Abstract
Background
Neoadjuvant chemotherapy (NAC) has been widely applied in operable breast cancer patients. This study aim to identify the predictive factors of overall survival(OS) and recurrence free survival (RFS) in breast cancer patients who received NAC from a single Chinese institution.
Patients and Methods
There were 646 patients recruited in this study. All the patients were treated at department of Surgical Oncology, Sir Run Run Shaw Hospital between February 25, 1999 and August 22, 2018. The relevant clinicopathological and follow-up data were collected retrospectively. RFS and OS were assessed using the Kaplan-Meier method. Multivariate Cox proportional hazards model was also employed. Multi-variate logistic regression model was simulated to predict pathologic complete response (pCR).
Results
In total, 118 patients (18.2%) achieved pCR during NAC. The 5-year OS was 94.6% versus 78.1% in patients with and without pCR, respectively (P < 0.001). The 5-year RFS was 95.3% and 72.7%, respectively (P < 0.001). No difference was detected among molecular subtypes of 5-year RFS in patients obtained pCR. Factors independently predicting RFS were HER2-positive subtype (hazard ratio(HR), 1.906; P = 0.004), triple-negative breast cancer (TNBC) (HR,2.079; P = 0.003), lymph node positive after NAC(HR,2.939; P < 0.001), pCR (HR, 0.396;P = 0.010), and clinical stage III (HR,2.950; P = 0.016). Multi-variate logistic regression model was simulated to predict the pCR rate after NAC, according to clinical stage, molecular subtype, ki-67, LVSI, treatment period and histology. In the ROC curve analysis, the AUC of the nomogram was 0.734 (95%CI,0.867–12.867).
Conclusions
Following NAC, we found that pCR positively correlated with prognosis and the molecular subtype was a prognostic factor.
Similar content being viewed by others
Introduction
Neoadjuvant chemotherapy (NAC) is increasingly being utilized as the frontline therapy for the management of high-risk early-stage breast cancer in operable patients [1, 2]. From a research perspective, neoadjuvant therapy was recognized as a human in vivo system for evaluating predictive biomarkers, alternative endpoints, and the therapeutic efficacy of including novel agents [3, 4]. Studies have demonstrated no difference in survival between adjuvant or neoadjuvant settings [1]. However, neoadjuvant therapy improves breast-conserving surgery success rates due to tumor downstaging [5,6,7] and allows for response assessment. Pathologic complete response (pCR) to neoadjuvant chemotherapy is associated with favorable long-term outcomes [8], however, most patients cannot achieve pCR in clinical practice. Whether pCR is accomplished or not, there are other factors that indicate poor prognosis in patients after NAC. The neoadjuvant therapy model provides a potentially efficient trial design to explore the efficacy of novel therapies utilizing pCR as a surrogate marker for disease-free survival (DFS) and overall survival [3]. Overall, pCR was found to have long-term benefits for patients, with the strongest association observed in triple-negative breast cancer (TNBC) and human epidermal growth receptor 2 (HER2)-positive breast cancer [9].
There has been a gap in the level of healthcare between developing and developed countries over the past 20 years, which has led to differences in drug availability. Although the NeoSphere study [10] published results as early as 2012 suggesting that pertuzumab combined with trastuzumab constituted an effective anti-HER2 treatment, pertuzumab was only approved for marketing in China in 2018. Such differences are bound to lead to variations in the prognosis of neoadjuvant chemotherapy for breast cancer. Thus, we reviewed data from a single institution in Asia to explore the long-term prognosis of neoadjuvant chemotherapy for breast cancer in developing countries.
