Abstract
Background
Lung cancer is the third most common type of cancer in the UK. Treatment outcomes are poor and UK deaths from lung cancer are higher than any other cancer. Prehabilitation has shown to be an important means of preparing patients both physically and psychologically for cancer treatment. However, little is understood about the context and mechanisms of prehabilitation that can impact physiological and psychological wellbeing.
Our aim was to review and summarise primary research on prehabilitation in the lung cancer pathway using a realist approach.
Methods
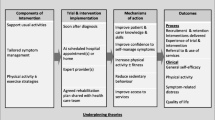
A scoping review of empirical primary research was conducted. Five online medical databases from 2016 – February 2023 were searched. All articles reporting on prehabilitation in lung cancer were included in the review. For this review, prehabilitation was defined as either a uni-modal or multi-modal intervention including exercise, nutrition and/or psychosocial support within a home, community or hospital based setting. A realist framework of context, mechanism and outcome was used to assist with the interpretation of findings.
Results
In total, 31 studies were included in the review, of which, three were published study protocols. Over 95% of studies featured an exercise component as part of a prehabilitation programme. Twenty-six of the studies had a surgical focus. Only two studies reported using theory to underpin the design of this complex intervention. There was large heterogeneity across all studies as well as a lack of clinical trials to provide definitive evidence on the programme design, setting, type of intervention, patient criteria, delivery, duration and outcome measures used.
Conclusion
A standardised prehabilitation programme for lung cancer patients does not yet exist. Future lung cancer prehabilitation programmes should take into account patient led values, needs, goals, support structures and beliefs, as these factors can affect the delivery and engagement of interventions. Future research should consider using a conceptual framework to conceptualise the living with and beyond cancer experience to help shape and inform personalised prehabilitation services.
Similar content being viewed by others
Background
Lung cancer is the third most common type of cancer in the UK [1]. Treatment outcomes are poor and UK deaths from lung cancer are higher than any other cancer [2]. Cancer incidence and mortality projections within the UK predict that although mortality rates are likely to increase over the next 10 years, there will also be more people living with and beyond cancer [3].
Prehabilitation has shown to be an important means of preparing patients both physically and psychologically for cancer treatment by mitigating deconditioning associated with cancer treatments between the time of cancer diagnosis and the beginning of acute treatment’ [4, 5].
Prehabilitation within cancer surgery has shown to reduce morbidity and improve health outcomes. For example, an improvement in functional capacity [6,7,8,9,10,11,12,13,14] and health reported quality of life (HRQOL) [9, 12, 15, 16] as well as a reduction in post-operative complications [9, 15] and length of hospital stay [9, 13, 15, 17].
Few prehabilitation pathways exist for people who do not have surgery, despite 50–60% of people with cancer in the UK being treated with primary, neo-adjuvant or palliative chemotherapy and/or radiotherapy treatment [18]. Along with the rise in the use of targeted agents and immunotherapy, there is potential to optimise quality of life within the lung cancer population. However, little is understood about the context and mechanisms of prehabilitation that can impact physiological and psychological wellbeing.
Prehabilitation is a complex intervention and it is widely understood that the success of a complex intervention depends on the theory underpinning its design [19], which helps to explain the mechanisms underlying an individual’s behaviour, based on what works for them and their circumstances [20,21,22,23]. Lung cancer treatment regimes can be prolonged and people may experience a range of toxicities, which could limit their ability to engage in prehabilitation interventions. Prehabilitation programmes should therefore be tailored to the individual to optimise symptom control, treatment tolerance and independence [24, 25].
The aim of this scoping review was to review and summarise primary research on prehabilitation in the lung cancer pathway using a realist approach. Realist approaches focus on the contexts and mechanisms that lead to particular outcomes. This approach enables a detailed exploration of factors likely to influence the success of a complex intervention, such as prehabilitation, thereby helping explain how and why interventions may or may not work [26, 27].
Methods
Scoping reviews are particularly relevant to examine the extent, range and nature of evidence on a certain topic and to identify concepts, theories and knowledge gaps from a heterogeneous body of research [28].
The PRISMA extension for scoping reviews was used for the conduct and reporting of this scoping review [28]. This enabled an examination of the extent, range and nature of the evidence on prehabilitation and lung cancer.
Following the Joanna Briggs Institute (JBI) framework [29], this scoping review addressed the following: 1. Define the review questions 2. Determine the inclusion criteria 3. Search strategy 4. Evidence screening and selection 5. Data extraction 6. Data analysis 7. Presentation of the results.
1. Define the review questions.
Prehabilitation is a complex intervention and it is important to understand what has worked or is perceived to work based on measured or predicted outcomes within the lung cancer pathway. Pre-surgical prehabilitation is often a linear process from baseline to a defined, one-off target (surgery). However, this is not the case for patients receiving oncological treatment where prehabilitation may be delivered immediately prior to, during ± after each treatment session or cycle. Our research questions were:
-
A)
How does the literature within the field of lung cancer describe the structure of prehabilitation?
