Abstract
Purpose
The PACIFIC study has demonstrated that the administration of durvalumab following concurrent chemoradiotherapy can significantly improve both overall survival and progression-free survival rates in patients with locally advanced unresectable non-small cell lung cancer. While the latest NCCN guidelines recommend this combination regimen, they do not specify the optimal timing for administering durvalumab after completing radiotherapy. The PACIFIC study suggested initiating durvalumab within 42 days of completing radiotherapy, but early administration of the drug may increase the incidence of pneumonitis. Therefore, we conducted this study to investigate whether the time interval between completion of radiotherapy and initiation of durvalumab treatment is associated with the risk of pneumonitis (Grade ≥ 3), which is the primary endpoint, as well as progression-free survival, which is the secondary endpoint.
Methods
A comprehensive search of clinical trials in PubMed and EMBASE was conducted up to March 2023 to identify clinical trials involving locally advanced unresectable non-small cell lung cancer patients who were treated with durvalumab following chemoradiotherapy. Meta-analysis was performed on single-arm studies to estimate the incidence of pneumonitis (Grade ≥ 3) and progression-free survival in all studies, as well as in studies that administered durvalumab within 42 days after completion of radiotherapy.
Results
This meta-analysis consisted of nine studies with a total of 2560 patients. The analysis showed that the incidence of pneumonitis (Grade ≥ 3) was 5.36% [95%CI (0.03, 0.08), I2 = 18.41%, p = 0.29], while the 1-year progression-free survival rate was 57.91% [95%CI (0.53, 0.63), I2 = 10.57%, p = 0.35]. Furthermore, when the duration between completion of radiotherapy and initiation of durvalumab treatment was shorter than 42 days, the incidence of pneumonitis (Grade ≥ 3) was 4.12% [95%CI (0.02, 0.06), I2 = 0.00%, p = 0.56], with a 1-year progression-free survival rate of 61.03% [95%CI (0.51, 0.71), I2 = 59.06%, p = 0.09].
Conclusion
Overall, based on the available evidence, it appears that there is no significant increase in pneumonitis or decrease in progression-free survival (PFS) when the time interval is less than 42 days and a shorter interval between treatment sessions does not necessarily have a detrimental effect on the rate of pneumonitis. We recommend that clinicians carefully evaluate the specific circumstances of each patient to determine the optimal timing for initiating immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overall, lung cancer remains one of the most dangerous malignancies in the world and is the leading cause of cancer-related death. Non-small cell lung cancer (NSCLC), a subtype of lung cancer, accounts for the majority of cases, with surgery being the primary treatment for locally advanced NSCLC [1]. However, due to factors such as tumor size and overall health status, surgery is often not possible. For patients with unresectable locally advanced NSCLC, concurrent chemoradiotherapy is standard treatment. Recent clinical studies, such as PACIFIC, suggest that immunotherapy with programmed cell death-1 (PD-1) receptor or programmed cell death-ligand 1 (PD-L1) inhibitors following chemoradiotherapy can significantly enhance patient outcomes in terms of overall survival (OS) and progression-free survival (PFS) [2,3,4,5]. This is especially applicable to patients with inoperable, locally advanced NSCLC. In the PACIFIC study, locally advanced NSCLC patients who received chemoradiotherapy and PD-L1 immune checkpoint inhibitor durvalumab had significantly longer OS and PFS than those treated with chemoradiotherapy alone [6]. The latest NCCN guidelines recommend incorporating durvalumab into NSCLC management after radiotherapy and chemotherapy completion, but there is no consensus on optimum timing for administering durvalumab after radiation therapy. As per the PACIFIC study, durvalumab treatment initiated up to 42 days after the final radiation session, and the greatest benefit is obtained when treatment commences between 1- and 14-days following radiation [6].
Radiation therapy can provide a robust immunomodulatory effect, creating a supportive immune microenvironment for anti-tumor immunity. Studies have revealed that radiation-induced anti-tumor immunity efficacy may depend on dose; higher doses demonstrate a stronger immune adjuvant effect [7, 8]. Indeed, radiation therapy offers several immunomodulatory effects such as increasing antigen presentation, releasing chemokines, attracting effector T-cells to the tumor microenvironment, and promoting immunogenic cell death mediated by lymphocytes [9]. Activation of negative T-cell regulation pathways like PD-1/PD-L1 axis following radiation may contribute to these effects [10]. It is crucial to note that radiation therapy can upregulate the expression of PD-1 and PD-L1 on immune and tumor cells, making its combined use with PD-1/PD-L1 inhibitors particularly relevant [10,11,12].