Materials & methods
Patient characteristics
The study has been approved by the Ethics approval of Medical Ethics Committee of Affiliated Sir Run Run Shaw Hospital, Zhejiang University (SRRSH), (No. 20210910-30). In this study, 646 patients with early or locally advanced stage breast cancer, who underwent neoadjuvant therapy and surgery at the Department of Surgical Oncology, SRRSH, were retrospectively analyzed. They were selected from the 695 patients diagnosed with breast cancer at the department between February 25,1999 and August 22, 2018. In this cohort, all neoadjuvant chemotherapy/targeted therapy regimens and prescriptions were followed the NCCN guidelines [11]. The excluded 49 patients: [1] had received neoadjuvant endocrine therapy (n = 10), [2] progressed during neoadjuvant therapy (n = 15), [3] had occult breast cancer (n = 6), [4] presented male breast cancer (n = 2), [5] had deputy breast cancer (n = 2), [6] were primarily diagnosed with bone metastases (n = 5), or [7] had no available follow-up data (n = 9).
All the demographic variables were assembled into a database. All patients were periodically followed up after surgery. The final date of diagnosis was defined as baseline data. The primary endpoint of this study was 5-year RFS, which was specified as a comprehensive indicator of local relapse or distant metastasis of breast cancer, contralateral breast cancer, or death from any cause. The primary endpoint was determined retrospectively by two oncologists. The secondary endpoint was defined as 5-year OS from diagnosis to death from any cause or censoring surviving patients.
Pathology review
All cases were diagnosed by core needle biopsy prior to treatment and estrogen receptor (ER), progesterone receptor (PR), HER2, and Ki-67 levels were determined by immunohistochemical staining. Our study used a Ki-67 cutoff of 25% for categorization. High Ki-67 expression was defined as Ki-67 > 25%.HER2-positivity was defined as having strong membrane staining patterns (3+) of the protein or gene amplification relative to the centromeric probe in ≥ 30% of tumor cells by fluorescence in situ hybridization (ERBB2/cep17 > 2.2) [12]. The ER positivity and PR positivity were defined as positive staining of tumor nuclei ≥ 10%. After the assessment of preoperative and postoperative pathological stages, the descending stage was defined as decreased postoperative pathological T stage or N stage compared with the preoperative stage. pCR is defined as the absence of invasive cancer in the breast and axillary nodes after surgery, following completion of neoadjuvant systemic therapy (i.e., ypT0/Tis ypN0 in the present American Joint Committee on Cancer (AJCC) staging system) [13]. The presence of lymphovascular space invasion (LVSI), extra-lymphatic dilatation, or pathologically positive lymph nodes were reported and evaluated by the pathologists from SRRSH.
Statistical methods
The time interval from diagnosis to death or the last follow-up was calculated. The survival endpoints for overall survival (OS) and relapse-free survival (RFS) were defined as starting from the date of diagnosis using the published standardized criteria [14]. The Kaplan-Meier method and log-rank tests were used to compare the difference of 5-year OS and RFS. Multivariate Cox regression modeling for proportional hazards was employed to calculate the hazard ratio and 95% CI to assess the effect of factors on the OS and RFS. The factors included in the model were clinical stage, molecular subtype, Ki-67, surgery type, lymph node positive after neoadjuvant chemotherapy, lymph vascular invasion (LVSI), neoadjuvant chemotherapy response and NAC regimens. Multi-variate logistic regression model was simulated to predict pCR, which incorporating clinical stage, molecular subtype, ki-67, LVSI, treatment period and histology. The treatment period was divided into two time-groups according to the time of diagnosis of breast cancer: 1999–2009 and 2010–2018. ROC curve (Receiver Operating Characteristic) analysis was performed and an AUC (area under the curve) was calculated to evaluate the model. The significance of p value was set at P < 0.05. SPSS Statistics version 21 (IBM) was applied for statistical analyses and the survival graphs were plotted using GraphPad Prism 7.
Results
Patient and treatment characteristics
A total of 646 patients were included for the final analysis, with a median follow-up of 5.0 years (ranging from 0.4 to 18.0 years). The pretreatment characteristics, treatment strategies, as well as response and follow-up information of patients are summarized in Table 1. The median age of the cohort at diagnosis was 49 years old (ranging from 22 to 84).