-
B)
How does the literature within the field of lung cancer describe the personalisation of prehabilitation interventions?
-
C)
What are the actual outcomes for lung cancer patients participating in a prehabilitation programme?
2. Determine the inclusion criteria.
All studies included in this review had to involve lung cancer patients who received a form of prehabilitation within a home, community or hospital based setting. For this review, prehabilitation was defined as either a uni-modal or multi-modal intervention or programme including either exercise, nutrition and/or psychological wellbeing. All study designs were included in this scoping review providing that they met the inclusion criteria as outlined in Table 1. Protocols for ongoing or upcoming lung cancer prehabilitation studies were included in the review, as the authors felt these provided key insights into the delivery and proposed outcomes for prehabilitation within this field. All articles available in English were included.
3. Search strategy.
The literature search was undertaken by a research librarian using pre-defined search terms between the period of 2016 and 03 February 2023. This time period was chosen due to a rapid emergence of the use of prehabilitation within cancer care to improve health outcomes and reduce healthcare costs since the publication of a key research paper by Silver in 2015 [30]. This was followed by the Macmillan prehabilitation evidence and insight review [31] and subsequent publication of the Macmillan prehabilitation guidance [32]. A total of five databases were searched incorporating medical, nursing, allied health and psychological literature relevant to prehabilitation and lung cancer: Cumulative Index to Nursing and Allied Health Literature (CINAHL), Embase, Emcare, Medical Literature Analysis and Retrieval System Online (MEDLINE) and Psychological Information Database (PsycINFO). The major search terms ‘lung’, ‘cancer’ and ‘prehabilitation’ were used.
4. Evidence screening and selection.
All duplications were removed using the Zotero deduplication function. All retrieved abstracts for possible inclusion were independently screened by the first and last author. There was a consensus between both authors and thus, a third reviewer was not required.
5&6. Data extraction and analysis.
All articles reviewed for inclusion were obtained in full text. The JBI reviewers manual for evidence synthesis was used to create a synthesis matrix for data extraction [33]. Data extraction included: study title, year of publication, country, study design, sample size, type of participants, study aim, type of prehabilitation intervention used, key findings, strengths and limitations.
Results
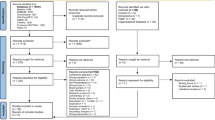
A total of 31 articles were included in this scoping review; see Fig. 1. In some studies, pulmonary rehabilitation was described as prehabilitation. After discussion with authors conducting the review, it was agreed that pulmonary rehabilitation is a separate intervention, acknowledging that it may complement prehabilitation in the long-term. Therefore, studies that focused on pulmonary rehabilitation were excluded from this review.
The results of this review are presented in a narrative form in Tables 2 and 3.
Overview of the studies
The 31 studies included in this review comprised of fourteen randomised controlled trials [34,35,36, 38, 40,41,42,43, 45,46,47,48,49,50], four feasibility studies [57,58,59,60], three registered protocols [37, 39, 44], two cohort studies [51, 52], two prospective studies [53, 54], two retrospective studies [55, 56], one qualitative study [61], one cross sectional survey [62], one proof of concept study [63] and one quality improvement study [64].
The largest number of studies originated from Canada (n = 6). The origins of the other studies included France (n = 4), United Kingdom (n = 4), China (n = 3), Switzerland (n = 3), United States of America (n = 3), Australia (n = 2), Denmark (n = 2), Spain (n = 2), Ireland (n = 1) and Turkey (n = 1).
Sample sizes ranged from 15 to 377 lung cancer patients with a mean age range of 46 – 72 years of age.
How does the literature within the field of lung cancer describe the structure of prehabilitation?
All studies except one [50] included in this review featured an exercise component as part of a prehabilitation programme. Sixteen studies were uni-modal, focusing solely on exercise prehabilitation [38, 40,41,42, 45, 47,48,49, 52, 54, 58,59,60, 62, 63]. Ten out of the 31 studies described multimodal prehabilitation interventions using a tri-modal approach, incorporating nutrition, exercise and psychological wellbeing [35, 36, 39, 43, 53, 55,56,57, 61, 64]. Other studies incorporated an exercise and psychological component (n = 3) [34, 44, 46] or an exercise and nutrition component (n = 1) [37]. A single study focused solely on nutrition prehabilitation [50], but there were no uni-modal psychological wellbeing prehabilitation intervention studies. See Table 3.
All prehabilitation interventions varied in terms of programme setting, type of intervention, patient criteria, intervention delivery, duration of prehabilitation and measured outcomes. Nine out of the 31 studies provided a comprehensive description of all aspects of the prehabilitation programme [36, 40, 44, 45, 47, 52, 59, 60, 63]. In relation to programme setting, most interventions were delivered in a hospital setting (n = 9) [37, 47,48,49, 51, 52, 58, 60, 64] or provided flexibility between a hospital setting and remote supervision (n = 9) [36, 39, 40, 44, 45, 54, 56, 61, 62]. Six of the 31 studies used remote supervision [35, 43, 55, 57, 59, 63]. In the remaining studies, the programme setting was unclear (n = 7) [34, 38, 41, 42, 46, 50, 53].
