Abstract
Background
The ALTA-1L study compared brigatinib with crizotinib in untreated ALK-rearranged non-small cell lung cancer (NSCLC) patients, demonstrating the efficacy of brigatinib. Although the median progression-free survival (PFS) of brigatinib group was 24.0 months, the one-year PFS rate was 70%. In the NEJ009 study, patients with EGFR mutations showed improved outcomes with gefitinib plus chemotherapy compared with gefitinib monotherapy. To evaluate the efficacy of the combination of brigatinib with chemotherapy for patients with ALK-rearranged NSCLC, we designed B-DASH study (WJOG 14720L).
Methods
B-DASH study is a multicenter, two-arm, phase II study. Eligible patients have untreated stage IIIB, stage IIIC, stage IV, or postoperative relapse ALK-rearranged nonsquamous NSCLC. Patients will be randomized in a 1:1 ratio to receive brigatinib (180 mg once daily with a 7-day lead-in period at 90 mg) monotherapy or carboplatin (area under the curve = 5 on day 1) plus pemetrexed (500 mg/m2 on day 1) and brigatinib in a 3-week cycle for up to four cycles, followed by pemetrexed and brigatinib as maintenance therapy. The target hazard ratio of 0.62 is set based on the NEJ009 study. With one-sided alpha = 0.20 and power = 0.8, the sample size for the B-DASH study was calculated to be 110, considering the possibility of patients dropping out. The primary endpoint is PFS. The key secondary endpoints are the overall response rate and overall survival. We will evaluate tumor-derived DNA from plasma specimens before treatment, 42 days after administering the study drug, and on the day of progressive disease. Recruitment began in November 2021 and is ongoing.
Discussion
The efficacy of combination therapy with tyrosine kinase inhibitors and cytotoxic chemotherapy was demonstrated in patients with EGFR mutations but remains unclear in patients with ALK-rearranged NSCLC. The B-DASH study is the only trial of brigatinib combined with chemotherapy in patients with untreated ALK-rearranged NSCLC.
Trial registration
jRCT identifier: jRCTs041210103.
Similar content being viewed by others
Background
Lung cancer is a leading cause of death worldwide. Non-small cell lung cancer (NSCLC) is a major pathological subtype of lung cancer. Many guidelines recommend that patients with stage IV NSCLC should be treated based on the presence of genetic alterations and expression of programmed death ligand 1 (PD-L1)[1,2,3,4]. Patients with anaplastic lymphoma kinase (ALK) fusion gene rearrangement account for 2–4% of lung adenocarcinoma [5, 6]. ALK tyrosine kinase inhibitors (TKI) are effective in patients with ALK-positive lung cancer. Alectinib, brigatinib, and lorlatinib are commonly used as first-line chemotherapy for patients with ALK-positive NSCLC based on the results of a phase III trial comparing crizotinib with new-generation ALK-TKI [7,8,9,10].
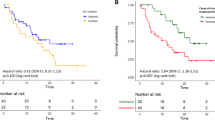
Brigatinib is a second-generation ALK-TKI. The results of the ALTA-1L trial, which compared crizotinib with brigatinib, showed that brigatinib significantly improved progression-free survival (PFS) (12-month PFS was 67% in the brigatinib group and 43% in the crizotinib group; hazard ratio (HR) = 0.49; 95% confidence interval (CI) = 0.33–0.74); p < 0.001) [9]. The final results of ALTA-1L were reported in 2021. Median PFS was 24.0 and 11.1 months in brigatinib and crizotinib groups, respectively [11]. Although brigatinib is effective as first-line chemotherapy for patients with ALK-positive NSCLC, approximately 30% of patients relapse at 12 months. Therefore, we need a more effective strategy for patients with ALK fusion gene rearrangements.