After NAC, 118 patients (18.2%) in the cohort experienced pCR, whereas 528 (81.7%) exhibited residual disease. The pCR rate for the period of 2010 to 2018 was significantly higher than that for the previous decade. The majority of pCR patients were HER2-positive (41.5%), and Ki-67 > 25% (50.8%). In patients without pCR, the tumors were usually ER + and/or PR+, HER2-negative type (49.6%) and Ki-67 < 25% (58.1%). Whether or not patients achieved pCR, the clinicopathological characteristics showed statistically significant differences, including molecular typing, Ki-67, pathological lymph node staging (ypN), LVSI, surgical treatment, anti-HER2-targeted therapy, and treatment period (P < 0.05). About half of the HER2-positive patients received anti-HER2-targeted therapy (117:214) (Table 1). Only 6 patients in pCR patients were ypTis, which had little effect on the analysis of the whole cohort, so it was not reflected in Table 1.Consistent with common clinical observations, there were a number of cases of carcinoma in situ with focal invasion in non-PCR patients.
Analysis of Relapse-Free Survival (RFS) and Overall Survival (OS)
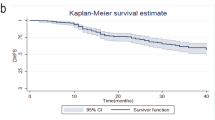
Based on a median follow-up of 5.0 years (ranging from 0.4 to 18.0 years), the 5-year RFS rates were 95.3% and 72.7% in patients with and without pCR, respectively (P < 0.001) (Fig. 1A). Meanwhile, the 5-year OS rates were 94.6% versus 78.1% in patients with and without pCR, respectively (P < 0.001) (Fig. 1B).
Among patients who achieved pCR, the 5-year RFS was 96.9% for luminal, 97.9% for HER2-positive tumors, and 90.2% for TNBC (P = 0.530) (Fig. 1C). There was no difference in subtype either for those not achieving pCR either, with 5-year RFS rates ranging from 77.3% for ER + and/or PR+, HER2- type, 69.9% for HER2-positive tumors to 66.9% for TNBC tumors (P = 0.035) (Fig. 1D).
Multivariate analysis
Factors independently predicting RFS in patients were HER2-positive subtype (hazard ratio, 1.906; 95% CI, 1.226–2.964; P = 0.004), TNBC (hazard ratio, 2.079; 95% CI, 1.280–3.378; P = 0.003), lymph node positive after neoadjuvant chemotherapy (hazard ratio, 2.939; 95% CI, 2.059–4.195; P < 0.001), pCR (hazard ratio, 0.396; 95% CI, 0.196–0.802; P = 0.010), and clinical stage III (hazard ratio, 2.950; 95% CI, 1.227–7.093; P = 0.016).
Factors independently predicting OS in patients were HER2-positive subtype (hazard ratio, 2.903; 95% CI, 1.785–4.720; P < 0.001), TNBC (hazard ratio, 2.688; 95% CI, 1.545–4.678; P < 0.001), lymph node positive after neoadjuvant chemotherapy (hazard ratio, 3.685; 95% CI, 2.438–5.570; P < 0.001), clinical stage III (hazard ratio, 4.241; 95% CI, 0.992–18.127; P = 0.051), pCR (hazard ratio, 0.378; 95% CI, 0.159–0.897; P = 0.027), and adjuvant targeted therapy (hazard ratio,0.380; 95% CI, 0.175–0.824; P = 0.014) (Table 2; Fig. 2).
Multi-variate logistic regression model to predict pCR
Multi-variate logistic regression model was simulated to was developed to predict the breast cancer pCR rate after NAC, according to clinical stage, molecular subtype, ki-67, LVSI, treatment period and histology. In the ROC curve analysis, the AUC of the nomogram was 0.752 (Table 3; Fig.3).