Incorporating a new treatment into standard cancer therapy is crucial, but balancing efficacy and safety is equally important. Immunotherapy with PD-1/PD-L1 inhibitors may affect other organ systems, and lead to immune-related pneumonitis, which poses the most significant potential fatalities among all reported adverse events [13,14,15]. The incidence of all grades of immune-related pneumonitis in clinical trial data ranges from 2 to 38%, while the incidence of Grade ≥ 3 immune-related pneumonitis ranges from 0.6 to 2.7% [16]. Additionally, based on real-world data, the incidence of immune-related pneumonitis in non-small cell lung cancer patients varies from 4.8 to 39.3%. Radiation therapy itself can also cause dose and radiation volume-dependent radiation pneumonitis in some patients [17]. In thoracic radiotherapy patients, the incidence of Grade 3 or higher radiation pneumonitis is between 1.8 and 10.0% [18,19,20,21,22]. Recently, a meta-analysis found that combining radiotherapy and chemotherapy with PD-1/PD-L1 inhibitors increases the incidence of Grades 1–2 immune-related pneumonitis or radiation pneumonitis but does not increase Grade ≥ 3 pneumonitis incidence [23]. Thus, PD-1/PD-L1 inhibitors offer excellent efficacy and good safety. The PACIFIC study initiated durvalumab treatment between 1 and 42 days after the final radiation therapy session. The latest NCCN guidelines recommend administering durvalumab treatment after the completion of radiotherapy and chemotherapy, but they do not specify when the therapy should be started after the final radiation therapy session. This study aims to investigate whether the time interval between the final radiation therapy session and durvalumab treatment is associated with the risk of pneumonitis, with the primary study endpoint being the incidence of Grade ≥ 3 pneumonitis. We believe that assessing the risk of pneumonitis alone is insufficient without considering survival. Hence, our original intention was to examine whether the timing of durvalumab treatment after chemoradiotherapy would impact survival, including overall survival and progression-free survival. However, due to the significant lack of overall survival data, we can only consider progression-free survival as a secondary endpoint for now.
Immunotherapy-related pneumonitis and radiation pneumonitis can be challenging to distinguish clinically. Many studies do not differentiate between the two when reporting adverse reactions. In addition to these two types of pneumonitis, patients may develop other forms of pneumonitis both during treatment and during the follow-up period. Whether it is immune-related pneumonitis, radiation pneumonitis, or other types of pneumonias that occur during treatment, they all have a significant impact on the treatment and prognosis of cancer patients. It should be noted that many studies do not specifically categorize pneumonitis cases. Therefore, this study aims to encompass various types of pneumonitis that can occur during treatment. This includes immune-related pneumonitis, radiation pneumonitis, and other forms of pneumonitis. By including these different types of pneumonitis, the study aims to provide a comprehensive assessment of the overall burden on cancer patients undergoing treatment. We also evaluate other factors that influence the risk of pneumonitis, such as radiation doses and populations. While many other factors can also impact the risk of pneumonitis, given the lack of extensive research data, this study still holds significant value and can contribute to optimizing combined treatment strategies.
Methods
Literature search
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [24,25,26] and has been registered on the PROSPERO database (CRD42023423736). A comprehensive search was conducted up to March 2023 in PubMed, Cochrane Library, and EMBASE using the algorithm “Non-Small Cell Lung cancer,” “Radiotherapy,” “Durvalumab” by Mr. Yang and Mrs. Zhong. Any conflicts and uncertainties were resolved by Mrs. Wu.
The inclusion criteria for this study were: (1) tumor: unresectable locally advanced non-small cell lung cancer; (2) treatment: chemoradiotherapy flowed by durvalumab; (3) time interval: the time interval between durvalumab and chemoradiotherapy was reported; (4) results: the incidence of pneumonitis (Grade ≥ 3) was reported.
The exclusion criteria for this study were: (1) tumor: other malignancies; (2) tumor progression: local recurrence or metastasis before durvalumab; (3) results: data could not be extracted, or the incidence of pneumonitis was 0%; (4) text: reviews, editorials, dispatches, protocols; (5) version: repeated reports (only the latest data was included).
The risk of bias
The assessment of bias risk was performed by Mr. Yang and Mrs. Zhong using the Cochrane Risk of Bias tool or MINORS. All trials were graded as high risk, unclear risk, or low risk. Data extraction was performed by Mr. Yang and Mrs. Zhong and validated independently by Mrs. Wu.
Statistical analysis
This meta-analysis was conducted using Stata 14. The incidence of pneumonitis (Grade ≥ 3) was analyzed using binary analysis. The heterogeneity between comparisons was estimated using the I-squared statistic.
Results
Characteristics of all trials
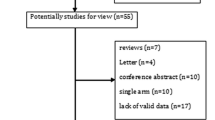
This meta-analysis included a total of 9 studies and 2560 patients [27,28,29,30,31,32,33,34,35]. All patients had completed chemoradiotherapy prior to receiving durvalumab, and no local recurrence or metastasis was present before durvalumab administration. Eight of the studies were retrospective single-arm trials [28,29,30,31,32,33,34,35], while one was a randomized controlled trial [27]. The time interval between completion of radiotherapy and durvalumab administration was reported in 3 studies to be within 1–42 days [27, 32, 33]. The time interval was reported as 0-157 days [28], 1.8–3.7 months [29], 35–981 days [30], 10–84 days [31], ≥ 14 days [34], and 13–103 days [35] in the remaining 6 studies. The study conducted by Girard et al. was indeed excluded from the analysis due to the wide range of interval durations, ranging from 35 to 981 days [30]. This considerable variation raised concerns about whether the 981-day period could still be considered as consolidation therapy. To ensure the credibility of the results, the decision was made to focus on studies with a more consistent and well-defined duration for consolidation therapy. By doing so, the study aimed to improve the reliability and accuracy of the findings. Harada conducted a subgroup analysis, where the time interval was reported as 10–14 days and 14–84 days [31]. The number of patients with pneumonitis (Grade ≥ 3) was reported as 3 and 0, respectively. Pneumonitis grading was evaluated according to the National Cancer Institute Standard Common Terminology for Adverse Events Pneumonia grading criteria. Figure 1 shows the flow diagram, and Table 1 summarizes the basic characteristics of all studies.
