifeline to Cancer: challenges and opportunities. Cancer Cell. 2018;33(6):965–83.
Radziwon-Balicka A, et al. Platelets increase survival of adenocarcinoma cells challenged with anticancer Drugs: mechanisms and implications for chemoresistance. Br J Pharmacol. 2012;167(4):787–804.
Mammadova-Bach E, et al. Platelet glycoprotein VI promotes Metastasis through interaction with cancer cell-derived galectin-3. Blood. 2020;135(14):1146–60.
Thies KA, Hammer AM, Hildreth BE 3. Stromal Platelet-Derived Growth Factor Receptor-β Signaling Promotes Breast Cancer Metastasis in the Brain. 2021;81(3):606–18.
Chen YP, et al. Pretreatment platelet count improves the prognostic performance of the TNM staging system and Aids in planning therapeutic regimens for nasopharyngeal carcinoma: a single-institutional study of 2,626 patients. Chin J Cancer. 2015;34(3):137–46.
Ikeda M, et al. Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann Surg Oncol. 2002;9(3):287–91.
Yuan Y, et al. Prognostic value of pretreatment platelet counts in Lung cancer: a systematic review and meta-analysis. BMC Pulm Med. 2020;20(1):96.
Bensalah K, et al. Prognostic value of thrombocytosis in renal cell carcinoma. J Urol. 2006;175(3 Pt 1):859–63.
Cacic D, Reikvam H. Platelet Microparticles Protect Acute Myelogenous Leukemia Cells against Daunorubicin-Induced Apoptosis 2021. 13(8).
Cacic D et al. Platelet microparticles decrease Daunorubicin-Induced DNA damage and modulate intrinsic apoptosis in THP-1 cells. Int J Mol Sci, 2021. 22(14).
Zhang Q, et al. Pretreatment platelet count predicts survival outcome of patients with de novo non-M3 acute Myeloid Leukemia. PeerJ. 2017;5:e4139.
Zhang Y, et al. Low platelet counts at Diagnosis Predict Better Survival for Patients with Intermediate-Risk Acute Myeloid Leukemia. Acta Haematol. 2020;143(1):9–18.
Swerdlow SH et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms 2016. 127(20): p. 2375-90.
Xuan L, et al. Effects of intensified conditioning on Epstein-Barr virus and cytomegalovirus Infections in allogeneic hematopoietic stem cell transplantation for hematological malignancies. J Hematol Oncol. 2012;5:46.
Cheson BD, et al. Revised recommendations of the International Working Group for Diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–9.
Cai SF, Levine RL. Genetic and epigenetic determinants of AML pathogenesis. Semin Hematol. 2019;56(2):84–9.
Patel JP, et al. Prognostic relevance of integrated genetic profiling in acute Myeloid Leukemia. N Engl J Med. 2012;366(12):1079–89.
Ley TJ, et al. DNA sequencing of a cytogenetically normal acute myeloid Leukaemia genome. Nature. 2008;456(7218):66–72.
Trafalis DT, et al. Platelet production and related pathophysiology in acute myelogenous Leukemia at first diagnosis: prognostic implications. Oncol Rep. 2008;19(4):1021–6.
Kuo KH, et al. A retrospective observational study of leucoreductive strategies to manage patients with acute myeloid Leukaemia presenting with hyperleucocytosis. Br J Haematol. 2015;168(3):384–94.
Wahlin A, et al. Results of risk-adapted therapy in acute myeloid Leukaemia. A long-term population-based follow-up study. Eur J Haematol. 2009;83(2):99–107.
Canaani J, et al. Long term impact of hyperleukocytosis in newly diagnosed acute Myeloid Leukemia patients undergoing allogeneic stem cell transplantation: an analysis from the acute Leukemia working party of the EBMT. Am J Hematol. 2017;92(7):653–9.
Malagola M, et al. A simple prognostic scoring system for newly diagnosed cytogenetically normal acute Myeloid Leukemia: retrospective analysis of 530 patients. Leuk Lymphoma. 2011;52(12):2329–35.
Schlenk RF, et al. Mutations and treatment outcome in cytogenetically normal acute Myeloid Leukemia. N Engl J Med. 2008;358(18):1909–18.
Shi L, et al. Predictable resistance and overall survival of Gemcitabine/Cisplatin by platelet activation index in Non-small Cell Lung Cancer. Med Sci Monit. 2018;24:8655–68.
Casagrande N, Borghese C, Agostini F. In Ovarian Cancer Multicellular Spheroids, Platelet Releasate Promotes Growth, Expansion of ALDH + and CD133 + Cancer Stem Cells, and Protection against the Cytotoxic Effects of Cisplatin, Carboplatin and Paclitaxel 2021. 22(6).
Giannakeas V. Trends in platelet count among cancer patients. Exp Hematol Oncol. 2022;11(1):16.