Discussion
In this study, we found that the pCR rate of breast cancer after neoadjuvant chemotherapy in our center was similar to the international level in the past 20 years. The rate of pCR in our cohort was 18.2%, which was close to most previous findings. A large meta-analysis [15] included a total of 52 studies and 27,895 women treated with the neoadjuvant approach. They showed an overall pCR rate of 21.1% (range: 10.1–74.2%). The highest rates of pCR were seen in HER2-positive tumors at 36.4% (range: 17.5–74.2%) and TNBC at 32.6% (range: 20.3–62.2%), and the hormone receptor positive (HR+)/HER2- tumors were the lowest at 9.3% (range: 5.5–31.3%). In particular, consistent with the above meta-analysis, HR+/HER2- patients were also associated with lower pCR rates than TNBC and HER2-positive subtypes [16]. Another meta-analysis showed that HER2-positive breast cancer also had an advantage in axillary pCR rate [17].
In a pooled analysis of 12 clinical trials by Cortazar et al. (2014), the authors demonstrated that pCR was associated with improved event-free survival (EFS), while the association between the magnitude of treatment-induced pCR change and corresponding improvement in EFS could not be established [9]. Nevertheless, the 3-year outcomes of the I-SPY2 trial showed that, regardless of subtype and treatment regimen, achieving pCR after neoadjuvant therapy including 9 novel therapeutic combinations implied an approximately 80% reduction in relapse rate [18]. As expected, Spring et al. [15] demonstrated the very strong correlation of pCR with EFS. The achievement of pCR following NAC is associated with significant better EFS and OS, particularly for triple-negative and HER2-positive breast cancer. Another meta-analysis also suggested that pCR in HER2-positive breast cancer is more likely [19]. The results in our study also suggest that HER2-positive and triple-negative breast cancers were sensitive to neoadjuvant therapy, however, there is no statistically significant survival advantage after neoadjuvant therapy compared with luminal subtype. In our study, the total treatment efficacy was comparable in the 5-year RFS rates of 95.3% and 72.7% in patients with and without pCR (P < 0.001) as compared with the studies of Spring et al. [15], where the pCR patients had a 5-year EFS of 88% (95% PI: 85-91%) while those without pCR exhibited a 5-year EFS of 67% (95% PI: 63-71%). In multivariate analysis, pCR indicated better OS (hazard ratio, 0.378; 95% CI, 0.159–0.897; P = 0.027) and RFS (hazard ratio, 0.396; 95% CI, 0.196–0.802; P = 0.010).
Furthermore, the HER2-positive breast cancer type does not present an advantage for prognosis, which is different from the results of many other studies [20]. As mentioned above, almost half of HER2-positive patients receive anti-HER2-targeted neoadjuvant therapy (117:214) (Table 1). For economic reasons, targeted therapies were still not widely used in China in early 2000s. The NeoSphere study [10] results indicated that pertuzumab combination therapy and trastuzumab plus docetaxel significantly increased pCR and 5-year DFS. The KATHERINE study [21], which further investigated the effects of adjuvant therapy after neoadjuvant targeted therapy on HER2-positive breast cancer, suggested that adjuvant T-DM1 can benefit patients whose neoadjuvant targeted therapy does not achieve pCR. While the case data in our paper was from the period of 1999 to 2018, pertuzumab was only applied in China at the end of 2018. In the past five years, targeted tumor therapy has been almost fully implemented in China.
The factors predicting non-pCR of NAC are still not clear. The most common prognostic factors for patients without successful pCR are residual cancer burden [22], Ki-67 [23], and tumor-infiltrating lymphocytes [24, 25]. In this study, we find LVSI, Ki-67, HER2 subtype were significant associated with pCR of patients who received NAC. However, the authors repeatedly wondered whether the prognostic indicator factors of residual cancer burden for patients without pCR could be simplified in the analysis. Neoadjuvant chemotherapy is an autologous drug sensitivity test and those sensitive to chemotherapy patients could have higher probability of achieving pCR. PCR to neoadjuvant chemotherapy is associated with favorable long-term outcomes.
