Abstract
Introduction
The optimal first-line immunotherapy regimen for advanced non-squamous non-small cell lung cancer (NS-NSCLC) patients with programmed cell death ligand 1 (PD-L1) expression ≥ 50% remains unclear. Our aim is to determine the most effective treatment regimen through a network meta-analysis (NMA) comparing these treatments.
Methods
A systematic search was performed in PubMed, Cochrane Library, Web of Science, and Embase databases, and a Bayesian network meta-analysis was conducted. To ensure transparency, the study was registered in the International Prospective Register of Systematic Reviews (CRD42022349712).
Results
The analysis included 11 randomized controlled trials (RCTs) with 2037 patients and 12 immunotherapy combinations. ICI-ICI, ICI alone, and chemotherapy-ICI showed significant advantages over chemotherapy in terms of overall survival (OS) and progression-free survival (PFS). Pembrolizumab plus chemotherapy showed the best OS results compared to chemotherapy. Tislelizumab plus chemotherapy and sintilimab plus chemotherapy provided the best PFS results.
Conclusions
For NS-NSCLC patients with PD-L1 ≥ 50%, pembrolizumab plus chemotherapy, tislelizumab plus chemotherapy, and sintilimab plus chemotherapy are recommended as good treatment options based on the results of this Network meta-analysis (NMA).
Similar content being viewed by others
Introduction
Lung cancer has the highest mortality rate of all cancers and is the second most common cancer, after female breast cancer[1]. NSCLC accounts for approximately 85% of all lung cancers, with NS-NSCLC comprising more than half of all NSCLC cases[2, 3]. Molecular targeted therapy is the standard first-line treatment for patients with advanced NSCLC with sensitive genetic mutations. In contrast, platinum-based dual chemotherapy is the standard first-line treatment for patients without targeted gene alterations or with unknown mutation status[4, 5]. While conventional chemotherapy is the mainstay of treatment for advanced NSCLC, its clinical benefits are limited. It has a median OS of less than one year and a five-year PFS rate of only 4%[6, 7]. However, the emergence of immune checkpoint inhibitors (ICIs) offers new hope for patients with advanced NSCLC.
ICIs work by activating the anti-tumor activity of T-lymphocytes through the inhibition of the interaction between PD-1, PD-L1, and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4). This interaction removes tumor cells and tumor tissue to achieve anti-tumor effects[8, 9]. Additionally, ICIs primarily target cancer antigens and prevent normal cells from being attacked [10]. In recent years, ICIs have been approved for use in advanced NSCLC and have been widely used in clinical practice [11, 12].
PD-L1 expression on tumor or immune cells has emerged as a potential predictive biomarker for sensitivity to ICIs and patient stratification[13]. According to National Comprehensive Cancer Network (NCCN) guidelines, pembrolizumab, pembrolizumab with chemotherapy, atezolizumab, and cemiplimab are the first-line immunotherapy regimens for advanced non-squamous NSCLC with PD-L1 expression ≥ 50%. These treatments have demonstrated better PFS and OS compared to platinum-based double chemotherapy[14,15,16,17]. However, direct comparisons of different PD-1/PD-L1 inhibitors, including sintilimab and tislelizumab, have not been performed as they have recently entered the market[18].
The aim of our study is to evaluate the effectiveness and ranking of ICIs in advanced NS-NSCLC with PD-L1 expression ≥ 50%. By doing so, our results may provide valuable insights into the most effective treatment for advanced NS-NSCLC with high PD-L1 expression.
Materials and methods
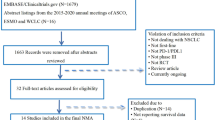
The NMA in this study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-NMA) extension statement, as shown in Fig. 1 [19]. Bayesian methods were employed to enable indirect comparisons between treatments that have not yet been directly compared in clinical trials, thus allowing for probabilistic predictions of treatment outcomes [20]. To ensure transparency, reliability, and originality, the protocol for this study was registered in the Prospective Register of Systematic Reviews (PROSPERO) under the reference number CRD42022349712.