The risk of bias
The risk of bias in the 8 studies included in the meta-analysis was assessed by Mr. Yang and Mrs. Zhong. They utilized the Cochrane Risk of Bias tool or the Methodological Index for Non-Randomized Studies (MINORS) to evaluate and determine the risk of detection, reporting, and attrition bias in each study. The assessment conducted by Mr. Yang and Mrs. Zhong concluded that all the included studies demonstrated a low risk of bias in these aspects. This rigorous evaluation process enhances the reliability and credibility of the meta-analysis results by ensuring that the included studies have minimized potential biases in their methodology and reporting.
Outcomes
A heterogeneity test was conducted on a set of 8 studies which showed that the I2 value was 70.46%, indicating moderate heterogeneity. A sensitivity analysis was performed where each study was excluded one-by-one, revealing that study Avrillon et al. was the source of the heterogeneity [28]. After removing this study, the I2 value dropped significantly, which suggests that there was a reduced level of heterogeneity. The incidence of pneumonitis (Grade ≥ 3) was 5.36% [95%CI (0.03, 0.08), I2 = 18.41%, p = 0.29], as shown in Fig. 2. To evaluate publication bias, a funnel plot and Egger’s test were used. The funnel plot, as depicted in Fig. 3, was used to assess publication bias in the meta-analysis. Additionally, Egger’s test was conducted, and the results indicated no significant publication bias, as evidenced by a p-value of 0.11. This suggests that there was no substantial asymmetry in the distribution of study effect sizes, indicating that the likelihood of publication bias influencing the results of the meta-analysis is minimal. These findings contribute to the overall validity and robustness of the study’s conclusions, as they suggest that the included studies were representative and not unduly influenced by publication bias. Furthermore, the trim-and-fill method demonstrated that there was no bias risk, indicating that the results were stable. Among the total of 7 studies that were analyzed for 1-year PFS rate, a heterogeneity test revealed I2 = 77.34%, indicating high heterogeneity. Upon sensitivity analysis, it was discovered that study Saad et al. was responsible for the heterogeneity [34]. After its removal, the I2 value dropped significantly, which suggests reduced heterogeneity and a 1-year PFS rate of 57.91% [95%CI (0.53, 0.63), I2 = 10.57%, p = 0.35].
A meta-analysis was conducted on 3 studies with time intervals ranging from 1 to 42 days, as well as a subgroup from Harada et al. with intervals less than 14 days [27, 31,32,33]. The results showed an I2 value of 67.77%, indicating moderate heterogeneity. A sensitivity analysis was performed, and it was found that Harada et al. was the source of heterogeneity [31]. Upon exclusion of this study, the I2 = 0.00%, while the incidence of pneumonitis (Grade ≥ 3) was 4.12% [95%CI (0.02, 0.06), I2 = 0.00%, p = 0.56] (Fig. 4) and the 1-year PFS rate was 61.03% [95%CI (0.51, 0.71), I2 = 59.06%, p = 0.09]. Among the studies with intervals greater than 42 days were Chu et al. and Saad et al. [29, 34]. For Chu et al., the incidence of grade 3 or higher pneumonitis and the 1-year PFS rate were 5.63% and 56.40% respectively. For Saad et al., the incidence of pneumonia (Grade ≥ 3) was 6.90%. Harada et al. showed that a shorter interval between radiotherapy and Durvalumab treatment resulted in a higher incidence of grade 3 or higher pneumonitis when compared to an interval greater than 14 days [31]. Unfortunately, since the only subgroup in the 9 studies with an interval less than 14 days was from study Harada et al., further analysis could not be performed.
All seven studies included in our study had administered a radiotherapy dose of 54 Gy or higher. Furthermore, within these seven studies, we additionally examined a subset of four studies that specifically administered a radiotherapy dose of 60 Gy or higher [29, 31, 32, 35]. This analysis allowed for a comparison between two groups based on radiotherapy dosage, specifically studying the potential effects and outcomes associated with a higher radiotherapy dose (≥ 60 Gy) compared to the overall group of studies that used a dose of ≥ 54 Gy. By conducting this subgroup analysis, we aimed to investigate whether the higher radiotherapy dose had any significant impact on the outcomes of interest. It allows for a more nuanced understanding of the relationship between radiotherapy dose and pneumonitis. The incidence of pneumonitis (Grade ≥ 3) was 9.75% [95%CI (0.05, 0.16), I2 = 0.00%, p = 0.73] (Fig. 5), and the 1-year PFS rate was 62.05% [95%CI (0.60, 0.65), I2 = 0.00%, p = 0.68].