The response to pre-surgery treatment was shown to have the ability to predict the subsequent outcome of breast cancer [2, 26, 27], which makes pCR a valuable intermediate endpoint for evaluating the efficacy of preoperative treatment regimens. The significance of neoadjuvant therapy is to improves breast-conserving surgery success rates due to tumor downstaging, and pCR to neoadjuvant chemotherapy is associated with favorable long-term outcomes. In other words, patients without pCR require further postoperative adjuvant intensive therapy to improve survival, which is currently the most important value of pCR: chemotherapy sensitivity screening. The CREATE-X [28] and KATHERINE [21] trials have transformed clinical practice by showing that capecitabine and T-DM1, respectively, significantly improve outcomes in TNBC and HER2-positive breast cancer patients who did not achieve pCR after neoadjuvant chemotherapy. To some extent, patients without pCR can benefit from additional post-surgical treatments, while the determination of adjuvant therapy intensity is the focus of future research.
The present study identified the following prognostic factors of breast cancer outcomes for patients: HER2-positive subtype, TNBC, pCR, Ki-67 > 25, higher pathologic nodal stage, later clinical staging, and treatment period. The Ki-67 protein has been reported to be an independent predictor of pCR, overall survival, and distant disease-free survival [29], which is consistent with the research results of this paper. The treatment period has an impact on OS, as the level of treatment in the past 10 years has significantly improved compared with that before 2010 due to the progress of international communication and the standardization of treatment in China. Studies on predictors without pCR after neoadjuvant chemotherapy for triple-negative breast cancer have demonstrated that pathological lymph node positivity and LVSI predict worse outcomes [30, 31]. In this study, however, LVSI was not a meaningful prognostic factor (hazard ratio, 1.241; 95% CI, 0.767–2.006; P = 0.379). The study cohort was sourced from a single facility in a tertiary level A hospital in a certain developing country, therefore the patient population may not be representative of various institutions in different developing countries. The regulatory variables used for multivariate analysis may be incomplete, and the absence of certain variables, including tumor infiltrating lymphocytes, p53 status, necrosis, etc., for which no data has been collected, may affect the results.
There was notable limitation in our study. It was conducted at a single center and was retrospective, which creates a susceptibility to selection bias. However, we studied a larger number of patients, and the choice of chemotherapy regimens followed the same principles. Therefore, we ‘re doing a multi-center data analysis.
Conclusions
This study concluded that the post-neoadjuvant chemotherapy pCR rate for breast cancer in our center has kept pace with the international level in the past 20 years. The correlation between pCR and better outcomes was consistent. Following NAC, HER2-positive subtype and TNBC molecular subtype had higher pCR rates.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in Breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(3):188–94.
Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical adjuvant breast and Bowel Project Protocols B-18 and B-27. J Clin Oncology: Official J Am Soc Clin Oncol. 2008;26(5):778–85.
Bardia A, Baselga J. Neoadjuvant therapy as a platform for drug development and approval in Breast cancer. Clin cancer Research: Official J Am Association Cancer Res. 2013;19(23):6360–70.
Berry DA, Hudis CA. Neoadjuvant therapy in Breast Cancer as a basis for drug approval. JAMA Oncol. 2015;1(7):875–6.
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable Breast cancer. J Clin Oncology: Official J Am Soc Clin Oncol. 1998;16(8):2672–85.
Bear HD, Anderson S, Smith RE, Geyer CE Jr., Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable Breast cancer:National Surgical adjuvant breast and Bowel Project Protocol B-27. J Clin Oncology: Official J Am Soc Clin Oncol. 2006;24(13):2019–27.
Mieog JS, van der Hage JA, van de Velde CJ. Preoperative chemotherapy for women with operable Breast cancer. Cochrane Database Syst Rev. 2007;2:CD005002.