Data sources and search strategy
To identify relevant studies, a systematic search was conducted in the PubMed, Cochrane Library, Web of Science, and Embase databases from the date of database creation to October 15, 2022, using the following keywords: “non-small-cell lung cancer,“ “randomized clinical trial,“ “immunotherapy,“ “PD-1,“ “PD-L1,“ “CTLA-4,“ “pembrolizumab,“ “atezolizumab,“ “nivolumab,“ “ipilimumab,“ “durvalumab,“ “tislelizumab,“ “camrelizumab,“ “cemiplimab,“ and “sintilimab.“
Selection criteria
For the inclusion criteria, we selected studies for this meta-analysis based on the following:
-
(1)
Phase II or Phase III RCTs were considered.
-
(2)
Patients with histologically or cytologically confirmed stage IV NSCLC were included in the RCT.
-
(3)
The RCT involved treatment with ICIs.
-
(4)
Availability of OS or PFS data was required for NS-NSCLC patients with PD-L1 expression ≥ 50%.
On the other hand, the following studies were excluded from this meta-analysis:
-
(1)
RCTs involving the same patient group were excluded.
-
(2)
Editorials, observational studies, and reviews were not included.
Data extraction and quality assessment
Data Extraction: The selected studies were subjected to a rigorous data extraction process in accordance with PRISMA guidelines. To ensure accuracy and completeness, three researchers independently extracted relevant data, with any discrepancies being resolved through discussion with a fourth author. The extracted data included details such as trial name, first author, source of publication, year of publication, trial phase, national clinical trial identification number, sample size, patient age and gender distribution, trial group, and control group. Clinical outcomes such as hazard ratios (HRs) and 95% confidence intervals (CIs) for OS and PFS were also extracted.
Quality Assessment: To ensure that the included studies met high-quality standards, the Cochrane Risk of Bias tool (2.0) was used to assess the quality of the RCTs. This tool evaluated the risk of bias in five key domains, including the randomization process, potential deviation from the intended intervention, missing outcome data, outcome measurement, and selection of reported results[21].
Based on the quality assessment results, the included studies were categorized as low-risk, high-risk, or unclear. This ensured that only studies with rigorous and robust methodologies were included in the meta-analysis.
Statistical analysis
The Bayesian framework was employed using R software (version 4.0.3) with the “JAGS” and “GeMTC” packages to conduct the NMA, which aimed to evaluate the effectiveness of various ICIs in treating advanced NSCLC[22, 23]. A fixed-effect consistency model was utilized, and 20,000 simultaneous iterations and 50,000 sample iterations per chain were run on three independent Markov chains. The NMA endpoints were OS and PFS, with effect sizes measured by HRs and corresponding 95% CIs. For the head-to-head meta-analysis, the Revman software (version 5.4) was used. The rank probability command was utilized to rank the treatments from best to worst, and statistical significance was determined at a bilateral alpha level of less than 0.05. One reviewer performed the statistical analysis, and the results were checked by three other reviewers to ensure accuracy.
Sensitivity analysis
In addition, to ensure the best fit for our analyses, we conducted a model comparison using the Deviance Information Criterion (DIC), which assesses the relative goodness-of-fit of the fixed-effect and random-effect models. A smaller DIC value indicates a better model fit. If the difference in DIC between the fixed-effect and random-effect models was less than 5, it was considered that the models were consistent. This approach helped us ensure that the most appropriate model was selected for each analysis cohort[24].
Heterogeneity analysis
We performed a heterogeneity analysis using the “anote” command to calculate I2 values. I2 values were interpreted as follows: if the I2 value was less than 25%, it was considered low heterogeneity; if it was between 25% and 50%, it was considered medium heterogeneity; and if it was greater than 75%, it was considered high heterogeneity. In cases of low heterogeneity, a fixed-effects model was used, while a random-effects model was used in cases of medium or high heterogeneity[25].