We conducted a meta-analysis to investigate how race impacts the risk of pneumonitis (Grade ≥ 3) and analyzed 5 studies that included Asian [29, 31,32,33,34]. Our analysis showed a 5.92% [95%CI (0.03, 0.10), I2 = 0.00%, p = 0.66] incidence of pneumonitis (Grade ≥ 3) (Fig. 6). The I2 for the 1-year PFS rate was 74.93%, indicating a high level of heterogeneity. We performed sensitivity analysis and identified that the study conducted by Saad et al. was responsible for the heterogeneity observed [34]. After omitting this study, the I2 value reduced significantly to 31.26%, indicative of a substantial decrease in heterogeneity. The 1-year PFS rate was 60.53% [95%CI (0.50, 0.70), I2 = 31.26%, p = 0.22].
Discussion
The PACIFIC study showed that using durvalumab treatment 1–42 days after chemoradiotherapy significantly improved overall survival, progression-free survival, and tumor response rates compared to using chemoradiotherapy alone in patients with unresectable locally advanced non-small cell lung cancer [6]. In the latest NCCN guidelines, receiving durvalumab treatment after chemoradiotherapy has become the standard treatment option for these patients, although the guidelines do not specify the optimal duration between the last radiation therapy session and durvalumab treatment. As a result, there is a new focus of research on the safety of using durvalumab treatment between 1 and 42 days after completion of radiation therapy. To evaluate the safety of this treatment regimen, we conducted a meta-analysis that mainly focused on whether the duration between completion of radiotherapy and durvalumab treatment was related to the risk of grade 3 or higher pneumonitis in patients with unresectable locally advanced non-small cell lung cancer.
The results of the meta-analysis showed that the incidence of pneumonitis (Grade ≥ 3) was 5.36% [95%CI (0.03, 0.08), I2 = 18.41%, p = 0.29], and the 1-year PFS was 57.91% [95%CI (0.53, 0.63), I2 = 10.57%, p = 0.35]. When the time interval between the completion of radiotherapy and durvalumab treatment was 1–42 days, the incidence of pneumonitis (Grade ≥ 3) was 4.12% [95%CI (0.02, 0.06), I2 = 0.00%, p = 0.56], and the 1-year PFS was 61.03% [95%CI (0.51, 0.71), I2 = 59.06%, p = 0.09]. The Chu et al. and Saad et al. studies had intervals longer than 42 days. The incidence of pneumonitis (Grade ≥ 3) and 1-year PFS were 5.63% and 56.40%, respectively, in the Chu et al. study [29], while the incidence of pneumonitis (Grade ≥ 3) was 6.90% in the Saad et al. study [34]. Girard et al. reported that an interval of less than 42 days between the completion of radiotherapy and durvalumab treatment was associated with higher PFS compared to intervals longer than 42 days [30]. Most of the included studies in this analysis were single-arm retrospective studies, making it difficult to compare the results and determine whether there is a statistically significant difference in the incidence of pneumonitis and PFS between the two different time intervals. However, based on these data, it appears that there is no significant increase in pneumonitis or decrease in progression-free survival (PFS) when the time interval is less than 42 days. Subgroup analysis of the PACIFIC trial also suggested that time interval less than 14 days may further improve OS, and there was no difference in the incidence of pneumonitis between patients with time interval less than or greater than 14 days [6]. This finding suggests that a shorter interval between treatment sessions does not appear to have a detrimental effect on these outcomes. Studies have shown that lung tissues from patients with radiation-induced pneumonitis exhibit significant infiltration of lymphocytes [36], while lung tissue and bronchoalveolar lavage fluid from patients with typical immune-related pneumonitis display increased lymphocytes rich in CD8 + T cells [37]. Another similar study found that CD4 + T cells dominate in the bronchoalveolar lavage fluid of patients with immune-related pneumonitis [38]. These findings underscore the cytotoxicity of T cells in inducing radiation-induced and immune-related pneumonitis. Radiation therapy can induce damage to tumor cell DNA and other cellular components, leading to clearance of damaged tumor cells by antigen-presenting cells and increased activation of T cells [39]. Most tumor-specific tumor-infiltrating lymphocytes express T cell receptors that were not identified before immunotherapy, suggesting that these tumor-infiltrating lymphocytes are newly recruited after treatment [40]. Both radiation therapy and immunotherapy recruit lymphocytes, which may be significant causes of radiation-induced and immune-related pneumonitis. Thus, we suggest that patients receive durvalumab treatment within 1–42 days after the completion of radiotherapy, with particular attention to patients receiving durvalumab treatment within 1–14 days after the completion of radiotherapy, and to remain vigilant for possible pneumonitis.