Exercise
All prehabilitation programmes that included an exercise intervention used a baseline physical fitness assessment to develop an individualised exercise prescription. The type of baseline physical fitness assessment used was variable throughout all of the studies, ranging from cardio-pulmonary exercise testing to more general elements such as muscle strength and activity questionnaires. More than 50% of the studies described the exercise intervention as a combination of both aerobic and resistance exercise to improve cardiorespiratory fitness and muscle strength. Other exercise interventions included High Intensity Interval Training (HIIT) and medical Qigong. Breathing exercises such as Respiratory Muscle Endurance Training (RMET) were also described under the term exercise, alongside aerobic exercise interventions.
Nutrition
Tailored dietetic advice was provided in all prehabilitation programmes which included a nutritional component (n = 12) [35,36,37, 39, 43, 50, 53, 55,56,57, 61, 64]. In eight studies, this was based on an individualised assessment, which was undertaken by a registered dietitian [35,36,37, 39, 53, 55, 56, 64]. Only three studies [35, 55, 64] stated using validated tools such as the Patient Generated Subjective Global Assessment (PG-SGA). However, the use of nutritional screening tools to assess malnutrition risk was not frequently described. The majority of studies (n = 8) [35,36,37, 39, 43, 53, 55, 56] focused on increasing dietary protein, often recommending the use of protein supplements. Two interventions focused on the use of a fish oil supplement [36, 50].
Psychological wellbeing
Only three studies described the validated tools that were used to establish baseline psychological wellbeing. These included the Hospital Anxiety and Depression Score (HADS) [53, 55] and the Short Form (SF) 36 questionnaire [47]. There was large variation in the description of the psychological interventions used. Some studies used the terms ‘support’ and ‘coping strategies’, whereas in other studies, more specific techniques such as relaxation, imagery, visualisation, cognitive behavioural therapy and motivational interviewing were described.
How does the literature within the field of lung cancer describe the personalisation of prehabilitation interventions?
A number of studies described tailoring the exercise, nutrition and/or psychological wellbeing prehabilitation intervention to an individual (n = 28), but there was large variation across all studies in how this was fully conceptualised and achieved. The personalisation of the prehabilitation intervention mostly referred to a starting point variation along a continuum, e.g. intensity of exercise in relation to baseline fitness. This was typically considered at a single point, usually at baseline and not reviewed.
Only two studies [44, 46] in this review reported using theory to underpin the design of this complex intervention. The two theories that were described were the theory of planned behaviour and social cognitive theory, respectively.
What are the actual outcomes for lung cancer patients participating in a prehabilitation programme?
Twenty-six out of the 31 studies included in this review focused on lung cancer surgery. The remaining studies included chemotherapy (n = 2) [38, 45], radiotherapy (n = 1) [60], neoadjuvant treatment (n = 1) [55] or had a specific focus on quality of life (n = 1) [46] for those with advanced lung cancer.
There was a wide variety of outcomes reported amongst all interventional studies. The majority of reported actual outcomes were positive. These included improvements in functional capacity (n = 16) [34, 40,41,42,43, 45, 47, 49, 51, 53, 55,56,57,58, 63, 64], high adherence to the intervention (n = 5) [36, 42, 57, 59, 60], improvements in psychological wellbeing (n = 3) [38, 53, 55], reductions in post-operative complications (n = 5) [40, 41, 50, 52, 53], improvements in muscle strength (n = 3) [38, 45, 47], no adverse events (n = 5) [34, 57, 58, 60, 64], qualitative outcomes including increased perceived physical and psychological health benefits (n = 2) [61, 62], reductions in length of hospital stay (n = 2) [41, 64] and improvements in quality of life (n = 1) [58]. Conversely, five studies reported no improvement in functional capacity [35, 36, 38, 46, 48]. Furthermore, some studies described no improvement in quality of life (n = 4) [36, 41, 45, 46], length of hospital stay (n = 5) [35, 43, 51, 52, 54], post-operative complications (n = 5) [43, 49, 51, 54, 64], survival (n = 3) [43, 46, 48] or psychological wellbeing (n = 2) [43, 45]. Only one study [59] reported an unintended outcome of their intervention which was related to an adverse reaction to the device used to monitor heart rate.
The intended outcomes described within the three protocol papers included in this review [37, 39, 44] are similar to the actual outcomes reported from the interventional studies as shown above. These include improvements in functional capacity (n = 2) [37, 44], improvements in quality of life (n = 2) [37, 44], reductions in hospital length of stay (n = 2) [39, 44], reductions in post-operative complications (n = 3) [37, 39, 44] and qualitative outcomes including self-efficacy (n = 1) [44]. All three randomised controlled trials for which protocols have been published [37, 39, 44] include health economics in their planned outcomes. These include treatment related costs as well as costs from a healthcare professional perspective. This will provide valuable information on the economic implication of the adoption of prehabilitation programmes for patients with lung cancer.