Results
Studies included in the NMA
After conducting a thorough search of four databases - namely PubMed, Web of Science, Embase, and the Cochrane Library - a total of 4643 articles were identified. Following the removal of duplicates, 2679 articles were excluded from the analysis. The final selection process is depicted in Fig. 1, resulting in the inclusion of 12 articles in this NSCLC NMA. This meta-analysis included 2,176 patients from 11 RCTs, which evaluated the following 12 treatment options for NSCLC: atezolizumab plus chemotherapy (atezo-chemo), atezolizumab plus bevacizumab plus chemotherapy (atezo-beva-chemo), pembrolizumab (pem), pembrolizumab plus ipilimumab (pem-ipi), pembrolizumab plus chemotherapy (pem-chemo), nivolumab plus bevacizumab plus chemotherapy (nivo-beva-chemo), camrelizumab plus chemotherapy (camre-chemo), sintilimab plus chemotherapy (sinti-chemo), tislelizumab plus chemotherapy (tisle-chemo), cemiplimab (cemi), chemotherapy (chemo), and bevacizumab plus chemotherapy (beva-chemo). The details of the RCTs are provided in Table 1.
Characteristics of studies
The experimental group of 3 RCTs including ICI monotherapy(KN-024[17], EMPOWER-Lung1[15]), and experimental groups in eight trials studied ICIs in combination with chemotherapy (Camel[26], IMpower130[27], IMpower132[16, 28], IMpower150[29], KN-189[30], ORIENT-11[31], RATIONALE304[32], TASUKI-52[33]). In addition, an RCT evaluated the combination of PD-1 inhibitors with CTLA-4 inhibitors(Pembrolizumab/ Ipilimumab.KN-598[34]). Figure 2 displays a network plot of the eligible comparisons.
Network plot for effectiveness of 9 and 8 different treatment modalities for patients with PD-L1 expression⩾50% for PFS (A) and OS (B), Respectively. Circles represent the intervention as a node in the network; lines represent direct comparisons within the frame of RCTs; the line thickness indicates the number of RCTs included in each comparison. Atezo, atezolizumab; Beva, bevacizumab; Camre, camrelizumab; Cemi, cemiplimab; Chemo, chemotherapy; Pem, pembrolizumab; Sinti, sintilimab; Tisle, Tislelizumab; Nivo, nivolumab
Assessment of included trials
Figure 3 presents the results of the risk of bias assessment for the 11 included trials. Overall, the risk of bias was low as all studies were well-designed randomized controlled trials. Trial protocols were accessed to confirm methodological information. In the selection bias domain, 10 trials were rated as low risk, while one trial (TASUKI-52) was rated as unclear. For the reporting bias domain, 10 trials were rated as low risk, while one trial (RATIONALE 304) was rated as unclear. In the performance bias domain, seven trials were rated as low risk, three trials (Camel, EMPOWER-Lung 1, KN-024) were rated as high risk, and one trial (RATIONALE 304) was rated as unclear. As for the detection bias domain, all trials were rated as low risk, given that lack of blinding is unlikely to affect this domain. In the attrition bias domain, 10 trials were rated as low risk, and one trial (EMPOWER-Lung 1) was rated as unclear. For the reporting bias domain, all trials were rated as low risk, mainly because they were analyzed based on the intention-to-treat population and reported sufficient endpoints. However, some trials allowed crossover, which was deemed a potential source of bias.
Risk of Bias Figure. (A) methodological quality summary: authors’ judgment about each methodological quality item for each included study. Performance bias and detection bias presented were for risk of bias; (B) Methodological quality graph: authors’ judgment about each methodological quality item presented as percentages across all included studies
Pairwise meta-analysis
Paired meta-analyses were performed for four trials that reported HRs for OS and seven trials that reported HRs for PFS when comparing ICIs to chemotherapy.
