Abstract
Background
Hepatocellular carcinoma (HCC) is highly malignant with a dismal prognosis, although the available therapies are insufficient. No efficient ubiquitinase has been identified as a therapeutic target for HCC despite the complicating role that of proteins ubiquitination plays in the malignant development of HCC.
Methods
The expression of ubiquitin carboxyl terminal hydrolase L5 (UCHL5) in HCC tumor tissue and adjacent normal tissue was determined using the cancer genome atlas (TCGA) database and was validated using real-time quantitative polymerase chain reaction (RT-qRCR), Western blot and immunohistochemistry (IHC), and the relation of UCHL5 with patient clinical prognosis was explored. The expression of UCHL5 was knocked down and validated, and the effect of UCHL5 on the biological course of HCC was explored using cellular assays. To clarify the molecular mechanism of action of UCHL5 affecting HCC, expression studies of Adenosine triphosphate adenosine triphosphate (ATP), extracellular acidification (ECAR), and glycolysis-related enzymes were performed. The effects of UCHL5 on β-catenin ubiquitination and Wnt signaling pathways were explored in depth and validated using cellular functionalities. Validation was also performed in vivo.
Results
In the course of this investigation, we discovered that UCHL5 was strongly expressed in HCC at both cellular and tissue levels. The prognosis of patients with high UCHL5 expression is considerably worse than that of those with low UCHL5 expression. UCHL5 has been shown to increase the degree of glycolysis in HCC cells with the impact of stimulating the proliferation and metastasis of HCC cells in both in vivo and in vitro. UCHL5 downregulates its degree of ubiquitination by binding to β-catenin, which activates the Wnt/β-catenin pathway and accelerates HCC cell glycolysis. Thereby promoting the growth of the HCC.
Conclusions
In summary, we have demonstrated for the first time that UCHL5 is a target of HCC and promotes the progression of hepatocellular carcinoma by promoting glycolysis through the activation of the Wnt/β-catenin pathway. UCHL5 may thus serve as a novel prognostic marker and therapeutic target for the treatment of HCC.
Similar content being viewed by others
Introduction
The second most prevalent cause of mortality worldwide and the sixth most common malignancy is hepatocellular carcinoma (HCC) [1, 2]. Hepatocellular carcinoma, which has one of the highest rates of occurrence in the world, especially in China, is a serious threat to human health and life expectancy [3]. The American Association for the Study of Liver Disease’s recommendations call for surgical resection (SR) and radiofrequency ablation (RFA) for early HCC. Patients who got RFA, however, exhibited worse long-term survival than those who received SR. For early-stage HCC, surgical excision is still regarded as the best course of action [4]. The majority of HCC patients are in advanced stages at the time of diagnosis and cannot receive surgical treatment because there are no distinct clinical symptoms. The conventional therapy for intermediate-stage, unresectable hepatocellular carcinoma is transarterial chemoembolization (TACE). A potential method called drug-eluting beads (DEB)-TACE is anticipated to increase the effectiveness and safety of traditional TACE [5]. Targeted therapy is one of the main treatment methods for advanced liver cancer [6], Sorafenib is the only drug approved by the FDA for targeted therapy of advanced hepatocellular carcinoma, but it will inevitably appear as primary or secondary drug resistance [7]. Immune checkpoint inhibitor seems to be a promising new direction, but only some patients with hepatocellular carcinoma are effective for Immunocheckpoint inhibitor [8]. Therefore, the creation of novel therapeutic targets and individualized precision medicine for hepatocellular carcinoma is urgently required.
The liver itself is an important metabolic organ of the human body. Metabolic abnormalities in hepatocellular carcinoma appear to be particularly prominent in comparison to other solid tumors. Tumor reprogramming by Metabolic is one of the important malignant hallmarks of cancer progression, which can meet the energetic demand of unlimited cancer cell proliferation by increasing glucose uptake [9]. Many of the molecules involved in the multistep process of glucose metabolism have been linked to both HCC formation and progression as well as being unfavourable prognostic indicators of disease, including GLUT1 [10], PKM2 [11], and HK2 [12] and so on. Aberrant expression of these key factors in HCC provides sufficient ATP to HCC cells via the “Warburg effect”. [13].
In addition to the abnormal protein expression caused by abnormal transcription, another mechanism of abnormal protein expression in tumors is the disorder of the protein degradation pathway. The most common is the ubiquitination degradation pathway. By reversibly attaching to the component of the 26 S proteasome, ubiquitin C-terminal hydrolase L5 (UCHL5) / UCH37 is a cysteine protease of the ubiquitin C-terminal hydrolase (UCHs) family, Ub can be removed from the distal end of multi Ub chain, resulting in reduced degradation of the deubiquitinated target protein and increased expression in cells [14,15,16,17]. However, UCHL5 has also been observed to specifically promote the destruction of inducible nitric oxide synthase and IκB-α on the proteasome [18]. UCHL5 is abnormally expressed in a variety of tumors, but its role in tumors is not completely consistent. In gastric cancer [19] and lymph node-positive renal cancer [20], However, in cases of lung adenocarcinoma [21] and ovarian cancer [22], its high expression is not linked to a positive prognosis, Its high expression is linked to a bad prognosis, demonstrating the complexity of UCHL5’s function in malignancies. Previous research on HCC has discovered that the expression of UCHL5 is elevated and encourages the spread of HCC cells [23], but its specific mechanism has not been completely clear, and further research is required to clarify its specific role and mechanism.
The aim of the present investigation was to investigate UCHL5’s function in HCC. The findings demonstrated that UCHL5 expression in HCC was substantially greater than in nearby tissues. Patients with high UCHL5 expression have a far poorer prognosis than those with low UCHL5 expression. In vivo and in vitro, UCHL5 encourages the growth and metastasis of HCC cells. This impact is accomplished by encouraging the cells’ glycolysis. UCHL5 reduces its amount of ubiquitination by binding to β-catenin, which in turn activates the Wnt/β-catenin pathway, resulting in accelerated HCC development. These findings offer a fresh window into the occurrence and progression of HCC and offer a foundation for therapeutic care.