Wang YH, et al. The pretreatment thrombocytosis may predict prognosis of patients with Colorectal cancer: a systematic review and meta-analysis. Biomark Med. 2017;11(2):195–210.
Foss B, Bruserud O. Platelet functions and clinical effects in acute myelogenous Leukemia. Thromb Haemost. 2008;99(1):27–37.
Paek SC, Min SK, Park JB. Effects of platelet-derived growth factor-BB on cellular morphology and cellular viability of stem cell spheroids composed of bone-marrow-derived stem cells. Biomed Rep. 2020;13(6):59.
Etxabe A, et al. Inhibition of serotonin receptor type 1 in acute Myeloid Leukemia impairs Leukemia stem cell functionality: a promising novel therapeutic target. Leukemia. 2017;31(11):2288–302.
Nara N, et al. Inhibition of the in vitro growth of blast progenitors from acute myeloblastic Leukemia patients by transforming growth factor-beta (TGF-beta). Leukemia. 1989;3(8):572–7.
Binder S, Luciano M, Horejs-Hoeck J. The cytokine network in acute Myeloid Leukemia (AML): a focus on pro- and anti-inflammatory mediators. Cytokine Growth Factor Rev. 2018;43:8–15.
Huang WK, et al. miR-125a-5p regulation increases phosphorylation of FAK that contributes to imatinib resistance in gastrointestinal stromal tumors. Exp Cell Res. 2018;371(1):287–96.
Hirao A, Sato Y. MiR-125b-5p Is Involved in Sorafenib Resistance through Ataxin-1-Mediated Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma 2021. 13(19).
Bai H, et al. Involvement of miR-125a in resistance to daunorubicin by inhibiting apoptosis in Leukemia cell lines. Tumour Biol. 2017;39(4):1010428317695964.
Zhang L, et al. Platelet-acute Leukemia interactions. Clin Chim Acta. 2022;536:29–38.
Chou FS, Mulloy JC. The thrombopoietin/MPL pathway in hematopoiesis and leukemogenesis. J Cell Biochem. 2011;112(6):1491–8.
Solar GP, et al. Role of c-mpl in early hematopoiesis. Blood. 1998;92(1):4–10.
Dong-Feng Z, et al. The TPO/c-MPL pathway in the bone marrow may protect Leukemia cells from chemotherapy in AML patients. Pathol Oncol Res. 2014;20(2):309–17.
Yoshihara H, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1(6):685–97.
Thol F, et al. Incidence and prognostic influence of DNMT3A mutations in acute Myeloid Leukemia. J Clin Oncol. 2011;29(21):2889–96.
Xing S et al. Cytogenetics and associated mutation profile in patients with acute monocytic Leukemia. 2019. 41(4): p. 485–92.
Yang L, et al. DNMT3A R882 mutation is associated with elevated expression of MAFB and M4/M5 immunophenotype of acute Myeloid Leukemia blasts. Leuk Lymphoma. 2015;56(10):2914–22.
Rauch PJ, et al. MPL expression on AML blasts predicts peripheral blood neutropenia and thrombocytopenia. Blood. 2016;128(18):2253–7.
Grigg AP, et al. Clinical, haematological and cytogenetic features in 24 patients with structural rearrangements of the Q arm of chromosome 3. Br J Haematol. 1993;83(1):158–65.
Sood R, Kamikubo Y, Liu P. Role of RUNX1 in hematological malignancies 2017. 129(15): p. 2070–2082.
Acknowledgements
We acknowledge the support from all staff members in the Department of Hematology, Nanfang Hospital of Southern Medical University, China.
Funding
This work was supported by the National Natural Science Foundation of China (82170165); the Natural Science Foundation of Guangdong Province (2023A1515012401); and the Natural Science Foundation of Guangdong Province (2021A1515011437).
Author information
Authors and Affiliations
Contributions
YZ: Development of methodology, acquisition of data, analysis and interpretation of data, writing the manuscript. QW and BY: Acquisition of data. YH and LJ: Technical and material support. FL, PY and YJ: Software. JY: Technical support. XJ: Conception and design, development of methodology, analysis and interpretation of data, revised the manuscript, administrative and technical support, and study supervision. Final approval of manuscript: All authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki. This study was in line with the laws and regulations of medical research and had been approved by the Ethic Committee of the Nanfang Hospital of Southern Medical University. The requirement to obtain informed consent was waived due to the retrospective nature of the study, and was deemed exempt from review by the Ethics Committee of the Nanfang Hospital of Southern Medical University.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, Y., Wu, Q., Yuan, B. et al. Influence on therapeutic outcome of platelet count at diagnosis in patients with de novo non-APL acute myeloid leukemia. BMC Cancer 23, 1030 (2023). https://doi.org/10.1186/s12885-023-11543-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11543-5