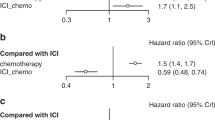
The head-to-head comparisons revealed that, in comparison to chemotherapy, patients treated with atezolizumab plus chemotherapy had improved OS (HR, 0.81; 95% CI, 0.52–1.26) and improved PFS (HR, 0.50; 95% CI, 0.35–0.71). Additionally, patients treated with pembrolizumab had improved OS (HR, 0.59; 95% CI, 0.40–0.87) and improved PFS (HR, 0.55; 95% CI, 0.39–0.77). Patients treated with cemiplimab had improved OS (HR, 0.64; 95% CI, 0.43–0.94) and improved PFS (HR, 0.60; 95% CI, 0.44–0.82). Moreover, patients treated with camrelizumab plus chemotherapy (HR, 0.39; 95% CI, 0.15–1.04), sintilimab plus chemotherapy (HR, 0.31; 95% CI, 0.20–0.49), and tislelizumab plus chemotherapy (HR, 0.31; 95% CI, 0.17–0.56) had improved PFS.
The forest plots used to compare the pairwise results of OS and PFS are displayed in Figs. 4 and 5, respectively.
Network Meta-Analysis
Regarding OS, the indirect comparison results are shown in Fig. 6, compared to chemotherapy, atezolizumab plus bevacizumab plus chemotherapy (HR, 0.77; 95%CI, 0.48–1.22).
Regarding PFS, the results of the indirect comparison are shown in Fig. 7, compared to chemotherapy, pembrolizumab plus ipilimumab (HR, 0.62; 95%CI, 0.41–0.93), nivolumab plus bevacizumab plus chemotherapy (HR, 0.55; 95%CI, 0.41–0.93).
Rankings
Figures 8 and 9 present the summary of treatment ranking probabilities for the comparative efficacy of OS and PFS, respectively.
For OS, pembrolizumab plus chemotherapy had the highest probability of being the most effective treatment (54.87%), followed by cemiplimab (40.27%) and atezolizumab plus bevacizumab plus chemotherapy (41.4%) in second and third place, respectively.
For PFS, tislelizumab plus chemotherapy was ranked first with a probability of 31.22%, followed by sintilimab plus chemotherapy in second place with a probability of 26.67%, and atezolizumab plus chemotherapy in third place with a probability of 15.78%.
Discussion
This groundbreaking study represents the most comprehensive NMA to date, providing an in-depth analysis of the efficacy of first-line immunotherapy in patients with NS-NSCLC and PD-L1 expression levels ≥ 50%. Notably, this study stands out from other NMAs in its inclusion of RCTs utilizing sintilimab and tislelizumab for treatment. The study’s analysis involved an impressive cohort of 2037 advanced non-squamous NSCLC patients with PD-L1 expression ≥ 50%, culled from 12 RCTs. The results showed that compared to chemotherapy alone, the use of chemotherapy-ICI, monotherapy ICI, and ICI-ICI regimens resulted in higher PFS and OS rates. Squamous NSCLC is a particularly complex disease, impacted by multiple factors, predominantly linked to smoking, which results in a high mutation rate in its genes[35]. Non-Small Cell Lung Adenocarcinoma is more common in non-smokers or light smokers and tends to occur in younger individuals[36]. It is more likely to develop in the outer regions of the lung.Non-Small Cell Lung Adenocarcinoma can present as a solitary nodule, multiple nodules, or as a diffuse infiltrative pattern. Compared to squamous NSCLC, non-squamous NSCLC is characterized by simpler mutations, which offers insight into why non-squamous NSCLC patients with PD-L1 expression ≥ 50% can benefit from various ICI treatment regimens[37]. These findings hold immense promise for improving the treatment of advanced NS-NSCLC patients with PD-L1 expression ≥ 50%, and bring us one step closer to more effective, personalized therapies for lung cancer[38].
The NMA is an expansion on the traditional meta-analysis, which indirectly compares interventions in RCTs through a common comparison group, in addition to the support of multitude of studies to ensure the validity of the results. To assess the relative effectiveness of chemotherapy-ICI and ICI-ICI in treating advanced non-squamous NSCLC with PD-L1 expression ≥ 50%, head-to-head clinical trials represent the most informative approach, providing valuable insights for clinical decision-making.