Materials and methods
Cell lines and cell culture
The Cell Resource Center of the Institute of Basic Medicine, Chinese Academy of Medical Sciences contributed a normal liver cell line (HL-7702) and HCC cell lines (BEL7402, PLC/PRF/5, Huh7, HepG2, HepG3B, and SNU449) (Beijing, China). The cell lines were grown in a 37 °C cell incubator with 5% CO2 using Roswell Park Memorial Institute 1640 (RPMI-1640) media from Glibco in Massachusetts, USA, supplemented with 10% fetal bovine serum (FBS) from HyClone in South Logan, UT, USA, 100 g/mL streptomycin, and 100 U/mL penicillin.
shRNA transfection and construction of stable knocked-down cell line
ViewSolid Biotech sold UCHL5 shRNAs and negative control shRNA (Beijing, China). The UCHL5 shRNA sequences were as follows: sh-RNA-1 UCHL5: GCAAAGAAAGCUCAGGAAATT, sh-RNA-2: 5’-GAUCAAGGUAAUAGUAUGUTT-3’, Control: 5’-UUCUCCGAACGUGUCACGUTT-3’. According to the manufacturer’s instructions, all of the aforementioned siRNAs were transfected into cells using jetPRIME reagent (Polyplus). Negative control and UCHL5-shRNA lentiviral particles were bought from Genechem to create a cell line that would permanently silence the UCHL5 gene (Shanghai, China). As directed by the manufacturer, cells were transfected with sh-Control or UCHL5-shRNA lentiviral particles. To choose stably transfected cells, puromycin (2 µg/mL, cat. no. P7130; Sigma-Aldrich; Merck Millipore) was utilized. The effectiveness of UCHL5’s knockdown was assessed by Western blotting analysis.
RNA isolation and quantitative real-time PCR (RT-qRCR)
Trizol reagent was used to extract total RNA (Invitrogen, Carlsbad, CA, USA). Then, using a NanoDrop ND-100 spectrophotometer, the absorbance at 260 nm was used to calculate the amount of RNA (NanoDrop Technologies, Rockland, DE, USA). Using the PrimeScript RT Reagent Kit, the RNA was reverse transcribed into complementary deoxyribose nucleic acid (cDNA) (Takara Bio, Kusatsu, Japan). SYBR Premix Ex Taq II (TaKaRa) was used for quantitative real-time PCR, which was conducted utilizing Applied Biosystems® 7500 Real-Time PCR Systems (Thermo Fisher Scientific, Waltham, MA, USA). Using the 2-ΔΔCt technique, the relative expression relative to internal parameters (GAPDH) was determined. The following PCR primers were utilized: UCHL5, forward: 5’GAGTGGTGCCTCATGGAAAG3’; reverse: 5’ CAAGTCGGGAGTCCTGAACC3’; GLUT1, forward: 5’GGCCAAGAGTGTGCTAAAGAA3’; reverse: 5’ACAGCGTTGATGCCAGACAG3’; HK2, forward: 5’GAGCCACCACTCACCCTACT3’; reverse: 5’ CCAGGCATTCGGCAATGTG3’; PKM2, forward: 5’ATGTCGAAGCCCCATAGTGAA3’; reverse: 5’TGGGTGGTGAATCAATGTCCA3’; LDHA, forward: 5’ATGGCAACTCTAAAGGATCAGC3’; reverse: 5’CCAACCCCAACAACTGTAATCT3’; LDHB, forward: 5’TGGTATGGCGTGTGCTATCAG3’; reverse: 5’TTGGCGGTCACAGAATAATCTTT3’; PDK1, forward: 5’CTGTGATACGGATCAGAAACCG3’; reverse: 5’TCCACCAAACAATAAAGAGTGCT.
Cell counting kit-8 (CCK-8) assay
The treated cells were plated in 96-well plates (5 × 103 cells/well) in 200 µL culture media in accordance with the experimental conditions. According to the manufacturer’s instructions, the CCK-8 assay (Dojindo, Kumamoto, Japan) was carried out.
Transwell assay
Transwell assays were carried out using transwell chambers on cells transfected for 24 h with sh-Control or UCHL5-shRNA in accordance with the experimental conditions (Corning Life Science, MA, USA). The cells were added to the upper chamber for the migration experiments at a density of 4 × 104 cells per 200 µL in RPMI-1640 media without FBS, and they were incubated there at 37 °C and 5% CO2. Matrigel (Corning, Corning, NY, USA) was pre-plated in the bottom of the top chamber of the Transwell chamber (50 µL) for invasion experiments and then incubated at 37 °C for 30 min. Cells that were still on the lower side of the filter were incubated for another 24–48 h before being fixed with ethanol, stained with Reiter coloring for 1 min, and then redyed with mixed Giemsa for 1 h. Using an Olympus microscope from Tokyo, Japan, the stained cells were counted and captured on camera at a magnification of about 200. At least five fields were tallied and statistically examined at random.
Colony formation experiment
The transfected HCC cells were seeded onto 12-well plates at a density of 300 cells per well, and they were subsequently cultured for two weeks at 5% CO2 and 37 °C. The generated cell colonies were stained for five minutes with 0.1% crystal violet after being fixed with 4% paraformaldehyde. Under a microscope, the number of colonies was counted and inspected.
Clinical samples
From January 2016 to December 2021, a total of 76 pairs of HCC tissues and matched normal liver tissues were collected from the Affiliated Cancer Hospital of Zhengzhou University Hospital. The patient had no operation, radiotherapy, or chemotherapy before the operation, and all patients knew and agreed. Ethical approval for this study was obtained from the ethics committee of the Affiliated Cancer Hospital of Zhengzhou University Hospital.