The incidence of pneumonitis may be related to different radiation therapy techniques and plans. Nonetheless, we evaluated the impact of radiation dose on the incidence of grade ≥ 3 pneumonitis and found that among the four studies with a radiation dose of ≥ 60 Gy, the incidence of grade 3 pneumonitis was 9.75% [95%CI (0.05, 0.16), I2 = 0.00%, p = 0.73]. Additionally, the 1-year progression-free survival (PFS) rate was 62.05% [95%CI (0.60, 0.65), I2 = 0.00%, p = 0.68].It appears that the incidence of grade ≥ 3 pneumonitis significantly increased in studies with a radiation dose of ≥ 60 Gy. However, subdividing studies based solely on the radiation dose allowed may not be sufficient to accurately assess the impact of the dose on pneumonitis rates. It is important to note that including patients from studies with minimum doses of 54 or 60 Gy does not provide a comprehensive quantification of the actual received dose.Therefore, while our analysis suggests an association between a radiation dose of ≥ 60 Gy and higher incidence of grade ≥ 3 pneumonitis, it is crucial to consider other factors, such as the actual received dose and individual patient characteristics, when assessing the impact of radiation dose on pneumonitis rates. Additionally, new technologies, such as respiratory motion management and image-guided radiation therapy, have the potential to further reduce the risk of pneumonitis. The lung volume receiving a dose of 20 Gy (V20) and the lung volume receiving a dose of 40 Gy (V40) [30, 41] may also be linked to the incidence of pneumonitis. However, due to the lack of relevant data, we cannot further assess the impact of radiation therapy dose, techniques, V20, and V40 on the incidence of pneumonitis. Indeed, conducting further research that includes more precise and comprehensive measurements of radiotherapy would be highly beneficial in gaining a more nuanced understanding of the relationship between radiation therapy and pneumonitis. By capturing detailed data on various aspects of radiotherapy, such as fractionation schedules, treatment techniques, target volumes, and dose distribution, researchers can better assess the impact of these factors on pneumonitis incidence and severity. A more comprehensive approach to data collection and analysis would enable the identification of potential dose-response relationships, the exploration of optimal dose thresholds for minimizing pneumonitis risk, and the development of tailored treatment strategies for patients. Additionally, by considering individual patient characteristics, such as pre-existing lung conditions or genetic susceptibility, future studies can provide a more personalized assessment of the risk associated with radiotherapy-induced pneumonitis. Ultimately, advancing our understanding of the intricacies between radiotherapy parameters and pneumonitis outcomes can contribute to improved treatment planning, individualized patient care, and the optimization of radiotherapy protocols to minimize the risk of pneumonitis while maximizing treatment efficacy. This study analyzed how different races impact the risk of pneumonitis (Grade ≥ 3). Five of the included studies focused on Asian populations, revealing a 5.92% [95%CI (0.03, 0.10), I2 = 0.00%, p = 0.66] incidence of pneumonitis (Grade ≥ 3) and a 60.53% [95%CI (0.50, 0.70), I2 = 31.26%, p = 0.22] 1-year PFS rate. This suggests that Asians may have a higher risk of pneumonitis (Grade ≥ 3) but may also benefit more from PFS. However, the study’s conclusion should be approached with caution due to the presence of unaccounted-for influencing factors.
Furthermore, factors such as age, sex, physical fitness score, smoking status, histopathological manifestations, lung dose-volume index, and PD-L1 expression level may also impact the incidence of pneumonitis. For example, the PACIFIC study found that Asian patients with non-squamous tumors and poor physical fitness scores were more likely to develop pneumonitis [5]. Additionally, current smokers may have a lower risk of pneumonitis [42]. Nonetheless, the influence of these factors on the risk of pneumonitis cannot be fully evaluated due to a lack of data.
Limitations
This study has several limitations: (1) The meta-analysis included seven studies with small sample sizes, which may have influenced the statistical results. (2) Due to the absence of randomized controlled trials with varying time intervals, it is not possible to conduct a direct comparison of the effects of different time intervals on the incidence of pneumonitis. (3) Six studies analyzed were retrospective, which may affect the reliability of the data. (4) Although we intended to conduct a subgroup analysis on aspects such as radiotherapy technology, radiotherapy plan, and PD-L1 status, the current data do not support such analysis.
Conclusion
Overall, based on the available evidence, it appears that there is no significant increase in pneumonitis or decrease in progression-free survival (PFS) when the time interval is less than 42 days and a shorter interval between treatment sessions does not necessarily have a detrimental effect on the rate of pneumonitis. However, it is important to note that this conclusion is based on the current data and may be subject to limitations and variability among studies. We strongly recommend that clinicians carefully evaluate the specific circumstances of each patient to determine the optimal timing for initiating immunotherapy. Developing an individualized treatment plan that considers various factors such as the patient’s overall health, disease stage, specific cancer type, and treatment goals is crucial. For patients who have started immunotherapy earlier, close monitoring is essential. Regular and thorough observation of these patients can help identify any potential adverse reactions or treatment-related complications, allowing for timely intervention and management.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. https://doi.org/10.1158/1055-9965.EPI-15-0578.
Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–35. https://doi.org/10.1056/NEJMoa1504627.
Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C, Horn L, et al. Five-year overall survival for patients with advanced nonsmall-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol. 2019;37(28):2518–27. https://doi.org/10.1200/JCO.19.00934.