Specific to the oncological lung cancer treatment pathway, a multi-modal prehabilitation programme delivered remotely during neoadjuvant treatment led to a significant improvement in functional capacity and psychological wellbeing [55]. In comparison, a uni-modal exercise prehabilitation intervention for 110 lung cancer patients with advanced inoperable lung cancer undergoing chemotherapy showed no significant difference in functional capacity. However, there were significant improvements in strength and psychological wellbeing [38]. Nevertheless, these results should be interpreted with caution owing to high attrition, poor adherence and anticipated attenuation of decline amongst this cohort.
Discussion
To our knowledge, this is the first scoping review that aims to summarise the evidence on prehabilitation in the lung cancer pathway using a realist approach.
Our review provides a summary of several interventional studies and three ongoing randomised controlled trials for which protocols have been published.
The majority of studies in this review had a surgical focus and demonstrated that prehabilitation before lung cancer surgery is feasible and is associated with physiological and psychological benefits. Our findings reveal that there are only a few studies involving lung cancer patients undergoing oncological treatment, despite 70–80% of people with lung cancer receiving non-surgical treatment within the UK [65].
Our analysis of the contexts, mechanisms and outcomes for prehabilitation provide useful insights into the factors that need to be considered in the design and implementation of prehabilitation for patients with lung cancer.
Prehabilitation is a complex intervention. It is widely understood that the success of a complex intervention depends on the theory underpinning its design [19], which helps to explain the mechanisms underlying an individual’s behaviour, based on what works for them and their circumstances [22]. However, only two studies in this review described using theory to underpin the design of this complex intervention [44, 46]. Similarly, there are only two completed studies which used a qualitative approach [61, 62], with only one evaluating the acceptability of their interventions, despite this being an important consideration for complex interventions [61, 66]. Although the qualitative literature in this field sheds some light on some of the factors which might influence engagement with prehabilitation, it does not fully illustrate the complexity of delivering prehabilitation.
Using a realist lens, this review has identified the importance of a personalised approach to prehabilitation. Whilst the personalisation of the prehabilitation intervention was often stated by the studies reviewed, and generally viewed as a positive factor, how it was fully conceptualised and achieved was less clear. The personalisation of the intervention mostly referred to a starting point variation along a continuum, e.g. intensity of exercise in relation to baseline fitness. No studies considered personalisation to patient-led values, needs, goals, support structures and beliefs. This is a considerable gap, given that initiation and adherence to any intervention is determined by behavioural, psychological, physiological, environmental and social factors [20,21,22,23], especially when research findings with well-intentioned patients need to be translated to clinical practice with the full range of real-world complexities and comorbidities [23].
Personalisation was mostly considered at a single point, typically at baseline. This is typical in pre-surgical prehabilitation, where it is a relatively brief and linear process from baseline to a defined, one-off target (surgery). However, prehabilitation during oncological treatment is a prolonged and undulating 'marathon', during which the patients other roles, values and needs have to be considered with learning and adaptation along the journey. An adaptable model and practice of ongoing, collaborative personalisation therefore needs to be explicitly defined and implemented. To address this, future research could utilise the Adversity, Restoration and Compatibility (ARC) framework [21] to help underpin a personalised and collaborative prehabilitation programme. The ARC framework provides a synthesised view of how people conceptualise the personal experience of living with and beyond cancer, namely as an ongoing process of learning about their evolving challenge (Adversity), learning how to cope effectively (Restoration) and adapting one's identity (Compatibility), in parallel. These broad themes, derived from qualitative synthesis of over 70 primary studies of patient narratives, are consistent with psychological adjustment theory [67] and may provide a structure for personalising prehabilitation that is patient-centred rather than a purely logistical or psychometric approach. This could be key to empower patients to maintain progress through the longer, more variable context of peri-oncological prehabilitation. This theory-led approach is further supported by Faithfull et al., 2019 [5] and The Medical Research Council (MRC) framework for developing and evaluating complex interventions [68] which suggests that future studies should use a conceptual framework to help guide intervention design and thereby maximise outcomes.
We acknowledge the limitations of this review. Firstly, we recognise that some studies may have been missed by database searching or were published after the search date. Secondly, the extrapolation of findings is limited owing to context dependency e.g. it may be difficult to extrapolate the results from a pre-surgical setting to those with a poor prognosis. Thirdly, only a few studies included in this scoping review provided a comprehensive description of all aspects of the prehabilitation programme, therefore the descriptions of the interventions are limited. To counterbalance this, a realist framework of context, mechanism and outcome has been used for reporting. Furthermore, our analysis of the mechanisms and outcomes for prehabilitation provide insight into the role of prehabilitation within the lung cancer pathway.
The coronavirus pandemic has accelerated the remote delivery of prehabilitation interventions. Completed studies suggest that home-based multimodal prehabilitation is feasible and leads to improvements in a range of outcomes [43, 55, 57, 59, 63]. However, there is limited qualitative data in this field to determine whether remote delivery of prehabilitation interventions is more or less favourable than face to face or a hybrid approach.