Western blot and immunoprecipitation
Radioimmunoprecipitation assay (RIPA) lysate that had been previously refrigerated was used to lyse the transfected cells or tissue. The BCA technique was used to measure the concentration of total protein (Beyotime, China). SDS-PAGE was used to separate the protein lysates and the membrane was subsequently coated with PVDF. The primary antibody was incubated with the PVDF membrane at 4 °C overnight as per the manufacturer’s instructions. The PVDF membrane was then incubated for 2 h at room temperature with a goat anti-rabbit secondary antibody that was HRP-labeled (1:5000, CAS #pr30011, Proteintech, China), after which the bands were identified using a Tianneng Tanon-5200 automated chemiluminescence image analysis equipment (China). Cell lysates were combined with antibody and protein G-sepharose beads (Cell Signaling Technology) for immunoprecipitation at 4 °C for at least 6 h. The immunoprecipitated proteins were then heated at 95 °C for 5 min while being treated with 2X sampling buffer. The blots were cut prior to hybridisation with antibodies during blotting.
Wound healing assays
Assays for the healing of scratch wounds were conducted 48 h after transfection. When confluence reached around 100%, the cells were planted on six-well plates and injured by making a straight scratch with a 200 µL pipette tip. Images of migration were taken at zero hours after scratching. The ImageJ program was used to evaluate and quantify the blank space. Three times each of the tests were run.
Animal experiments
After being acclimated for a week, ten BALB/c female nude mice (4–6 weeks old, 18-20 g) were obtained from Beijing Vital River Laboratory Animal Technology Co, Ltd. Mice were subcutaneously implanted with the cells that were suspended in 200 µl of PBS (1 × 107 stable shUCHL5). The weight of the animals and the tumors’ long diameter and short meridian were frequently checked after cell injection, which took place around a week later. All mice were sacrificed via cervical dislocation under anesthesia using isoflurane. These mice were euthanized after 5 weeks in accordance with the criteria set by the Zhengzhou University Committee on Animal Care, and the weights of the tumors were measured, along with the tumor tissues being removed aseptically. 2 × 106 cells were injected into the caudal vein of mice in order to identify cell metastasis. Daily observations of the mice’s health were made, and after four weeks of feeding, the animals were put to death via cervical dislocation. The lungs were then removed, and HE staining was carried out. The Zhengzhou University Institutional Review Board gave its approval to animal testing.
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of UCHL5 co-expressed genes in The Cancer Genome Atlas (TCGA)
It is necessary to download the raw data files of TCGA data (https://portal.gdc.cancer.gov), which contain expression matrices, annotation files and related metadata of all samples. KEGG enrichment analysis was performed on 100 correlated genes, with the parameter setting: p < 0.05. The enrichment analysis was performed by the DAVID database (https://david.ncifcrf.gov/home.jsp).
Immunohistochemistry (IHC) staining
Purchased from Maixin Biotechnology were the reagents (Fuzhou, China). Execute particular experimental procedures in accordance with the guidelines. According to the staining intensity, IHC grading was done (0, negative; 1, weak; 2, moderated; 3, strong).
Protein identification by mass spectrometry
The gel was stained with silver as per the instructions, and the isolated peptides were examined using nano-LC-MS/MS (AB SCIEX TripleT OF 5600, USA) as per the instructions.
Lactate production, glucose uptake and ATP levels measurement
Hepatocellular carcinoma cells are handled in accordance with the needs of the experiment. Using a Lactate Colorimetric Assay kit II, the buildup of lactate in the medium was found (K627-100, BioVision, USA). Utilizing a glucose colorimetric assay kit, the quantity of glucose was determined (K606-100, Bio Vision, USA). The ENLITEN® ATP Assay System (Promega) was used to quantify ATP release in order to examine the ATP in supernatants.
Extracellular acidification rate (ECAR) analysis
Indicated cells were plated onto Seahorse XFe24 Cell Culture Microplates for the ECAR assay (Agilent, Palo Alto, CA, USA). According to the manufacturer’s instructions, the test was carried out using the Seahorse XF Glycolysis Stress Test Kit (103020-100; Agilent). The XFe24 wave program was used to gather and analyze the data (Agilent).
Luciferase assay
HCC cells were transfected with TOPflash, a luciferase reporter plasmid with Wnt luciferase activity, or control FOPflash. TOPflash encodes the three primary untranslated regions (3′-UTR) of UCHL5, whereas FOPflash is an empty vector. The Dual-Luciferase Reporter Assay System (Promega, Madison, WI) was used to quantify luciferase activities in accordance with the manufacturer’s instructions.
Statistical analysis
GraphPad Prism was used to perform all statistical analyses. Three different experiments’ means and standard deviations (SD) were used to illustrate the results of the cell culture experiment. The Student’s t-tests were used to evaluate the differences between the two groups. The threshold for statistical significance was P < 0.05.
Results
Hepatocellular carcinoma patients who have high levels of UCHL5 expression have a bad prognosis
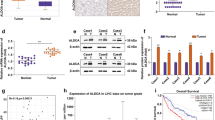
We initially used GEPIA online TCGA-LIHC data to identify the expression level of UCHL5 in HCC and surrounding tissues in order to investigate the function of UCHL5 in HCC (Fig. 1A), the expression of UCHL5 in HCC was significantly increased, and the accuracy of 1-year prognosis prediction was high. The AUC value of a single UCHL5 gene was 0.898 (Fig. 1B). We further confirmed the UCHL5 expression differentially in fresh tissues between HCC and normal tissues. In HCC tissues, UCHL5 mRNA was highly up-regulated (Fig. 1C), and the protein level followed a similar pattern (Fig. 1D F and S1A). We utilized the Kaplan Meier plotter to assess the prognostic differences of HCC patients with varying levels of UCHL5 expression in order to understand the function of UCHL5 expression in the prognosis of HCC. The outcomes demonstrated that patients with high UCHL5 expression in their HCC had a bad prognosis (Fig. 1E). Then, we discovered that hepatocytes and other HCC cells expressed UCHL5 mRNA and protein. The findings demonstrated that hepatoma cells expressed more UCHL5 mRNA and protein than normal hepatocytes did and the most significant differential expression of UCHL5 was observed in HepG2 and Hep3B cell lines (Fig. 1G H and S1B). Therefore, HepG2 and Hep3B cell lines were selected as target cell lines for subsequent cell experiments.