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim Y-C, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Faivre-Finn C, Reck M, Vansteenkiste J, Spigel DR, Wadsworth C, Melillo G, Taboada M, Dennis PA, Özgüroğlu M. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–50. https://doi.org/10.1056/NEJMoa1809697.
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim Y-C, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–29. https://doi.org/10.1056/NEJMoa1709937.
Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, Cho BC, Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, Cho BC, Planchard D, Paz-Ares L, Faivre-Finn C, Vansteenkiste JF, Spigel DR, Wadsworth C, Taboada M, Dennis PA, Özgüroğlu M, Antonia SJ. Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC-update from PACIFIC. J Thorac Oncol. 2020;15(2):288–93. https://doi.org/10.1016/j.jtho.2019.10.002.
Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 2012;83(4):1306–10. https://doi.org/10.1016/j.ijrobp.2011.09.049.
Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–71. https://doi.org/10.1084/jem.20052494.
Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16(13):e498–509. https://doi.org/10.1016/S1470-2045(15)00007-8.
Gong J, Le TQ, Massarelli E, Hendifar AE, Tuli R. Radiation therapy and PD-1/PD-L1 blockade: the clinical development of an evolving anticancer combination. J Immunother Cancer. 2018;6(1):46. https://doi.org/10.1186/s40425-018-0361-7.
Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–95. https://doi.org/10.1172/JCI67313.
Gong X, Li X, Jiang T, Xie H, Zhu Z, Zhou F, Zhou C. Combined radiotherapy and anti-PD-L1 antibody synergistically enhances antitumor effect in non-small cell lung cancer. J Thorac Oncol. 2017;12(7):1085–97. https://doi.org/10.1016/j.jtho.2017.04.014.
Suresh K, Naidoo J, Lin CT, Danoff S. Immune checkpoint immunotherapy for non-small cell lung cancer: benefits and pulmonary toxicities. Chest. 2018;154(6):1416–23. https://doi.org/10.1016/j.chest.2018.08.1048.
Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin AM, Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin AM, Rosenberg J, Voss MH, Rudin CM, Rizvi H, Hou X, Rodriguez K, Albano M, Gordon R-A, Leduc C, Rekhtman N, Harris B, Menzies AM, Guminski AD, Carlino MS, Kong BY, Wolchok JD, Postow MA, Long GV, Hellmann MD. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709–17. https://doi.org/10.1200/JCO.2016.68.2005.
Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2(12):1607–16. https://doi.org/10.1001/jamaoncol.2016.2453.
Zhang Q, Tang L, Zhou Y, He W, Li W. Immune checkpoint inhibitor-associated pneumonitis in non-small cell lung cancer: current understanding in characteristics, diagnosis, and management. Front Immunol. 2021;12: 663986. https://doi.org/10.3389/fimmu.2021.663986.
Choi YW, Munden RF, Erasmus JJ, Park KJ, Chung WK, Jeon SC, Park CK. Effects of radiation therapy on the lung: radiologic appearances and differential diagnosis. Radiographics. 2004;24(4):985–97. https://doi.org/10.1148/rg.244035160. discussion 998.
Senan S, Brade A, Wang LH, Vansteenkiste J, Dakhil S, Biesma B, Martinez Aguillo M, Aerts J, Govindan R, Rubio-Viqueira B, Senan S, Brade A, Wang L-H, Vansteenkiste J, Dakhil S, Biesma B, Martinez Aguillo M, Aerts J, Govindan R, Rubio-Viqueira B, Lewanski C, Gandara D, Choy H, Mok T, Hossain A, Iscoe N, Treat J, Koustenis A, San Antonio B, Chouaki N, Vokes E. PROCLAIM: randomized phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2016;34(9):953–62. https://doi.org/10.1200/JCO.2015.64.8824.
Sasaki T, Seto T, Yamanaka T, Kunitake N, Shimizu J, Kodaira T, Nishio M, Kozuka T, Takahashi T, Harada H, Sasaki T, Seto T, Yamanaka T, Kunitake N, Shimizu J, Kodaira T, Nishio M, Kozuka T, Takahashi T, Harada H, Yoshimura N, Tsutsumi S, Kitajima H, Kataoka M, Ichinose Y, Nakagawa K, Nishimura Y, Yamamoto N, Nakanishi Y. A randomised phase II trial of S-1 plus cisplatin versus vinorelbine plus cisplatin with concurrent thoracic radiotherapy for unresectable, locally advanced non-small cell lung cancer: WJOG5008L. Br J Cancer. 2018;119(6):675–82. https://doi.org/10.1038/s41416-018-0243-2.
Yamamoto N, Nakagawa K, Nishimura Y, Tsujino K, Satouchi M, Kudo S, Hida T, Kawahara M, Takeda K, Katakami N, Yamamoto N, Nakagawa K, Nishimura Y, Tsujino K, Satouchi M, Kudo S, Hida T, Kawahara M, Takeda K, Katakami N, Sawa T, Yokota S, Seto T, Imamura F, Saka H, Iwamoto Y, Semba H, Chiba Y, Uejima H, Fukuoka M. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan thoracic Oncology Group WJTOG0105. J Clin Oncol. 2010;28(23):3739–45. https://doi.org/10.1200/JCO.2009.24.5050.