Despite the promising results of immune checkpoint inhibitors for the treatment of NSCLC, it is evident that certain patients may not respond optimally to these therapies. As such, the identification of predictive biomarkers has emerged as a critical strategy to guide the personalized selection of immunotherapy, ensuring that patients receive the most effective treatment available. These biomarkers provide valuable insights into the unique molecular characteristics of individual patient’s tumors, enabling oncologists to make informed decisions regarding the use of immune checkpoint inhibitors in the management of NSCLC[39]. PD-L1 has been identified as a good predictive biomarker and NSCLC patients with high PD-L1 expression tend to respond better to the use of immune checkpoint inhibitors [40, 41]. After conducting a comprehensive analysis of seven RCTs, we have determined that pembrolizumab in combination with chemotherapy outperforms all other included therapeutic agents in terms of OS benefit. In fact, the findings of the Dafni et al. meta-analysis support the superiority of pembrolizumab, particularly in the management of NSCLC patients with a PD-L1 expression level of 50% or higher[42]. This is consistent with our findings, suggesting that the beneficial immunotherapy approach for NSCLC and NS-NSCLC with PD-L1 expression ≥ 50% does not differ significantly. Our comprehensive analysis of nine RCTs has revealed that tislelizumab in combination with chemotherapy and sintilimab in combination with chemotherapy offers superior PFS benefits compared to chemotherapy and all other included therapeutic agents. Interestingly, our analysis also revealed no significant difference in PFS between tisle-chemo and sinti-chemo. Furthermore, our findings indicate that PD-1 inhibitors in combination with chemotherapy are more efficacious than PD-L1 inhibitors in combination with chemotherapy. This observation can be attributed to the fact that PD-1 antibodies can block the binding of PD-1 to both PD-L1 and PD-L2, thus more fully inhibiting the occurrence of immune escape, leading to better treatment outcomes for patients with advanced cancers, including non-small cell lung cancer[43].
While our study provides valuable insights into the efficacy of chemotherapy and immune checkpoint inhibitors (ICI) in the management of cancer, there are still some limitations that should be considered. Firstly, the included trials used different methods to detect PD-L1 expression cut-offs, and in several trials, the investigators did not specify the method used to detect PD-L1 expression cut-offs. This inconsistency in measurement could lead to some unintentional misclassification, resulting in an underestimation or overestimation of the benefits of chemotherapy and ICI. For example, the SP142 method, which is used to measure PD-L1 expression in tumor cells, is less sensitive than other methods, potentially impacting the accuracy of our findings[44]. Secondly, four of the included studies reported reporting both OS and PFS, with the remaining eight included studies reporting only OS or PFS. As a result, it was impossible to conduct direct comparisons between many treatment modalities, leading to some limitations in our ability to draw definitive conclusions. Thirdly, it’s important to acknowledge that there is no universally accepted metric for assessing the efficacy of immune checkpoint inhibitor (ICI) treatments for lung cancer. While we used overall survival and progression-free survival as the primary endpoints in our study, it’s important to note that they do not capture the full range of treatment benefits that may be experienced by patients. Other important metrics, such as health-related quality of life, should also be considered when evaluating the efficacy of ICI treatments. It’s worth emphasizing that health-related quality of life is an especially valuable metric for patients undergoing ICI treatment for lung cancer. Beyond the clinical endpoints of overall survival and progression-free survival, patients often prioritize their physical and emotional well-being. As such, assessing treatment efficacy through patient-reported outcomes that capture the quality of life can provide a more comprehensive understanding of the benefits and limitations of ICI treatments in real-world settings. Fourth, we did not evaluate the safety of the ICIs due to the lack of safety data for PD-L1 expression ≥ 50% in advanced NS-NSCLC reported in the included studies. Lastly, it’s important to acknowledge that the results of our network meta-analysis (NMA) should be interpreted with caution due to the limited number of randomized controlled trials and participants included in our study. While our analysis provides valuable insights into the relative efficacy and safety of the various immune checkpoint inhibitor therapies for non-small cell lung cancer, it’s essential to note that further research is needed to confirm and expand upon our findings. Given the complexity and heterogeneity of lung cancer, it’s crucial to approach treatment selection on an individualized basis. In this regard, targeted histological staging and stratification of PD-L1 expression may be key factors to consider when selecting an appropriate immune checkpoint inhibitor therapy for a given patient. By tailoring treatment based on individual patient characteristics, we can optimize treatment outcomes and improve overall survival rates in this patient population.