UCHL5is highly expressed in hepatocellular carcinoma, and the prognosis of patients with high expression is poor. (A) Expression of the UCHL5 was validated in 369 HCC tissues and 160 normal tissues with GEPIA. Tumor tissue is shown in red, and normal tissue is shown in gray; (B) The AUC value of a single UCHL5 gene in hepatocellular carcinoma; (C, D) The mRNA and protein expression of UCHL5 in hepatocellular carcinoma and adjacent normal tissues were analyzed by using RT-qPCR and immunohistochemistry; (E) Prognosis of patients with different UCHL5 expression levels; (F) The expression of UCHL5 in 14 cases of hepatocellular carcinoma and adjacent tissues was detected by Western blot; (G, H) The expression of UCHL5 mRNA and protein expression in human normal hepatocytes (HL-7702) and six hepatocellular carcinoma cells (BEL7402, PLC/PRF/5, Huh-7, HepG2, HepG3B and SNU449). Data represent the mean ± SD of three independent experiments. GAPDH as internal parameter. (*p < 0.05, **p < 0.01 and ***p < 0.001)
UCHL5 promotes the growth and metastasis of HCC cells in vitro
The function of UCHL5 in HCC cells was then investigated. In the beginning, we reduced UCHL5 expression in HCC cells. At the mRNA and protein levels, respectively, Fig. 2A, B and S1C show the knockdown effectiveness. The capacity of cells to proliferate changed when UCHL5 expression in cells was altered, and CCK-8 was utilized to identify this shift. The findings demonstrated that UCHL5 knockdown greatly reduced HCC cell growth and that this effect was time-dependent (Fig. 2C), and the colony experiment confirmed that the same trend was obtained in long-term proliferation (Fig. 2E). Further, explore whether UCHL5 affects HCC cell metastasis. The results show that the knockdown of UCHL5 significantly inhibited HCC cell metastasis. The Wound healing test (Fig. 2D) and Transwell migration and invasion test (Fig. 2F) both supported this finding. In HCC cells, UCHL5 knockdown dramatically reduced the capacity for cell metastasis. These findings demonstrate that UCHL5 promotes the in vitro proliferation and metastasis of HCC cells.
UCHL5promotes the proliferation and metastasis of HCC cells in vitro. (A, B) ShUCHL5 or sh-control were transfected into HepG2 and Hep3B cells. The mRNA and protein levels of UCHL5 were examined by RT-qPCR and western blot; (C, E) The effect of UCHL5 on HCC cell proliferation was determined by CCK-8 (C) and colony formation (E); (D) Cell scratch assay to determine the effect of UCHL5 on the migratory ability of HCC cells; (F) The effect of UCHL5 on migration (left) and invasion (right) were evaluated by Transwell assays. Data represent the mean ± SD of three independent experiments. GAPDH as internal parameter. (*p < 0.05, ** p < 0.01 and *** p < 0.001)
UCHL5 promotes glycolysis of HCC cells
We examined the pathway enrichment of UCHL5 in HCC in order to better understand how UCHL5 encourages the growth and metastasis of HCC cells [24,25,26]. The findings demonstrated that the glycolysis route underwent the greatest multiple of change (Fig. 3A), which led us to speculate whether UCHL5 promoted tumor progression by regulating the glycolysis process. In order to verify this conjecture, we measured and detected the change in glucose uptake after the knockdown of UCHL5 in HCC cells using fluorescently labeled glucose 2-NBDG. The results showed that glucose uptake decreased significantly after the knockdown of UCHL5 (Fig. 3B). In addition, the intracellular L-lactic acid concentration and ATP concentration decreased significantly in the knockdown UCHL5 group (Fig. 3C and D). These findings imply that UCHL5 promotes tumor cell glycolysis, and the drop in intracellular metabolites and ATP following UCHL5 knockdown supports this hypothesis. In addition, we indirectly reflected the glycolytic ability under different expression states of UCHL5 by detecting the intracellular acid production ability. The results also found that UCHL5 significantly improved the glycolytic ability of tumor cells (Fig. 3E). Furthermore, we detected metabolism-related target markers, including GLUT1, PKM2, HK2, LDHA, LDHB, and PDK1. Results from RT-qRCR revealed that UCHL5 expression significantly reduced the expression of the target genes GLUT1, PKM2, HK2, and LDHA, but did not affect the expression of LDHB and PDK1 (Fig. 3F). These findings imply that the high expression of UCHL5 in tumors stimulates the expression of genes involved in metabolism, allowing for significant glycolysis and increased energy generation to sustain tumor development.