There is potential for digital interventions within this field. Two completed studies in this review used an app for the delivery of their prehabilitation interventions [59, 63], with one study demonstrating high recruitment and attrition rates [59] and the other showing a minimally clinically meaningful improvement in aerobic capacity prior to surgery as a result of participation in the intervention [63]. However, it is important to note that in the latter study, approximately 50% did not achieve an improvement in aerobic capacity with the majority of patients falling short of achieving the prescribed weekly exercise target. Technology access, skillset and confidence strongly need to be considered prior to implementation of delivery of prehabilitation interventions [69]. Furthermore, the cost-effectiveness of a technology supported multimodal prehabilitation programme needs to be evaluated. The study by Barberan-Garcia et al. [39] aims to address this, as detailed in their published study protocol.
Whilst there will be emerging evidence from ongoing randomised controlled trials, the heterogeneity in study designs, programme setting, type of intervention, patient criteria, intervention delivery, duration of prehabilitation and measured outcomes is significant. There are no trials which have the same set of primary and secondary outcomes. The lack of standardisation across interventions and outcome measures makes it difficult to conclude benefit across the whole lung cancer pathway. The inability to draw significant improvement benefit of prehabilitation due to the heterogeneity of studies has also been seen in systematic reviews in breast cancer [70], pancreatic cancer [71] and hepatobiliary cancers [72].
Conclusion
This scoping review demonstrates that there is evidence for providing prehabilitation for patients with lung cancer, particularly in the surgical domain. However, there is a lack of clinical trials which provide definitive evidence on the programme design, setting, type of intervention, patient criteria, delivery and duration. This therefore makes it difficult to conclude significant improvement benefit.
The design and implementation of future lung cancer prehabilitation programmes should take into account factors such as patient led values, needs, goals, support structures and beliefs which can affect the delivery and engagement of interventions. The findings of this review provide important insights into these issues.
Furthermore, future research should consider the use of a conceptual framework such as ARC [21] to conceptualise the living with and beyond cancer experience to help shape and inform personalised prehabilitation services. This will enable personalised care to be given from the outset and help support identification of the ideal prehabilitation model and delivery options to optimise both health and economic outcomes. This will enable patient empowerment and engagement towards self-managed behaviours and thus, optimise long-term health.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
References
Cancer Research UK. Cancer Research UK. Lung cancer incidence statistics. 2016–2018. 2016 2018. Cited 2023 Mar 17. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer/incidence#heading-Zero.
Cancer Research UK. Cancer Research UK. Lung cancer mortality statistics 2017–2019. 2017 2019. Cited 2022 Oct 17. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer#heading-Five.
Smittenaar CR, Petersen KA, Stewart K, Moitt N. Cancer incidence and mortality projections in the UK until 2035. Br J Cancer. 2016;115(9):1147–55.
Silver JK, Baima J. Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil. 2013;92(8):715–27.
Faithfull S, Turner L, Poole K, Joy M, Manders R, Weprin J, et al. Prehabilitation for adults diagnosed with cancer: A systematic review of long‐term physical function, nutrition and patient‐reported outcomes. Eur J Cancer Care (Engl). 2019;28(4). Cited 2021 Oct 27. Available from: https://onlinelibrary.wiley.com/doi/10.1111/ecc.13023.
Boereboom C, Doleman B, Lund JN, Williams JP. Systematic review of pre-operative exercise in colorectal cancer patients. Tech Coloproctology. 2016;20(2):81–9.
Carli F, Gillis C, Scheede-Bergdahl C. Promoting a culture of prehabilitation for the surgical cancer patient. Acta Oncol. 2017;56(2):128–33.
Barberan-Garcia A, Ubré M, Roca J, Lacy AM, Burgos F, Risco R, et al. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg. 2018;267(1):50–6.
Piraux E, Caty G, Reychler G. Effects of preoperative combined aerobic and resistance exercise training in cancer patients undergoing tumour resection surgery: a systematic review of randomised trials. Surg Oncol. 2018;27(3):584–94.
Doganay E, Moorthy K. Prehabilitation for esophagectomy. J Thorac Dis. 2019;11(S5):S632–8.
van Rooijen SJ, Molenaar CJL, Schep G, van Lieshout RHMA, Beijer S, Dubbers R, et al. Making patients fit for surgery: introducing a four pillar multimodal prehabilitation program in colorectal cancer. Am J Phys Med Rehabil. 2019;98(10):888–96.
Fulop A, Lakatos L, Susztak N, Szijarto A, Banky B. The effect of trimodal prehabilitation on the physical and psychological health of patients undergoing colorectal surgery: a randomised clinical trial. Anaesthesia. 2021;76(1):82–90.
Waterland JL, Chahal R, Ismail H, Sinton C, Riedel B, Francis JJ, et al. Implementing a telehealth prehabilitation education session for patients preparing for major cancer surgery. BMC Health Serv Res. 2021;21(1):443.