Hatzis C, Symmans WF, Zhang Y, Gould RE, Moulder SL, Hunt KK, et al. Relationship between Complete Pathologic Response to Neoadjuvant Chemotherapy and Survival in Triple-negative Breast Cancer. Clin cancer Research: Official J Am Association Cancer Res. 2016;22(1):26–33.
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in Breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72.
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive Breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32.
Shaunfield S, Jensen S, Fisher AP, Webster K, Shahabi S, Ganguli A, et al. Further content validation of the 18-item NCCN/FACT ovarian Symptom Index and its Disease Related Symptom-Physical (DRS-P) subscale for use in advanced Ovarian cancer clinical trials. Health Qual Life Outcomes. 2019;17(1):185.
Senkus EKS, Penault-Llorca F, Poortmans P, Thompson A, Zackrisson S, Cardoso F. ESMO Guidelines Working Group. Primary Breast cancer: ESMO Clinical Practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. Suppl 2013;6:vi7–23.
US Department of Health and Human Services Food and Drug Administration CfDEaRC. Guidance for Industry: Pathological Complete Response in Neoadjuvant Treatment of High-Risk Early-Stage Breast Cancer: Use as an Endpoint to Support Accelerated Approval. 2020 [Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pathological-complete-response-neoadjuvant-treatment-high-risk-early-stage-breast-cancer-use.
Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman JA, et al. Proposal for standardized definitions for efficacy end points in adjuvant Breast cancer trials: the STEEP system. J Clin Oncology: Official J Am Soc Clin Oncol. 2007;25(15):2127–32.
Spring LM, Fell G, Arfe A, Sharma C, Greenup R, Reynolds KL, et al. Pathologic Complete Response after neoadjuvant chemotherapy and impact on Breast Cancer recurrence and survival: a Comprehensive Meta-analysis. Clin cancer Research: Official J Am Association Cancer Res. 2020;26(12):2838–48.
Esserman LJ, Berry DA, DeMichele A, Carey L, Davis SE, Buxton M, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL–CALGB 150007/150012, ACRIN 6657. J Clin Oncology: Official J Am Soc Clin Oncol. 2012;30(26):3242–9.
Samiei S, Simons JM, Engelen SME, Beets-Tan RGH, Classe JM, Smidt ML et al. Axillary Pathologic Complete Response after Neoadjuvant systemic therapy by Breast Cancer subtype in patients with initially clinically node-positive Disease: a systematic review and Meta-analysis. JAMA Surg. 2021:e210891.
Consortium IST, Yee D, DeMichele AM, Yau C, Isaacs C, Symmans WF et al. Association of event-free and distant recurrence-free Survival with Individual-Level Pathologic Complete response in Neoadjuvant Treatment of Stages 2 and 3 Breast Cancer: three-year follow-up analysis for the I-SPY2 adaptively randomized clinical trial. JAMA Oncol. 2020.
Broglio KR, Quintana M, Foster M, Olinger M, McGlothlin A, Berry SM, et al. Association of Pathologic Complete Response to Neoadjuvant Therapy in HER2-Positive Breast Cancer with Long-Term outcomes: a Meta-analysis. JAMA Oncol. 2016;2(6):751–60.
Haque W, Verma V, Hatch S, Suzanne Klimberg V, Brian Butler E, Teh BS. Response rates and pathologic complete response by Breast cancer molecular subtype following neoadjuvant chemotherapy. Breast Cancer Res Treat. 2018;170(3):559–67.
von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab Emtansine for residual invasive HER2-Positive Breast Cancer. N Engl J Med. 2019;380(7):617–28.
Muller HD, Posch F, Suppan C, Bargfrieder U, Gumpoldsberger M, Hammer R, et al. Validation of residual Cancer burden as prognostic factor for Breast Cancer patients after neoadjuvant therapy. Ann Surg Oncol. 2019;26(13):4274–83.