UCHL5promotes the glycolysis of HCC cells. (A) UCHL5 co-expression genes in TCGA were enriched in KEGG pathways; (B, C, D) Detection of glucose consumption (B), lactate production (C), intracellular ATP level (D) and ECAR(E) in transfected cells; (F) The mRNA levels of GLUT1, PKM2, HK2, LDHA, LDHB, and PDK1 were examined by RT-qPCR after transfected with shUCHL5 or sh-control. Data represent the mean ± SD of three independent experiments. (*p < 0.05)
UCHL5 reduces β-catenin degradation through deubiquitination
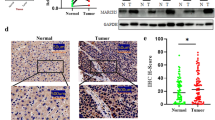
We screened the proteins binding to UCHL5 by mass spectrometry in HCC cells, it was found that β-catenin is a potential binding protein of UCHL5 (Fig. 4A). We further conducted a Co-IP experiment to evaluate whether UCHL5 binds to β-catenin. UCHL5 binds to β-catenin in HCC cells (Fig. 4B and S1D). Cells’ expression of the β-catenin protein was up-regulated or down-regulated when UCHL5 was knocked down or overexpressed (Fig. 4C and S1E). IHC further showed that UCHL5 was positively linked with β-catenin expression in HCC tissues (Fig. 4D), although this expression shift was not brought on by a change in the β-catenin mRNA that UCHL5 regulates. β-catenin mRNA did not significantly alter following UCHL5 overexpression or knockdown (Fig. 4E). It showed that the alteration in UCHL5 expression had no impact on the alteration in mRNA levels for β-catenin. We wonder if UCHL5, a protein that is typically deubiquitinated, is involved in the ubiquitination modification of β-catenin so that it can take part in the breakdown process. As a result, we measured the ubiquitination level of the β-catenin protein after overexpressing UCHL5. It was found that this resulted in a significant decrease in the ubiquitination of β-catenin (Fig. 4F and S1F). The fact that β-catenin did not substantially decrease with the addition of the protein synthesis inhibitor CHX in the UCHL5 overexpression group (Fig. 4G) shows that β-catenin protein was more rapidly destroyed in cells without UCHL5. Following the alteration of UCHL5 in cells, the TOP/FOP-flash approach was used to further determine the activity of the Wnt/β-catenin signaling pathway. The findings demonstrated that UCHL5 dramatically increased the Wnt/β-catenin signaling pathway’s activity (Fig. 4H and I). Additionally, UCHL5 promotes the mRNA and protein expression levels of CyclinD1, c-Myc, VEGF and Survivin (Fig. 4J, K, L and S1G). These findings imply that UCHL5 promotes β-catenin ‘s deubiquitination by binding with β-catenin, preventing β-catenin protein from being degraded by ubiquitination, increasing the amount of β-catenin protein in tumor cells, and activating the Wnt/β-catenin signaling pathway, which in turn accelerates the development of HCC.
UCHL5reduces β-catenin degradation through deubiquitination. (A) Immunoprecipitation of UCHL5 or IgG was carried out in HCC cells. The precipitated protein was analyzed by SDS-PAGE, silver staining was carried out, and the differential bands were identified by mass spectrometry (MS); (B) Co-IP demonstrated the binding between UCHL5 and β-catenin protein; (C, E) The mRNA and protein levels of β-catenin were examined by RT-qPCR (E) and western blot (C) after knockdown or overexpression of UCHL5; (D) The expression level of β-catenin in different groups (UCHL5 high or UCHL5 low) were showed by IHC; (F) The protein levels of β-catenin and the ubiquitination level of β-catenin were examined by western blot after overexpression of UCHL5 or addition of MG132; (G) The protein levels of β-catenin were examined in different time points after adding CHX by western blot and gel blots quantification; (H, I) The TOP/FOP fold change after knockdown or overexpression of UCHL5; (J, K, L) The mRNA and protein levels of cyclinD1, c-Myc, VEGF and survivin were examined by RT-qPCR (J, K) and western blot (L) after knockdown or overexpression of UCHL5. Data represent the mean ± SD of three independent experiments. GAPDH as internal parameter. (*p < 0.05)
UCHL5 promotes HCC progression through β-catenin
We overexpressed UCHL5 while inhibiting β-catenin or adding a β-catenin inhibitor to further determine if UCHL5 accelerates HCC development by stimulating the Wnt/β-catenin pathway. The findings demonstrated that the proliferation stimulation brought on by the overexpression of UCHL5 could be reversed by either knocking down β-catenin or adding a β-catenin inhibitor (Fig. 5A), the same conclusion can be obtained in the long-term proliferation of the set (Fig. 5C). Similar patterns were also discovered in trials involving cell scratching, Tranwell invasion and migration assays. The ability of UCHL5 to promote metastasis can be increased by using β-catenin inhibitors or decreased by using β-catenin (Fig. 5B and D). Our previous results showed that UCHL5 promoted the glycolysis of HCC cells. Therefore, we further detected the changes in glucose uptake and lactic acid content in HCC cells after the knockdown of β-catenin or the addition of inhibitors after overexpression of UCHL5. The results showed that overexpression of UCHL5 could promote glucose uptake and lactic acid production in cells, that is, promote glycolysis in cells (Fig. 5E, F). However, its content drastically dropped once β-catenin was knocked down or an inhibitor was added, and it essentially reverted to the level of the control group. Similar to this, ATP produced by cells follows the same trend (Fig. 5G). The mRNA levels of UCHL5’s primary metabolic target genes, GLUT1, PKM2, HK2, and LDHA, were up-regulated after UCHL5 was overexpressed, and they were almost down-regulated to the control group after β-catenin was knocked down or inhibitors were added (Fig. 5H). These findings demonstrate that the promotion of intracellular glycolysis, which is accomplished by activating the intracellular Wnt/β-catenin pathway in cells to activate the transcription of metabolic-related genes, is responsible for the proliferation and metastasis of UCHL5 on HCC cells.
UCHL5promotes HCC progression through β-catenin. After changing the expression ofUCHL5, β-catenin or adding IWP-4. (A, C) The change of HCC cell proliferation was determined by CCK-8 (A) and colony formation (C) in different time points; (B) Cell scratch assay to determine the migration ability of HCC cells; (D) The change of migration and invasion ability was evaluated byTranswell assays; (E, F, G) Detection of glucose consumption (E), lactate production (F) and intracellular ATP level by RT-qPCR (G); The mRNA levels of GLUT1, PKM2, HK2, and LDHA were examined by RT-qPCR. Data represent the mean ± SD of three independent experiments. (*p < 0.05)
UCHL5 promotes the proliferation and metastasis of HCC cells in vivo
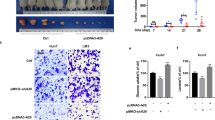
We employed the xenograft model to validate subcutaneous carcinogenesis in vivo in order to further support the effect of UCHL5 on HCC. The capacity of tumor cells to proliferate dramatically diminished in comparison to the control group when UCHL5 was knocked down (Fig. 6A). After the knockdown of UCHL5 expression, the volume and weight of tumors were significantly lower than the control group (Fig. 6B C). Immunohistochemical results showed that knockdown of UCHL5 expression was followed by a significant decrease in the expression of β-catenin, and the expression of proliferation-related markers KI67 and PCNA (Fig. 6D). Lung metastasis analysis showed that down-regulation of UCHL5 expression significantly reduced the diameter and number of metastatic foci in lung tissue (Fig. 6E F). These outcomes demonstrated that UCHL5 can indeed promote tumor development in vivo.