Bradley P, Merchant Z, Rowlinson-Groves K, Taylor M, Moore J, Evison M. Feasibility and outcomes of a real-world regional lung cancer prehabilitation programme in the UK. Br J Anaesth. 2023;130(1):e47-55.
Treanor C, Kyaw T, Donnelly M. An international review and meta-analysis of prehabilitation compared to usual care for cancer patients. J Cancer Surviv. 2018;12(1):64–73.
Chmelo J, Navidi M, Sinclair RC, Greystoke A, Phillips AW. 522 Can prehabilitation improve health-related quality of life of patients during Neoadjuvant chemotherapy for Esophagogastric adenocarcinoma? Dis Esophagus. 2022;35(Supplement_2):doac051.522.
Gillis C, Buhler K, Bresee L, Carli F, Gramlich L, Culos-Reed N, et al. Effects of nutritional prehabilitation, with and without exercise, on outcomes of patients who undergo colorectal surgery: a systematic review and meta-analysis. Gastroenterology. 2018;155(2):391-410.e4.
Cancer Research UK. Cancer Research UK. Cancer treatment statistics 2013–2016. 2013 2016. Cited 2022 Oct 3. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/treatment.
Dhiman A, Ray MD. Enhanced recovery after gynecological/oncological surgeries: current status in India. Indian J Gynecol Oncol. 2020;18(4):127.
Ferreira V, Agnihotram RV, Bergdahl A, van Rooijen SJ, Awasthi R, Carli F, et al. Maximizing patient adherence to prehabilitation: what do the patients say? Support Care Cancer. 2018;26(8):2717–23.
Le Boutillier C, Archer S, Barry C, King A, Mansfield L, Urch C. Conceptual framework for living with and beyond cancer: A systematic review and narrative synthesis. Psychooncology. 2019;28(5):948–59.
Bhandoria G, Solanki SL, Bhavsar M, Balakrishnan K, Bapuji C, Bhorkar N, et al. Enhanced recovery after surgery (ERAS) in cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC): a cross-sectional survey. Pleura Peritoneum. 2021;6(3):99–111.
Grimmett C, Bradbury K, Dalton SO, Fecher-Jones I, Hoedjes M, Varkonyi-Sepp J, et al. The Role of behavioral science in personalized multimodal prehabilitation in cancer. Front Psychol. 2021;12:261.
Bayly J, Edwards BM, Peat N, Warwick G, Hennig IM, Arora A, et al. Developing an integrated rehabilitation model for thoracic cancer services: views of patients, informal carers and clinicians. Pilot Feasibility Stud. 2018;4(1):160.
Bayly J, Fettes L, Douglas E, Teixiera MJ, Peat N, Tunnard I, et al. Short-term integrated rehabilitation for people with newly diagnosed thoracic cancer: a multi-centre randomized controlled feasibility trial. Clin Rehabil. 2020;34(2):205–19.
Rycroft-Malone J, McCormack B, Hutchinson AM, DeCorby K, Bucknall TK, Kent B, et al. Realist synthesis: illustrating the method for implementation research. Implement Sci. 2012;7(1):33.
Wong G, Greenhalgh T, Westhorp G, Buckingham J, Pawson R. RAMESES publication standards: realist syntheses. BMC Med. 2013;11(1):21.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467–73.
Peters MDJ, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. 2020;18(10):2119–26.
Silver J. Cancer prehabilitation and its role in improving health outcomes and reducing health care costs. Semin Oncol Nurs. 2015;31(1):13–30.
Macmillan Cancer Support. Prehabilitation evidence and insight review . Macmillan Cancer Support; 2017. Cited 2022 May 3. Available from: https://www.macmillan.org.uk/_images/prehabilitation-evidence-and-insight-review_tcm9-335025.pdf.
Macmillan Cancer Support, NIHR Cancer and Nutrition Collaboration, Royal College of Anaesthetists. Principles and guidance for prehabilitation within the management and support of people with cancer. Macmillan Cancer Support; 2019. Cited 2021 Oct 27. Available from: https://www.macmillan.org.uk/healthcare-professionals/news-and-resources/guides/principles-and-guidance-for-prehabilitation.
Peters M, Godfrey C, McInerney P, Munn Z, Trico A, Khalil H. Chapter 11: Scoping Reviews. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. JBI; 2020. Cited 2022 Sep 28. Available from: https://wiki.jbi.global/display/MANUAL/Chapter+11%3A+Scoping+reviews.
Gravier FE, Smondack P, Boujibar F, Prieur G, Medrinal C, Combret Y, et al. Prehabilitation sessions can be provided more frequently in a shortened regimen with similar or better efficacy in people with non-small cell lung cancer: a randomised trial. J Physiother. 2022;68(1):43–50.
Ferreira V, Minnella EM, Awasthi R, Gamsa A, Ferri L, Mulder D, et al. Multimodal prehabilitation for lung cancer surgery: a randomized controlled trial. Ann Thorac Surg. 2021;112(5):1600–8.