A study comparing epidermal growth factor receptor (EGFR)-TKI monotherapy with EGFR-TKI combined with cytotoxic chemotherapy has been reported in patients with EGFRmutations. The NEJ 009 trial, which compared gefitinib monotherapy (monotherapy group) with gefitinib combined with carboplatin plus pemetrexed (combination group), was reported in 2019 [12]. The study hierarchically analyzed PFS, progression-free survival 2 (PFS2), and overall survival (OS) as co-primary endpoints. Although there were no significant differences in PFS2 and OS between the two groups, PFS was significantly prolonged in the combination group (median PFS was 20.9 and 11.2 months in the combination and monotherapy groups, respectively; HR = 0.49, 95% CI = 0.39–0.62; p < 0.001). And overall response rate (ORR) was improved in the combination group compared with the monotherapy group. In 2022, the updated results of NEJ009 were reported. The median survival time was 38.5 months and 49.0 months in the monotherapy group and the combination group, respectively (HR = 0.82. 95% CI = 0.62–1.06; p = 0.127) [13]. Although the rate of grade ≥ 3 adverse events was higher in the combination group, there was no significant difference in the incidence of protocol treatment discontinuation due to adverse events between the two groups. A phase III trial with the same design was carried out in India and reported in 2020, showing that the addition of chemotherapy to gefitinib significantly prolonged PFS and OS. The ORR was also improved in combination therapy compared with gefitinib monotherapy [14]. The efficacy of the EGFR-TKI combined with the cytotoxic chemotherapy treatment strategy was reproducible. This treatment strategy will be tested in patients with EGFR mutations, and the FLAURA 2 study comparing osimertinib monotherapy to osimertinib combined with cytotoxic chemotherapy is ongoing. However, few trials assessing the efficacy of ALK-TKI plus chemotherapy have been performed for patients with ALK-positive NSCLC. Although the S1300 study (NCT02134912) assessed the efficacy of crizotinib plus pemetrexed, patients with NSCLC that had progressed after crizotinib were eligible, not previously untreated patients. It was reported that greater depth of response was associated with longer PFS and OS for patients with NSCLC treated with ALK-TKI [15]. Because gefitinib plus chemotherapy improved ORR compared with gefitinib monotherapy in patients with EGFR mutations, we hypothesize that ALK-TKI plus chemotherapy improved ORR leading to prolong PFS as patients with EGFR mutations.
Subgroup analysis of the PROFILE 1007 study, which compared crizotinib with chemotherapy for previously treated patients with ALK-positive NSCLC, showed that median PFS was 4.2 and 2.6 months for pemetrexed and docetaxel, respectively [16]. And, a retrospective study also showed that ALK-positive NSCLC patients on pemetrexed had a significantly longer PFS than EGFR wild type or KRASwild type patients [17]. These reports suggested that pemetrexed was more effective than other cytotoxic agents in patients with ALK-positive NSCLC. Since pemetrexed is a key drug for patients with ALK rearrangement, we must check its efficacy with other drugs.
Therefore, we designed the B-DASH study, which compares the efficacy of brigatinib monotherapy and brigatinib combined with carboplatin and pemetrexed in patients with ALK-positive NSCLC.
Methods / design
Study design
Figure 1 shows an overview of the B-DASH study—a multicenter, two-arm, phase II study. Eligible patients will be randomized in a 1:1 ratio to receive brigatinib (monotherapy group) or brigatinib combined with carboplatin and pemetrexed (combined group). Randomization is stratified according to the disease stage (stage IIIB, IIIC, or IV vs. postoperative relapse) and brain metastasis (present vs. absent), because it is reported that these factors are prognostic factors. Patients in the monotherapy group will receive 180 mg of brigatinib orally once daily after 90 mg once daily for seven days as the lead-in period until disease progression and unacceptable toxicities. In the combined group, patients will receive four cycles of carboplatin (area under the curve, 5 on day 1) plus pemetrexed (500 mg/m2 on day 1) and brigatinib (same dose as in the monotherapy group), followed by pemetrexed and brigatinib as maintenance therapy.
Before registration in this trial, contrast-enhanced computed tomography (CT) of the chest and abdomen and contrast-enhanced magnetic resonance imaging (MRI) of the brain are required. CT and MRI will be performed every six weeks for 24 weeks after registration and every nine weeks after that.
The study is being conducted in compliance with the principles of the Declaration of Helsinki. Also. The study was approved by the central review board of the Shizuoka Cancer Center. This trial has been registered in the Japan Registry of Clinical Trials (jRCTs041210103).