UCHL5promotes the proliferation and metastasis of HCC cells in vivo. (A) Images of xenograft tumors derived from transfected cells in nude mice; (B) Tumor volume curves were summarized in the line chart. The average tumor volume was expressed as the mean standard deviation of 5 mice; (C) The tumor weight was assessed in different groups; (D) The expression level of UCHL5, β-catenin, KI67, and PCNA in different groups were shown by IHC. (E) Representative images of the tail vein-injected mouse models. (F) The lung foci number was evaluated. Data represent the mean ± SD of three independent experiments. (**p < 0.01)
Discussion
Most protein breakdown in all eukaryotic cells depends on the ubiquitin-proteasome system (UPS) pathway [27]. The degraded protein is specifically labeled by ubiquitin (Ub), and the proteasome accurately recognizes and degrades the labeled protein [28]. This ubiquitin labeling process depends on ubiquitinase. Of course, this process is reversible. Ubiquitinase prolongs the Ub chain and deubiquitinase (DUBs) mediates cutting multi Ub chain to reversibly regulate [28, 29]. Previous research has demonstrated that both ubiquitinates and deubiquitinases are crucial for tumor growth and perform a variety of roles in the development of tumors [30], which depends on their important role in the regulation of specific protein expression. UCHL5 belongs to the ubiquitin C-terminal hydrolases (UCHs) family, which can remove Ub from the ubiquitin chain of the target protein, resulting in protein deubiquitination, and thereby reducing degradation [14,15,16,17]. In line with earlier research, we also found in this work that silencing UCHL5 in HCC cells increased the degree of protein ubiquitination. Similarly, the target protein β-catenin is down-regulated, indicating that UCHL5 is likely to inhibit protein degradation through the classical deubiquitination pathway. However, whether UCHL5 directly binds to β-catenin or is related to the role of 26 S proteasome requires further experimental exploration. This regulatory relationship between UCHL5 and β-catenin also exists in endometrial cancer. The presence of UCHL5 up regulates and activates β-catenin, thereby promoting the proliferation of endometrial cancer [21, 31]. In this study, we confirmed that this regulatory mechanism also exists in HCC cells, and we first proposed that UCHL5 regulates β-catenin in hepatocellular carcinoma by regulating its ubiquitination level in combination with β-catenin, which may provide a new potential direction for inhibiting β-catenin pathway.
The main protein of the Wnt/β-catenin pathway, β-catenin protein, plays a crucial role in the proliferation and spread of tumors by controlling a number of genes’ transcription [32]. Numerous studies have demonstrated that the Wnt/β-catenin pathway functions abnormally in HCC [33, 34]. In this work, we discovered that the amount of UCHL5 expression regulated the activity of the Wnt/β-catenin pathway. Additionally, elevated Wnt/β-catenin pathway related proteins, including CyclinD1, c-Myc, Survivin, and VEGF, were also activated. Concerning these genes, the β-catenin inhibitor IWP-4 counteracted their overexpression. Additionally, the Wnt/β-catenin pathway promotes HCC cells to enhance glycolysis.
One of the major hallmarks of cancer is the unlimited proliferation and spread of tumor cells, which may encounter the energy needed for their proliferation and metastasis via abnormal glucose metabolism. This effect is called the “Warburg effect”, which is characterized by the transfer of energy production from oxidative phosphorylation to glycolysis [35, 36]. Enhanced cell glycolysis leads to the accumulation of its product lactic acid in the tumor microenvironment, this can increase tumor cell survival and assist tumor cells in evading the host’s immune surveillance. [37,38,39,40]. This metabolic abnormality affects the progression of HCC [41]. The Wnt/β-catenin pathway is a crucial mechanism for controlling metabolism, particularly glycolysis, in HCC, as demonstrated by earlier research [42]. This is in line with the findings of this investigation. Hepatocellular carcinoma-educated macrophages’ promotion of epithelial-mesenchymal transformation via Wnt2b/β-catenin /c-Myc signaling and reprogramming glycolysis [43]. In hepatocellular carcinoma cells, autophagy increases MCT1 expression and activates the Wnt/β-catenin signaling pathway, promoting metastasis and glycolysis [44]. Through blocking the Warburg Effect through the PPARγ-Dependent WNT/β-catenin /Pyruvate Dehydrogenase Kinase Isozyme 1 Axis, PPARγ Coactivator-1 Suppresses Metastasis of Hepatocellular Carcinoma [45]. Gankyrin stimulates glycolysis and glutaminolysis via upregulating c-Myc and activating β-catenin signaling to enhance tumorigenesis, metastasis, and treatment resistance in human HCC [46]. Our study’s findings demonstrate that altering UCHL5 expression can control the mRNA expression levels of markers associated with metabolism, including GLUT1, PKM2, HK2, and LDHA, and further encourage the growth of glycolysis.
In this work, we discovered that UCHL5 triggers Wnt/β-catenin to activate glycolysis. The final intuitive effect is that high UCHL5 expression promotes glycolysis of HCC cells to provide energy for the proliferation and metastasis of HCC cells, this is unreported in earlier investigations. At the same time, we also found that the intracellular metabolite lactic acid is also increased. This finding provides some basis for further clarifying the molecular mechanism of HCC development, while targeting UCHL5 may be a potential predictive and therapeutic strategy for the clinical treatment of HCC. Unfortunately, we have not evaluated whether the metabolite lactic acid is discharged into the microenvironment and whether it reshapes the microenvironment through lactic acid, leading to cell metastasis, which requires us to further explore the detailed mechanism of UCHL5. Meanwhile, given the relatively high incidence of HCC, the clinical samples we collected were limited, and we need to further expand the sample size for more in-depth mechanistic and clinical studies.