Ferreira V, Lawson C, Carli F, Scheede-Bergdahl C, Chevalier S. Feasibility of a novel mixed-nutrient supplement in a multimodal prehabilitation intervention for lung cancer patients awaiting surgery: A randomized controlled pilot trial. Int J Surg. 2021;93:106079.
Sheill G, Guinan E, O’Neill L, Normand C, Doyle SL, Moore S, et al. Preoperative exercise to improve fitness in patients undergoing complex surgery for cancer of the lung or oesophagus (PRE-HIIT): protocol for a randomized controlled trial. BMC Cancer. 2020;20(1):321.
Quist M, Langer SW, Lillelund C, Winther L, Laursen JH, Christensen KB, et al. Effects of an exercise intervention for patients with advanced inoperable lung cancer undergoing chemotherapy: a randomized clinical trial. Lung Cancer. 2020;145:76–82.
Barberan-Garcia A, Navarro-Ripoll R, Sánchez-Lorente D, Moisés-Lafuente J, Boada M, Messaggi-Sartor M, et al. Cost-effectiveness of a technology-supported multimodal prehabilitation program in moderate-to-high risk patients undergoing lung cancer resection: randomized controlled trial protocol. BMC Health Serv Res. 2020;20(1):207.
Laurent H, Aubreton S, Galvaing G, Pereira B, Merle P, Richard R, et al. Preoperative respiratory muscle endurance training improves ventilatory capacity and prevents pulmonary postoperative complications after lung surgery. Eur J Phys Rehabil Med. 2020;56(1). Cited 2022 Oct 17. Available from: https://www.minervamedica.it/index2.php?show=R33Y2020N01A0073.
Lai Y, Wang X, Zhou K, Su J, Che G. Impact of one-week preoperative physical training on clinical outcomes of surgical lung cancer patients with limited lung function: a randomized trial. Ann Transl Med. 2019;7(20):544–544.
Bhatia C, Kayser B. Preoperative high-intensity interval training is effective and safe in deconditioned patients with lung cancer: a randomized clinical trial. J Rehabil Med. 2019;51(9):712–8.
Liu Z, Qiu T, Pei L, Zhang Y, Xu L, Cui Y, et al. Two-week multimodal prehabilitation program improves perioperative functional capability in patients undergoing thoracoscopic lobectomy for lung cancer: a randomized controlled trial. Anesth Analg. 2020;131(3):840–9.
Ulrich CM, Himbert C, Boucher K, Wetter DW, Hess R, Kim J, et al. Precision-Exercise-Prescription in patients with lung cancer undergoing surgery: rationale and design of the PEP study trial. BMJ Open. 2018;8(12):e024672.
Vanderbyl BL, Mayer MJ, Nash C, Tran AT, Windholz T, Swanson T, et al. A comparison of the effects of medical Qigong and standard exercise therapy on symptoms and quality of life in patients with advanced cancer. Support Care Cancer. 2017;25(6):1749–58.
Dhillon HM, Bell ML, van der Ploeg HP, Turner JD, Kabourakis M, Spencer L, et al. Impact of physical activity on fatigue and quality of life in people with advanced lung cancer: a randomized controlled trial. Ann Oncol. 2017;28(8):1889–97.
Sebio García R, Yáñez-Brage MI, Giménez Moolhuyzen E, Salorio Riobo M, Lista Paz A, Borro Mate JM. Preoperative exercise training prevents functional decline after lung resection surgery: a randomized, single-blind controlled trial. Clin Rehabil. 2017;31(8):1057–67.
Karenovics W, Licker M, Ellenberger C, Christodoulou M, Diaper J, Bhatia C, et al. Short-term preoperative exercise therapy does not improve long-term outcome after lung cancer surgery: a randomized controlled study†. Eur J Cardiothorac Surg. 2017;52(1):47–54.
Licker M, Karenovics W, Diaper J, Frésard I, Triponez F, Ellenberger C, et al. Short-term preoperative high-intensity interval training in patients awaiting lung cancer surgery: a randomized controlled trial. J Thorac Oncol. 2017;12(2):323–33.
Kaya SO, Akcam TI, Ceylan KC, Samancılar O, Ozturk O, Usluer O. Is preoperative protein-rich nutrition effective on postoperative outcome in non-small cell lung cancer surgery? A prospective randomized study. J Cardiothorac Surg. 2016;11(1):14.
Gravier FE, Bonnevie T, Boujibar F, Médrinal C, Prieur G, Combret Y, et al. Effect of prehabilitation on ventilatory efficiency in non–small cell lung cancer patients: a cohort study. J Thorac Cardiovasc Surg. 2019;157(6):2504-2512.e1.
Boujibar F, Bonnevie T, Debeaumont D, Bubenheim M, Cuvellier A, Peillon C, et al. Impact of prehabilitation on morbidity and mortality after pulmonary lobectomy by minimally invasive surgery: a cohort study. J Thorac Dis. 2018;10(4):2240–8.
Yao L, Chen H, Xue B. Application and practice of trimodal prehabilitation model in preoperative management of patients with lung cancer undergoing video-assisted thoracoscopic surgery. Front Surg. 2023;6(9):1047977.