Eligibility criteria
The primary patient inclusion and exclusion criteria are presented in Table 1. Patients with untreated ALK-rearranged nonsquamous NSCLC are eligible for the study. ALK-rearrangement is diagnosed on the basis of locally approved ALK testing in Japan, such as immunohistochemistry, fluorescence in situ hybridization and next-generation sequencing. Other inclusion criteria are as follows: stage IIIB, stage IIIC, stage IV, or postoperative relapse for which definitive radiotherapy is impossible; Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–1; patients aged ≥ 20 years; and patients with at least one measurable lesion based on RECIST v1.1. Baseline brain metastasis is permitted if the patient is asymptomatic.
Study endpoints
The B-DASH study will evaluate the efficacy and safety of brigatinib combined with carboplatin and pemetrexed in patients with untreated ALK-rearranged NSCLC. The primary endpoint is progression-free survival assessed using the RECIST criteria. The secondary endpoints include the ORR, OS, and the rate of adverse events. To assess the resistance mechanism, we will analyze tumor-derived DNA from plasma specimens before treatment, 42 days after the administration of the study drug, and on the date of progressive disease. The tumor-derived DNA analysis will be performed with next generation sequencing using the AVENIO ctDNA Surveillance Kit (Roche Diagnostics, Basel, Switzerland).
Statistical considerations
The primary endpoint of the B-DASH study is progression-free survival assessed using the RECIST criteria assessed by investigator. Progression-free survival is the time from randomization to confirmation of progressive disease or death, whichever occurs first. The sample size of B-DASH study is calculated based on the median PFS of brigatinib monotherapy and hazard ration. We set the median PFS of brigatinib monotherapy in this study of 24.0 months based on the results of ALTA-1L study. The target hazard ratio of 0.62 is set based on the NEJ009 study. With one-sided alpha = 0.20 and power = 0.8, the sample size for the B-DASH study was calculated to be 110, considering drop out rate as 5%.
Discussion
The efficacy of combination therapy with tyrosine kinase inhibitors and cytotoxic chemotherapy was demonstrated in patients with EGFR mutations but remains unclear in patients with ALK-rearranged NSCLC. The B-DASH study is the only trial of brigatinib combined with chemotherapy in patients with untreated ALK-rearranged NSCLC.
Availability of data and materials
Not applicable.
Abbreviations
- NSCLC:
-
Non-small cell lung cancer
- PD-L1:
-
Programmed death ligand 1
- ALK:
-
Anaplastic lymphoma kinase
- TKI:
-
Tyrosine kinase inhibitors
- PFS:
-
Progression-free survival
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- EGFR:
-
Epidermal growth factor receptor
- OS:
-
Overall survival
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- ECOG:
-
Eastern Cooperative Oncology Group
- PS:
-
Performance status
- DNA:
-
Deoxyribonucleic acid
References
Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–237. https://doi.org/10.1093/annonc/mdy275.
Akamatsu H, Ninomiya K, Kenmotsu H, et al. The Japanese Lung Cancer Society Guideline for non-small cell lung cancer, stage IV. Int J Clin Oncol. 2019;24(7):731–70. https://doi.org/10.1007/s10147-019-01431-z.
Singh N, Temin S, Baker S Jr, et al. Therapy for Stage IV Non-Small-Cell Lung Cancer Without Driver Alterations: ASCO Living Guideline. J Clin Oncol. 2022;40(28):3323–43. https://doi.org/10.1200/JCO.22.00825.
Singh N, Temin S, Baker S Jr, et al. Therapy for Stage IV Non-Small-Cell Lung Cancer With Driver Alterations: ASCO Living Guideline. J Clin Oncol. 2022;40(28):3310–22. https://doi.org/10.1200/JCO.22.00824.
Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res. 2013;19(15):4273–81. https://doi.org/10.1158/1078-0432.CCR-13-0318.
Saito M, Shiraishi K, Kunitoh H, Takenoshita S, Yokota J, Kohno T. Gene aberrations for precision medicine against lung adenocarcinoma. Cancer Sci. 2016;107(6):713–20. https://doi.org/10.1111/cas.12941.
Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390(10089):29–39. https://doi.org/10.1016/S0140-6736(17)30565-2.
Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377(9):829–38. https://doi.org/10.1056/NEJMoa1704795.
Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2018;379(21):2027–39. https://doi.org/10.1056/NEJMoa1810171.
Shaw AT, Bauer TM, de Marinis F, et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N Engl J Med. 2020;383(21):2018–29. https://doi.org/10.1056/NEJMoa2027187.
Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib Versus Crizotinib in ALK Inhibitor-Naive Advanced ALK-Positive NSCLC: Final Results of Phase 3 ALTA-1L Trial. J Thorac Oncol. 2021;16(12):2091–108. https://doi.org/10.1016/j.jtho.2021.07.035.
Hosomi Y, Morita S, Sugawara S, et al. Gefitinib Alone Versus Gefitinib Plus Chemotherapy for Non-Small-Cell Lung Cancer With Mutated Epidermal Growth Factor Receptor: NEJ009 Study. J Clin Oncol. 2020;38(2):115–23. https://doi.org/10.1200/JCO.19.01488.
Miyauchi E, Morita S, Nakamura A, et al. Updated Analysis of NEJ009: Gefitinib-Alone Versus Gefitinib Plus Chemotherapy for Non-Small-Cell Lung Cancer With Mutated EGFR. J Clin Oncol. 2022;40(31):3587–92. https://doi.org/10.1200/JCO.21.02911.
Noronha V, Patil VM, Joshi A, et al. Gefitinib Versus Gefitinib Plus Pemetrexed and Carboplatin Chemotherapy in EGFR-Mutated Lung Cancer. J Clin Oncol. 2020;38(2):124–36. https://doi.org/10.1200/JCO.19.01154.
McCoach CE, Blumenthal GM, Zhang L, et al. Exploratory analysis of the association of depth of response and survival in patients with metastatic non-small-cell lung cancer treated with a targeted therapy or immunotherapy. Ann Oncol. 2017;28(11):2707–14. https://doi.org/10.1093/annonc/mdx414.
Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94. https://doi.org/10.1056/NEJMoa1214886.
Camidge DR, Kono SA, Lu X, et al. Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. J Thorac Oncol. 2011;6(4):774–80. https://doi.org/10.1097/JTO.0b013e31820cf053.
Acknowledgements
Data management and monitoring for this study were conducted by the West Japan Oncology Group under a funding contract with Takeda Pharmaceutical Company Limited.
Funding
The B-DASH study was funded by Takeda Pharmaceutical Company Limited.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design of the study. KW and HK are the principal investigators of the study. YS, AN, HA, MT, SM, TY, KN, and NY will be involved in participant recruitment. KM was responsible for statistical analysis. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The B-DASH study is being conducted in compliance with the principles of the Declaration of Helsinki, and it was approved by the central review board of Shizuoka Cancer Center. Written informed consent will be obtained from participants.
Consent for publication
Not applicable.
Competing interests
KW, HK, HA, MT, TY, KN, and NY have received personal fees and / or grants from Takeda Pharmaceutical Company Limited. Disclosures other than Takeda Pharmaceutical Company Limited are follows; KW reports grants and personal fees from Chugai Pharmaceutical Co.,Ltd, Astrazeneca, Novartis, Abbvie, AMGEN, MSD, Taiho Pharmaceutical, Boehringer Ingelheim, Eli Lilly K.K, Ono Pharmaceutical, Janssen Pharmaceutical K.K., and Daiichi Sankyo. HK reports grants and personal fees from AstraZeneca, Chugai Pharmaceutical Co, Ltd., Eli Lilly K.K, LOXO Oncology, Novartis Pharma K.K., Ono Pharmaceutical Co, Ltd., AMGEN, MSD, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Merk, Pfizer, and Taiho Pharma. YS reports personal fees from AstraZeneca, MSD, Novartis, Chugai Pharmaceutical, Ono Pharmaceutical, Pfizer, Taiho Pharmaceutical, Nippon Kayaku, Bristol Myers Squibb, Eli Lilly, and Kyowa Kirin. AN reports personal fees from AstraZeneca, Thermo Fisher Scientific, Chugai Pharmaceutical CO., LTD., Eli Lilly Japan K.K., Pfizer Inc., NIPPON KAYAKU, Merck, and Novartis. HA reports grants and personal fees from Amgen Inc, Chugai Pharmaceutical Co. Ltd., MSD K.K., AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Bristol-Myers Squibb, Eli Lilly Japan K.K., Nippon Kayaku. Co. Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co. Ltd., Pfizer Inc., and Taiho Pharmaceutical Co. Ltd. MT reports personal fees from Eli Lilly Japan Co Ltd, Ono Pharmaceutical Co Ltd, Bristol-Myers Squibb Co Ltd, Chugai Pharmaceutical Co Ltd, AstraZeneca KK, MSD KK, and Novartis pharmaceuticals K.K. SM reports honoraria from Chugai Pharmaceutical, Taiho Pharmaceutical, Ono Pharmaceutical, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, and Boehringer-Ingelheim Japan. TY reports grants and personal fees from MSD, Chugai Pharmaceutical Co. Ltd., Bristol-Myers Squibb Co. Ltd., Boehringer Ingelheim Japan Inc., AstraZeneca K.K., Eli Lilly Japan K.K., Pfizer Japan Inc, Ono Pharmaceutical Co. Ltd., and Nippon Kayaku Co., Ltd. KM reports no competing-interest. KN reports grants, personal fees from AstraZeneca K.K., MSD K.K., Ono Pharmaceutical Co.,Ltd., Nippon Boehringer Ingelheim Co.,Ltd., Novartis Pharma K.K., Pfizer Japan Inc., Bristol Myers Squibb Company, Eli Lilly Japan K.K., Chugai Pharmaceutical Co.,Ltd., Daiichi Sankyo Co., Ltd., Merck Biopharma Co., Ltd., PAREXEL International Corp., PRA HEALTHSCIENCES, EPS Corporation., Kissei Pharmaceutical Co., Ltd., EPS International Co.,Ltd,, Taiho Pharmaceutical Co.,Ltd., PPD-SNBL K.K, SymBio Pharmaceuticals Limited., IQVIA Services JAPAN K.K., SYNEOS HEALTH CLINICAL K.K., Nippon Kayaku Co.,Ltd., EP-CRSU Co., Ltd., Mebix, Inc., Janssen Pharmaceutical K.K., AbbVie Inc., Bayer Yakuhin, Ltd, Eisai Co., Ltd., Mochida Pharmaceutical Co., Ltd., Covance Japan Inc., Japan Clinical Research Operations, GlaxoSmithKline K.K., Sanofi K.K., Sysmex Corporation, Medical Reserch Support, Otsuka Pharmaceutical Co., Ltd., SRL, Inc., Pfizer R&D Japan G.K., Amgen Inc., KYORIN Pharmaceutical Co., Ltd., CMIC ShiftZero K.K., Life Technologies Japan Ltd., Neo Communication, Roche Diagnostics K.K., Kyowa Kirin Co., Ltd., 3H Clinical Trial Inc., Care Net, Inc., Medical Review Co., Ltd., Medical Mobile Communications co., Ltd, YODOSHA CO., LTD., Nikkei Business Publications, Inc., Japan Clinical Research Operations, and CMIC Co., Ltd. NY reports grants and personal fees from Boehringer-Ingelheim, Taiho, Chugai, Shionogi, Eli Lilly Japan, Daiichi-Sankyo, Tumura, Nippon Kayaku, Asahikasei-pharma, AstraZeneca, Janssen, Sanofi, AMGEN, Novartis, Astellas, MSD, Esai, Bristol Myers Squibb, Abbvie, Tosoh, Ono, Otsuka, Guardant Health Japan, Kyowa-Kirin, Kyorin, GlaxoSmithKline, Sanofi, Daiichi-Sankyo, Pfizer, and Miyarisan, Merck.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wakuda, K., Kenmotsu, H., Sato, Y. et al. Randomized, open-label phase II study of brigatinib and carboplatin plus pemetrexed and brigatinib alone for chemotherapy-naive patients with ALK-rearranged non-squamous non-small cell lung cancer: treatment rationale and protocol design of the B-DASH study (WJOG 14720 L). BMC Cancer 23, 902 (2023). https://doi.org/10.1186/s12885-023-11417-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11417-w