Conclusions
In summary, our study revealed that UCHL5 is significantly expressed in hepatocellular carcinoma and speeds up tumor growth by encouraging hepatocellular carcinoma cell proliferation and metastasis. First, we discovered that UCHL5 stimulates the Wnt/β-catenin pathway in tumor cells to enhance glycolysis. By interacting with β-catenin, UCHL5 lowers its amount of ubiquitination, preventing β-catenin from being degraded and increasing its intracellular concentration. After activating the Wnt/β-catenin pathway, many genes including cell cycle detection points are activated, which eventually promotes the progression of HCC and has a poor prognosis. This opens new possibilities for clinical prediction and treatment of HCC.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109(4):542–53.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, Colombo M, Craxi A, Crespo J, Day CP, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69(4):896–904.
Wang H, Liu Y, Shen K, Dong Y, Sun J, Shu Y, Wan X, Ren X, Wei X, Zhai B. A comparison between radiofrequency ablation combined with transarterial chemoembolization and surgical resection in hepatic carcinoma: a meta-analysis. J Cancer Res Ther. 2019;15(7):1617–23.
Wang H, Cao C, Wei X, Shen K, Shu Y, Wan X, Sun J, Ren X, Dong Y, Liu Y, et al. A comparison between drug-eluting bead-transarterial chemoembolization and conventional transarterial chemoembolization in patients with hepatocellular carcinoma: a meta-analysis of six randomized controlled trials. J Cancer Res Ther. 2020;16(2):243–9.
Bhayani NH, Jiang Y, Hamed O, Kimchi ET, Staveley-O’Carroll KF. Gusani NJ: advances in the pharmacologic treatment of Hepatocellular Carcinoma. Curr Clin Pharmacol. 2015;10(4):299–304.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90.
Federico P, Petrillo A, Giordano P, Bosso D, Fabbrocini A, Ottaviano M, Rosanova M, Silvestri A, Tufo A, Cozzolino A et al. Immune checkpoint inhibitors in Hepatocellular Carcinoma: current status and novel perspectives. Cancers 2020, 12(10).
Pavlova NN, Thompson CB. The emerging Hallmarks of Cancer Metabolism. Cell Metabol. 2016;23(1):27–47.
Amann T, Hellerbrand C. GLUT1 as a therapeutic target in hepatocellular carcinoma. Expert Opin Ther Targets. 2009;13(12):1411–27.
Liu Y, Wu H, Mei Y, Ding X, Yang X, Li C, Deng M, Gong J. Clinicopathological and prognostic significance of PKM2 protein expression in cirrhotic hepatocellular carcinoma and non-cirrhotic hepatocellular carcinoma. Sci Rep. 2017;7(1):15294.
DeWaal D, Nogueira V, Terry AR, Patra KC, Jeon SM, Guzman G, Au J, Long CP, Antoniewicz MR, Hay N. Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat Commun. 2018;9(1):446.
Zuo Q, He J, Zhang S, Wang H, Jin G, Jin H, Cheng Z, Tao X, Yu C, Li B, et al. PPARγ Coactivator-1α suppresses metastasis of Hepatocellular Carcinoma by Inhibiting Warburg Effect by PPARγ-Dependent WNT/β-Catenin/Pyruvate dehydrogenase kinase isozyme 1 Axis. Hepatology (Baltimore MD). 2021;73(2):644–60.
Lam YA, Xu W, DeMartino GN, Cohen RE. Editing of ubiquitin conjugates by an isopeptidase in the 26S proteasome. Nature. 1997;385(6618):737–40.
Tian Z, D’Arcy P, Wang X, Ray A, Tai YT, Hu Y, Carrasco RD, Richardson P, Linder S, Chauhan D, et al. A novel small molecule inhibitor of deubiquitylating enzyme USP14 and UCHL5 induces apoptosis in multiple myeloma and overcomes bortezomib resistance. Blood. 2014;123(5):706–16.
Matilainen O, Arpalahti L, Rantanen V, Hautaniemi S, Holmberg CI. Insulin/IGF-1 signaling regulates proteasome activity through the deubiquitinating enzyme UBH-4. Cell Rep. 2013;3(6):1980–95.
Chen X, Walters KJ. Structural plasticity allows UCH37 to be primed by RPN13 or locked down by INO80G. Mol Cell. 2015;57(5):767–8.
Mazumdar T, Gorgun FM, Sha Y, Tyryshkin A, Zeng S, Hartmann-Petersen R, Jorgensen JP, Hendil KB, Eissa NT. Regulation of NF-kappaB activity and inducible nitric oxide synthase by regulatory particle non-ATPase subunit 13 (Rpn13). Proc Natl Acad Sci U S A. 2010;107(31):13854–9.
Arpalahti L, Laitinen A, Hagström J, Mustonen H, Kokkola A, Böckelman C, Haglund C, Holmberg CI. Positive cytoplasmic UCHL5 tumor expression in gastric cancer is linked to improved prognosis. PLoS ONE. 2018;13(2):e0193125.
Arpalahti L, Hagstrom J, Mustonen H, Lundin M, Haglund C, Holmberg CI. UCHL5 expression associates with improved survival in lymph-node-positive rectal cancer. Tumour Biol. 2017;39(7):1010428317716078.
Liu D, Song Z, Wang X, Ouyang L. Ubiquitin C-Terminal hydrolase L5 (UCHL5) accelerates the growth of Endometrial Cancer via activating the Wnt/β-Catenin signaling pathway. Front Oncol. 2020;10:865.