In light of these considerations, we recommend that clinicians and researchers continue to explore the differences in efficacy and safety of immune checkpoint inhibitor therapies for non-small cell lung cancer through well-designed, rigorous studies that take into account the full range of patient factors and clinical outcomes. Only through such efforts can we achieve truly personalized and effective treatments for this devastating disease.
Conclusions
In conclusion, this NMA demonstrated that for NS-NSCLC with PD-L1 ≥ 50%, pembrolizumab plus chemotherapy, tislelizumab plus chemotherapy and sintilimab plus chemotherapy appear to be good treatment options. For this group of patients, ICI alone, ICI in combination with chemotherapy drugs, or a combination of two ICIs is more effective than chemotherapy drugs.
Data availability
All data generated or analysed during this study are included in this published article.
Abbreviations
- NSCLC:
-
Non-small Cell Lung Cancer
- NS-NSCLC:
-
Non-squamous Non-small Cell Lung Cancer
- PD-1:
-
Programmed Cell Death-1
- PD-L1:
-
Programmed Cell Death 1 Ligand 1
- ICIs:
-
Immune Checkpoint Inhibitors
- NMA:
-
Network Meta-analysis
- RCT:
-
Randomized Controlled Trials
- OS:
-
Overall Survival
- PFS:
-
Progression-free Survival
- CTLA-4:
-
Cytotoxic T Lymphocyte-Associated Antigen-4
- MCMC:
-
Markov chain Monte Carlo
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: a cancer journal for clinicians 2021, 71(1):7–33.
Langer CJ, Besse B, Gualberto A, Brambilla E, Soria JC. The evolving role of histology in the management of advanced non-small-cell lung cancer. J Clin oncology: official J Am Soc Clin Oncol. 2010;28(36):5311–20.
Carlisle JW, Steuer CE, Owonikoko TK, Saba NF. An update on the immune landscape in lung and head and neck cancers. Cancer J Clin. 2020;70(6):505–17.
Mok T, Yang JJ, Lam KC. Treating patients with EGFR-sensitizing mutations: first line or second line–is there a difference? J Clin oncology: official J Am Soc Clin Oncol. 2013;31(8):1081–8.
Cetin K, Ettinger DS, Hei YJ, O’Malley CD. Survival by histologic subtype in stage IV nonsmall cell lung cancer based on data from the Surveillance, Epidemiology and End results Program. Clin Epidemiol. 2011;3:139–48.
Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM classification for Lung Cancer. J Thorac oncology: official publication Int Association Study Lung Cancer. 2016;11(1):39–51.
Hudson K, Cross N, Jordan-Mahy N, Leyland R. The extrinsic and intrinsic roles of PD-L1 and its receptor PD-1: implications for immunotherapy treatment. Front Immunol 2020:2362.
Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1 (PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207–12.
Johdi NA, Sukor NF. Colorectal cancer immunotherapy: options and strategies. Front Immunol. 2020;11:1624.
Low JL, Walsh RJ, Ang Y, Chan G, Soo RA. The evolving immuno-oncology landscape in advanced lung cancer: first-line treatment of non-small cell lung cancer. Therapeutic Adv Med Oncol. 2019;11:1758835919870360.
Ackermann CJ, Reck M, Paz-Ares L, Barlesi F, Califano R. First-line immune checkpoint blockade for advanced non-small-cell lung cancer: travelling at the speed of light. Lung cancer (Amsterdam Netherlands). 2019;134:245–53.
Brody R, Zhang Y, Ballas M, Siddiqui MK, Gupta P, Barker C, Midha A, Walker J. PD-L1 expression in advanced NSCLC: insights into risk stratification and treatment selection from a systematic literature review. Lung cancer (Amsterdam Netherlands). 2017;112:200–15.
Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D’Amico TA, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J Natl Compr Cancer Network: JNCCN. 2021;19(3):254–66.
Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, Turk HM, Cicin I, Bentsion D, Gladkov O, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet (London England). 2021;397(10274):592–604.
Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, Longeras PD, Goldschmidt J Jr, Novello S, Orlandi F, et al. Atezolizumab Plus Chemotherapy for First-Line treatment of Nonsquamous NSCLC: results from the Randomized phase 3 IMpower132 trial. J Thorac oncology: official publication Int Association Study Lung Cancer. 2021;16(4):653–64.
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive non-small-cell Lung Cancer. N Engl J Med. 2016;375(19):1823–33.
Theelen W, Baas P. Pembrolizumab monotherapy for PD-L1 ≥ 50% non-small cell lung cancer, undisputed first choice? Annals of translational medicine. 2019;7(Suppl 3):140.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84.
Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med. 2017;12(1):103–11.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed). 2019;366:l4898.
Neupane B, Richer D, Bonner AJ, Kibret T, Beyene J. Network meta-analysis using R: a review of currently available automated packages. PLoS ONE. 2014;9(12):e115065.
Shim SR, Kim SJ, Lee J, Rücker G. Network meta-analysis: application and practice using R software. Epidemiol health. 2019;41:e2019013.
Lin L, Chu H, Hodges JS. Sensitivity to excluding treatments in Network Meta-analysis. Epidemiol (Cambridge Mass). 2016;27(4):562–9.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003;327(7414):557–60.
Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, Wang Z, Shu Y, Shi J, Hu Y, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. The Lancet Respiratory medicine. 2021;9(3):305–14.
West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924–37.
Nishio M, Barlesi F, Ball S, Bordoni R, Cobo M, Dubray-Longeras P, Goldschmidt J, Novello S, Orlandi F, Sanborn R. 375O final efficacy results from IMpower132: first-line atezolizumab + chemotherapy in patients with stage IV non-squamous NSCLC. Ann Oncol. 2020;31:1386–S1387.
Socinski MA, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, et al. IMpower150 final overall survival analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in First-Line Metastatic Nonsquamous NSCLC. J Thorac oncology: official publication Int Association Study Lung Cancer. 2021;16(11):1909–24.
Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, Hui R, Hochmair MJ, Clingan P, Powell SF, et al. Updated analysis from KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for previously untreated metastatic nonsquamous non-small-cell Lung Cancer. J Clin oncology: official J Am Soc Clin Oncol. 2020;38(14):1505–17.
Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang S, Chen G, Mei X, Yang Z, Ma R, et al. Efficacy and safety of Sintilimab Plus Pemetrexed and Platinum as First-Line treatment for locally advanced or metastatic nonsquamous NSCLC: a Randomized, Double-Blind, phase 3 study (Oncology pRogram by InnovENT anti-PD-1-11). J Thorac oncology: official publication Int Association Study Lung Cancer. 2020;15(10):1636–46.
Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X, Ma Z, Li X, Zhuang W, Liu Y, et al. Tislelizumab Plus Chemotherapy as First-Line treatment for locally advanced or metastatic nonsquamous NSCLC (RATIONALE 304): a randomized phase 3 trial. J Thorac oncology: official publication Int Association Study Lung Cancer. 2021;16(9):1512–22.
Sugawara S, Lee JS, Kang JH, Kim HR, Inui N, Hida T, Lee KH, Yoshida T, Tanaka H, Yang CT, et al. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Annals of oncology: official journal of the European Society for Medical Oncology. 2021;32(9):1137–47.
Boyer M, Şendur MAN, Rodríguez-Abreu D, Park K, Lee DH, Çiçin I, Yumuk PF, Orlandi FJ, Leal TA, Molinier O, et al. Pembrolizumab Plus Ipilimumab or Placebo for metastatic non-small-cell lung Cancer with PD-L1 tumor proportion score ≥ 50%: Randomized, double-blind phase III KEYNOTE-598 study. J Clin oncology: official J Am Soc Clin Oncol. 2021;39(21):2327–38.