Miglietta F, Dieci MV, Tsvetkova V, Griguolo G, Vernaci G, Menichetti A et al. Validation of Residual Proliferative Cancer Burden as a Predictor of Long-Term Outcome Following Neoadjuvant Chemotherapy in Patients with Hormone Receptor-Positive/Human Epidermal Growth Receptor 2-Negative Breast Cancer. The oncologist. 2020.
Asano Y, Kashiwagi S, Goto W, Takada K, Takahashi K, Hatano T, et al. Prediction of survival after neoadjuvant chemotherapy for Breast cancer by evaluation of tumor-infiltrating lymphocytes and residual cancer burden. BMC Cancer. 2017;17(1):888.
Hwang HW, Jung H, Hyeon J, Park YH, Ahn JS, Im YH, et al. A nomogram to predict pathologic complete response (pCR) and the value of tumor-infiltrating lymphocytes (TILs) for prediction of response to neoadjuvant chemotherapy (NAC) in Breast cancer patients. Breast Cancer Res Treat. 2019;173(2):255–66.
Komenaka IK, Hsu CH, Martinez ME, Bouton ME, Low BG, Salganick JA, et al. Preoperative chemotherapy for operable Breast cancer is associated with better compliance with adjuvant therapy in matched stage II and IIIA patients. Oncologist. 2011;16(6):742–51.
Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, et al. The effect on Tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical adjuvant breast and Bowel Project Protocol B-27. J Clin Oncology: Official J Am Soc Clin Oncol. 2003;21(22):4165–74.
Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant capecitabine for Breast Cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–59.
Fasching PA, Heusinger K, Haeberle L, Niklos M, Hein A, Bayer CM, et al. Ki67, chemotherapy response, and prognosis in Breast cancer patients receiving neoadjuvant treatment. BMC Cancer. 2011;11:486.
Kennedy WR, Tricarico C, Gabani P, Weiner AA, Altman MB, Ochoa LL, et al. Predictors of distant metastases in Triple-negative Breast Cancer without pathologic complete response after Neoadjuvant Chemotherapy. J Natl Compr Cancer Network: JNCCN. 2020;18(3):288–96.
Gabani P, Merfeld E, Srivastava AJ, Weiner AA, Ochoa LL, Mullen D, et al. Predictors of Locoregional Recurrence after failure to Achieve Pathologic Complete response to Neoadjuvant Chemotherapy in Triple-negative Breast Cancer. J Natl Compr Cancer Network: JNCCN. 2019;17(4):348–56.
Acknowledgements
Breast cancer cohort study and authors would like to thank all the patients and their family members for their cooperation and willingness to take part in this study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Danzhi Chen. Qinchuan Wang. and Fei Chen. wrote the main manuscript text, Aihua Huang. Cong Chen. and Yi Lu. performed the data curation, Danzhi Chen. Fei Chen. and Minjun Dong. performed the data analysis, Danzhi Chen. Cong Chen. prepared Figs. 1 and 2; Table 1, and 2. Qinchuan Wang. prepared Fig. 3; Table 3, Wenhe Zhao. and Linbo Wang. reviewed & edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests as defined by BMC, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Ethics approval and consent to participate
Breast cancer cohort study in department of Surgical Oncology, Sir Run Run Shaw Hospital was approved by the Ethics approval of Medical Ethics Committee of Sir Run Run Shaw Hospital affiliated to Zhejiang University Medical College. This ethics committee is independent and adheres to IGH GCP principles, Chinese GCP and local regulations. All methods were carried out in accordance with relevant guidelines and regulations. Written informed consent was obtained from all study subjects.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, D., Wang, Q., Dong, M. et al. Analysis of neoadjuvant chemotherapy for breast cancer: a 20-year retrospective analysis of patients of a single institution. BMC Cancer 23, 984 (2023). https://doi.org/10.1186/s12885-023-11505-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11505-x