Zhang J, Xu H, Yang X, Zhao Y, Xu X, Zhang L, Xuan X, Ma C, Qian W, Li D. Deubiquitinase UCHL5 is elevated and associated with a poor clinical outcome in lung adenocarcinoma (LUAD). J Cancer. 2020;11(22):6675–85.
Fang Y, Fu D, Tang W, Cai Y, Ma D, Wang H, Xue R, Liu T, Huang X, Dong L, et al. Ubiquitin C-terminal hydrolase 37, a novel predictor for hepatocellular carcinoma recurrence, promotes cell migration and invasion via interacting and deubiquitinating PRP19. Biochim Biophys Acta. 2013;1833(3):559–72.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30.
Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019;28(11):1947–51.
Kanehisa M, Furumichi M, Sato Y, Kawashima M, Ishiguro-Watanabe M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023;51(D1):D587–92.
Ciechanover A. The ubiquitin proteolytic system: from a vague idea, through basic mechanisms, and onto human diseases and drug targeting. Neurology. 2006;66(2 Suppl 1):7–19.
Bader N, Jung T, Grune T. The proteasome and its role in nuclear protein maintenance. Exp Gerontol. 2007;42(9):864–70.
Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol. 2006;17(7):1807–19.
Welchman RL, Gordon C, Mayer RJ. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat Rev Mol Cell Biol. 2005;6(8):599–609.
Li Z, Zhou L, Jiang T, Fan L, Liu X, Qiu X. Proteasomal deubiquitinase UCH37 inhibits degradation of β-catenin and promotes cell proliferation and motility. Acta Biochim Biophys Sin. 2019;51(3):277–84.
Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–205.
He S, Tang S. WNT/β-catenin signaling in the development of liver cancers. Biomed Pharmacotherapy = Biomedecine Pharmacotherapie. 2020;132:110851.
Perugorria MJ, Olaizola P, Labiano I, Esparza-Baquer A, Marzioni M, Marin JJG, Bujanda L, Banales JM. Wnt-β-catenin signalling in liver development, health and disease. Nat Reviews Gastroenterol Hepatol. 2019;16(2):121–36.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Liberti MV, Locasale JW. The Warburg Effect: how does it Benefit Cancer cells? Trends Biochem Sci. 2016;41(3):211–8.
Choi SY, Collins CC, Gout PW, Wang Y. Cancer-generated lactic acid: a regulatory, immunosuppressive metabolite? J Pathol. 2013;230(4):350–5.
Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109(9):3812–9.
Romero-Garcia S, Moreno-Altamirano MM, Prado-Garcia H, Sanchez-Garcia FJ. Lactate Contribution to the Tumor Microenvironment: mechanisms, Effects on Immune cells and therapeutic relevance. Front Immunol. 2016;7:52.
Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et al. LDHA-Associated Lactic Acid Production blunts Tumor Immunosurveillance by T and NK cells. Cell Metab. 2016;24(5):657–71.
Zhang Z, Li TE, Chen M, Xu D, Zhu Y, Hu BY, Lin ZF, Pan JJ, Wang X, Wu C, et al. MFN1-dependent alteration of mitochondrial dynamics drives hepatocellular carcinoma metastasis by glucose metabolic reprogramming. Br J Cancer. 2020;122(2):209–20.
Fan Q, Yang L, Zhang X, Ma Y, Li Y, Dong L, Zong Z, Hua X, Su D, Li H, et al. Autophagy promotes metastasis and glycolysis by upregulating MCT1 expression and Wnt/β-catenin signaling pathway activation in hepatocellular carcinoma cells. J Experimental Clin cancer Research: CR. 2018;37(1):9.
Jiang Y, Han Q, Zhao H, Zhang J. Promotion of epithelial-mesenchymal transformation by hepatocellular carcinoma-educated macrophages through Wnt2b/beta-catenin/c-Myc signaling and reprogramming glycolysis. J Exp Clin Cancer Res. 2021;40(1):13.
Fan Q, Yang L, Zhang X, Ma Y, Li Y, Dong L, Zong Z, Hua X, Su D, Li H, et al. Autophagy promotes metastasis and glycolysis by upregulating MCT1 expression and Wnt/beta-catenin signaling pathway activation in hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2018;37(1):9.
Zuo Q, He J, Zhang S, Wang H, Jin G, Jin H, Cheng Z, Tao X, Yu C, Li B, et al. PPARgamma Coactivator-1alpha suppresses metastasis of Hepatocellular Carcinoma by Inhibiting Warburg Effect by PPARgamma-Dependent WNT/beta-Catenin/Pyruvate dehydrogenase kinase isozyme 1 Axis. Hepatology. 2021;73(2):644–60.
Liu R, Li Y, Tian L, Shi H, Wang J, Liang Y, Sun B, Wang S, Zhou M, Wu L, et al. Gankyrin drives metabolic reprogramming to promote tumorigenesis, metastasis and drug resistance through activating beta-catenin/c-Myc signaling in human hepatocellular carcinoma. Cancer Lett. 2019;443:34–46.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China, No. 61802350.
Author information
Authors and Affiliations
Contributions
The research was thought of and created by W-BS and Z L. W-BS carried out the experiments, analyzed the data, and wrote the report. C M and H T carried out the data screening and analysis. Experimental guiding was undertaken by W-BS, C M, and H T. Z L made edits to the paper.
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the ethics committee of the Affiliated Cancer Hospital of Zhengzhou University. All methods were performed in accordance with the Declaration of Helsinki. All subjects provided written informed consent. The study was carried out in compliance with the ARRIVE guidelines. All animal studies were approved by the Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University.
Consent for publication
Not applicable.
Conflict of interest
The study’s authors affirm that there were no financial or commercial ties that may be viewed as having a possible conflict of interest.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wan, B., Cheng, M., He, T. et al. UCHL5 promotes hepatocellular carcinoma progression by promoting glycolysis through activating Wnt/β-catenin pathway. BMC Cancer 24, 618 (2024). https://doi.org/10.1186/s12885-023-11317-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11317-z