Socinski MA, Obasaju C, Gandara D, Hirsch FR, Bonomi P, Bunn PA Jr, Kim ES, Langer CJ, Natale RB, Novello S. Current and emergent therapy options for advanced squamous cell lung cancer. J Thorac Oncol. 2018;13(2):165–83.
Wang C, Yu Q, Song T, Wang Z, Song L, Yang Y, Shao J, Li J, Ni Y, Chao N. The heterogeneous immune landscape between lung adenocarcinoma and squamous carcinoma revealed by single-cell RNA sequencing. Signal Transduct Target Therapy. 2022;7(1):289.
Bai X, Wu D-H, Ma S-C, Wang J, Tang X-R, Kang S, Fu QJ, Cao C-H, Luo H-S, Chen Y-H. Development and validation of a genomic mutation signature to predict response to PD-1 inhibitors in non-squamous NSCLC: a multicohort study. J Immunother Cancer 2020, 8(1).
Pennell NA, Arcila ME, Gandara DR, West H. Biomarker testing for patients with advanced non–small cell lung cancer: real-world issues and tough choices. Am Soc Clin Oncol Educational Book. 2019;39:531–42.
Xia L, Liu Y, Wang Y. PD-1/PD-L1 blockade therapy in Advanced Non-Small-Cell Lung Cancer: current status and future directions. Oncologist. 2019;24(Suppl 1):31–s41.
Voong KR, Feliciano J, Becker D, Levy B. Beyond PD-L1 testing-emerging biomarkers for immunotherapy in non-small cell lung cancer. Annals of translational medicine. 2017;5(18):376.
Herbst R, Jassem J, Abogunrin S, James D, McCool R, Belleli R, Giaccone G, De Marinis F. A network meta-analysis of cancer immunotherapies versus chemotherapy for first-line treatment of patients with non-small cell lung cancer and high programmed death-ligand 1 expression. Front Oncol. 2021;11:676732.
Dafni U, Tsourti Z, Vervita K, Peters S. Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first-line treatment for advanced non-small cell lung cancer. A systematic review and network meta-analysis. Lung cancer (Amsterdam Netherlands). 2019;134:127–40.
Chen L, Han X. Anti–PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Investig. 2015;125(9):3384–91.
Tsao MS, Kerr KM, Kockx M, Beasley M-B, Borczuk AC, Botling J, Bubendorf L, Chirieac L, Chen G, Chou T-Y. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol. 2018;13(9):1302–11.
Acknowledgements
I would like to thank my supervisor, Dr. Junxian Yu, for his guidance through each stage of the process.
Funding
None.
Author information
Authors and Affiliations
Contributions
Wei Chen (Co-first author): Conducted literature searches and screened articles for inclusion. Performed data extraction and quality assessment of studies. Analyzed and interpreted the data. Drafted and revised the manuscript. Jiayi Chen (Co-first author): Conducted literature searches and screened articles for inclusion. Performed data extraction and quality assessment of studies. Analyzed and interpreted the data. Drafted and revised the manuscript. Lin Zhang: Advised on study design and data analysis. Reviewed and provided feedback on manuscript drafts. Sheng Cheng: Contributed to the interpretation of the data. Reviewed and provided feedback on manuscript drafts. Juxian Yu (Corresponding author): Conceptualized the study and secured funding. Provided guidance on study design and data analysis. Facilitated communication among the authors. Ensured adherence to ethical standards and manuscript guidelines. Reviewed and provided feedback on manuscript drafts. Submitted the manuscript for publication. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, W., Chen, J., Zhang, L. et al. Network meta-analysis of first-line immune checkpoint inhibitor therapy in advanced non-squamous non-small cell lung cancer patients with PD-L1 expression ≥ 50%. BMC Cancer 23, 791 (2023). https://doi.org/10.1186/s12885-023-11285-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11285-4