Segawa Y, Kiura K, Takigawa N, Kamei H, Harita S, Hiraki S, Watanabe Y, Sugimoto K, Shibayama T, Yonei T, Segawa Y, Kiura K, Takigawa N, Kamei H, Harita S, Hiraki S, Watanabe Y, Sugimoto K, Shibayama T, Yonei T, Ueoka H, Takemoto M, Kanazawa S, Takata I, Nogami N, Hotta K, Hiraki A, Tabata M, Matsuo K, Tanimoto M. Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non-small-cell lung cancer: OLCSG 0007. J Clin Oncol. 2010;28(20):3299–306. https://doi.org/10.1200/JCO.2009.24.7577.
Kwa SL, Lebesque JV, Theuws JC, Marks LB, Munley MT, Bentel G, Oetzel D, Spahn U, Graham MV, Drzymala RE, et al. Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys. 1998;42(1):1–9. https://doi.org/10.1016/s0360-3016(98)00196-5.
Geng Y, Zhang Q, Feng S, Li C, Wang L, Zhao X, Yang Z, Li Z, Luo H, Liu R, et al. Safety and efficacy of PD-1/PD-L1 inhibitors combined with radiotherapy in patients with non-small-cell lung cancer: a systematic review and meta-analysis. Cancer Med. 2021;10(4):1222–39. https://doi.org/10.1002/cam4.3718.
Moher D, Liberati A, Tetzlaff J, Altman DG, P Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. https://doi.org/10.1136/bmj.b2535.
Ge L, Tian JH, Li YN, Pan JX, Li G, Wei D, Xing X, Pan B, Chen YL, Song FJ, Ge L, Tian J-H, Li Y-N, Pan J-X, Li Ge, Wei D, Xing X, Pan B, Chen Y-L, Song F-J, Yang K-h. Association between prospective registration and overall reporting and methodological quality of systematic reviews: a meta-epidemiological study. J Clin Epidemiol. 2018;93:45–55. https://doi.org/10.1016/j.jclinepi.2017.10.012.
Wang X, Chen Y, Yao L, Zhou Q, Wu Q, Estill J, Wang Q, Yang K, Norris SL. Reporting of declarations and conflicts of interest in WHO guidelines can be further improved. J Clin Epidemiol. 2018;98:1–8. https://doi.org/10.1016/j.jclinepi.2017.12.021.
Paz-Ares L, Spira A, Raben D, Planchard D, Cho BC, Ozguroglu M, Daniel D, Villegas A, Vicente D, Hui R, et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann Oncol. 2020;31(6):798–806. https://doi.org/10.1016/j.annonc.2020.03.287.
Avrillon V, Daniel C, Boisselier P, Le Pechoux C, Chouaid C. Nationwide real-life safety and treatment exposure data on durvalumab after concurrent chemoradiotherapy in unresectable stage III, locally advanced, non-small cell lung cancer: analysis of patients enrolled in the French Early Access Program. Lung. 2022;200(1):95–105. https://doi.org/10.1007/s00408-022-00511-8.
Chu CH, Chiu TH, Wang CC, Chang WC, Huang AC, Liu CY, Wang CL, Ko HW, Chung FT, Hsu PC, et al. Consolidation treatment of durvalumab after chemoradiation in real-world patients with stage III unresectable non-small cell lung cancer. Thorac Cancer. 2020;11(6):1541–9. https://doi.org/10.1111/1759-7714.13426.
Girard N, Bar J, Garrido P, Garassino MC, McDonald F, Mornex F, Filippi AR, Smit HJM, Peters S, Field JK, Girard N, Bar J, Garrido P, Garassino MC, McDonald F, Mornex F, Filippi AR, Smit HJM, Peters S, Field JK, Christoph DC, Sibille A, Fietkau R, Haakensen VD, Chouaid C, Markman B, Hiltermann TJN, Taus A, Sawyer W, Allen A, Chander P, Licour M, Solomon B. Treatment characteristics and real-world progression-free survival in patients with unresectable stage III NSCLC who received durvalumab after chemoradiotherapy: findings from the PACIFIC-R study. J Thorac Oncol. 2023;18(2):181–93. https://doi.org/10.1016/j.jtho.2022.10.003.
Harada D, Shimonishi A, Saeki K, Ninomiya T, Kanzaki H, Nagasaki K, Ogura C, Tsutsui Y, Kojin K, Hamamoto Y, et al. Early administration of durvalumab after chemoradiotherapy increased risk of pneumonitis in patients with locally advanced non-small cell lung cancer. Asia Pac J Clin Oncol. 2023;19(2):e111–e117. https://doi.org/10.1111/ajco.13803.
Huang Y, Zhao JJ, Soon YY, Wong A, Aminkeng F, Ang Y, Asokumaran Y, Low JL, Lee M, Choo JRE, et al. Real-world experience of consolidation durvalumab after concurrent chemoradiotherapy in stage III non-small cell lung cancer. Thorac Cancer. 2022;13(22):3152–61. https://doi.org/10.1111/1759-7714.14667.
Miura Y, Mouri A, Kaira K, Yamaguchi O, Shiono A, Hashimoto K, Nishihara F, Shinomiya S, Akagami T, Murayama Y, et al. Chemoradiotherapy followed by durvalumab in patients with unresectable advanced non-small cell lung cancer: management of adverse events. Thorac Cancer. 2020;11(5):1280–7. https://doi.org/10.1111/1759-7714.13394.
Saad A, Goldstein J, Appel S, Daher S, Urban D, Onn A, Gantz-Sorotsky H, Lobachov A, Gottfried T, Spieler B, et al. Chemoradiation followed by adjuvant durvalumab in stage III non-small cell lung cancer: real-world comparison of treatment outcomes to historical controls treated with chemoradiation alone. Thorac Cancer. 2022;13(12):1763–71. https://doi.org/10.1111/1759-7714.14452.
Taugner J, Kasmann L, Eze C, Ruhle A, Tufman A, Reinmuth N, Duell T, Belka C, Manapov F. Real-world prospective analysis of treatment patterns in durvalumab maintenance after chemoradiotherapy in unresectable, locally advanced NSCLC patients. Invest New Drugs. 2021;39(4):1189–96. https://doi.org/10.1007/s10637-021-01091-9.
Zhang W, Becciolini A, Biggeri A, Pacini P, Muirhead CR. Second malignancies in breast cancer patients following radiotherapy: a study in Florence, Italy. Breast Cancer Res. 2011;13(2):R38. https://doi.org/10.1186/bcr2860.
Shea M, Rangachari D, Hallowell RW, Hollie NI, Costa DB, VanderLaan PA. Radiologic and autopsy findings in a case of fatal immune checkpoint inhibitor-associated pneumonitis. Cancer Treat Res Commun. 2018;15:17–20. https://doi.org/10.1016/j.ctarc.2018.02.004.
Suresh K, Naidoo J, Zhong Q, Xiong Y, Mammen J, de Flores MV, Cappelli L, Balaji A, Palmer T, Forde PM, Suresh K, Naidoo J, Zhong Q, Xiong Ye, Mammen J, de Flores MV, Cappelli L, Balaji A, Palmer T, Forde PM, Anagnostou V, Ettinger DS, Marrone KA, Kelly RJ, Hann CL, Levy B, Feliciano JL, Lin C-T, Feller-Kopman D, Lerner AD, Lee H, Shafiq M, Yarmus L, Lipson EJ, Soloski M, Brahmer JR, Danoff SK, D’Alessio F. The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis. J Clin Invest. 2019;129(10):4305–15. https://doi.org/10.1172/JCI128654.
Wang Y, Deng W, Li N, Neri S, Sharma A, Jiang W, Lin SH. Combining immunotherapy and radiotherapy for cancer treatment: current challenges and future directions. Front Pharmacol. 2018;9: 185. https://doi.org/10.3389/fphar.2018.00185.
Callahan MK, Wolchok JD. Recruit or reboot? How does anti-PD-1 therapy change tumor-infiltrating lymphocytes? Cancer Cell. 2019;36(3):215–7. https://doi.org/10.1016/j.ccell.2019.08.009.
Mayahara H, Uehara K, Harada A, Kitatani K, Yabuuchi T, Miyazaki S, Ishihara T, Kawaguchi H, Kubota H, Okada H, Mayahara H, Uehara K, Harada A, Kitatani K, Yabuuchi T, Miyazaki S, Ishihara T, Kawaguchi H, Kubota H, Okada H, Ninomaru T, Shindo C, Hata A. Predicting factors of symptomatic radiation pneumonitis induced by durvalumab following concurrent chemoradiotherapy in locally advanced non-small cell lung cancer. Radiat Oncol. 2022;17(1):7. https://doi.org/10.1186/s13014-021-01979-z.
Tucker SL, Liu HH, Liao Z, Wei X, Wang S, Jin H, Komaki R, Martel MK, Mohan R. Analysis of radiation pneumonitis risk using a generalized Lyman model. Int J Radiat Oncol Biol Phys. 2008;72(2):568–74. https://doi.org/10.1016/j.ijrobp.2008.04.053.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of Liaoning Province (NO. 2021‑MS‑181 and 2019-MS-06 to Chunli Wu) and Young and middle-aged scientific and technological talents support program of Shenyang City (NO. RC200554 to Chunli Wu).
Author information
Authors and Affiliations
Contributions
Mr. Yang had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.Concept and design: Yang. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: Yang. Critical revision of the manuscript for important intellectual content: Yang, Zhong, Luo. Statistical analysis: Yang, Zhong. Obtained funding: Wu. Administrative, technical, or material support: Yang, Zhong, Luo. Supervision: Yang, Wu.
Authors’ information
Not applicable.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, Z., Zhong, W., Luo, Y. et al. The timing of durvalumab administration affects the risk of pneumonitis in patients with locally advanced non-small cell lung cancer: a systematic review and meta-analysis. BMC Cancer 23, 962 (2023). https://doi.org/10.1186/s12885-023-11472-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11472-3