Goldsmith I, Chesterfield-Thomas G, Toghill H. Pre-treatment optimization with pulmonary rehabilitation in lung cancer: making the inoperable patients operable. EClinicalMedicine. 2021;31:100663.
Schmid S, Minnella EM, Pilon Y, Rokah M, Rayes R, Najmeh S, et al. Neoadjuvant prehabilitation therapy for locally advanced non–small-cell lung cancer: optimizing outcomes throughout the trajectory of care. Clin Lung Cancer. 2022;23(7):593–9.
Ferreira V, Lawson C, Gillis C, Scheede-Bergdahl C, Chevalier S, Carli F. Malnourished lung cancer patients have poor baseline functional capacity but show greatest improvements with multimodal prehabilitation. Nutr Clin Pract. 2021;36(5):1011–9.
Bradley P, Merchant Z, Rowlinson-Groves K, Taylor M, Moore J, Evison M. Feasibility and outcomes of a real-world regional lung cancer prehabilitation programme in the UK. Br J Anaesth. 2022;S0007091222002951.
Ricketts WM, Bollard K, Streets E, Hutton K, Hornby C, Lau K. Feasibility of setting up a pre-operative optimisation ‘pre-hab’ service for lung cancer surgery in the UK. Perioper Med. 2020;9(1):14.
Finley DJ, Fay KA, Batsis JA, Stevens CJ, Sacks OA, Darabos C, et al. A feasibility study of an unsupervised, pre‐operative exercise program for adults with lung cancer. Eur J Cancer Care (Engl). 2020;29(4). Cited 2023 Mar 8. Available from: https://onlinelibrary.wiley.com/doi/10.1111/ecc.13254.
Egegaard T, Rohold J, Lillelund C, Persson G, Quist M. Pre-radiotherapy daily exercise training in non-small cell lung cancer: a feasibility study. Rep Pract Oncol Radiother. 2019;24(4):375–82.
Collaço N, Henshall C, Belcher E, Canavan J, Merriman C, Mitchell J, et al. Patients’ and healthcare professionals’ views on a pre- and post-operative rehabilitation programme (SOLACE) for lung cancer: a qualitative study. J Clin Nurs. 2022;31(1–2):283–93.
Shukla A, Granger CL, Wright GM, Edbrooke L, Denehy L. Attitudes and perceptions to prehabilitation in lung cancer. Integr Cancer Ther. 2020;19:153473542092446.
Finley DJ, Stevens CJ, Emond JA, Batsis JA, Fay KA, Darabos C, et al. Potential effectiveness of a surgeon-delivered exercise prescription and an activity tracker on pre-operative exercise adherence and aerobic capacity of lung cancer patients. Surg Oncol. 2021;37:101525.
Minnella EM, Baldini G, Quang ATL, Bessissow A, Spicer J, Carli F. Prehabilitation in thoracic cancer surgery: from research to standard of care. J Cardiothorac Vasc Anesth. 2021;35(11):3255–64.
Cancer Research UK. Cancer Research UK. Lung cancer treatment statistics 2013–2014. 2013 2014. Cited 2022 Oct 17. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer/diagnosis-and-treatment#heading-Three.
Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res. 2017;17(1):88.
Brennan J. Adjustment to cancer? coping or personal transition? Psychooncology. 2001;10(1):1–18.
Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ. 2021;30: n2061.
Lambaudie E, Bannier/Braticevic C, Villaron/Goetgheluck C, Zemmour C, Boher JM, Ben Soussan P, et al. TRAINING-Ovary 01 (connecTed pRehabiliAtIoN pelvIc caNcer surGery): multicenter randomized study comparing neoadjuvant chemotherapy for patients managed for ovarian cancer with or without a connected pre-habilitation program. Int J Gynecol Cancer. 2021;31(6):920–4.
Toohey K, Hunter M, McKinnon K, Casey T, Turner M, Taylor S, et al. A systematic review of multimodal prehabilitation in breast cancer. Breast Cancer Res Treat. 2023;197(1):1–37.
Bundred JR, Kamarajah SK, Hammond JS, Wilson CH, Prentis J, Pandanaboyana S. Prehabilitation prior to surgery for pancreatic cancer: a systematic review. Pancreatology. 2020;20(6):1243–50.
Dagorno C, Sommacale D, Laurent A, Attias A, Mongardon N, Levesque E, et al. Prehabilitation in hepato-pancreato-biliary surgery: a systematic review and meta-analysis. A necessary step forward evidence-based sample size calculation for future trials. J Visc Surg. 2022;159(5):362–72.
Acknowledgements
The authors would like to acknowledge and thank Health Education England for the funding and support provided to undertake this scoping review.
Funding
This review was supported by Health Education England through a Cancer Prehabilitation Service Transformation Fellowship. The funders were not involved in the data collection, analysis, interpretation and writing of the study.
Author information
Authors and Affiliations
Contributions
K.W-M: conceptualization, methodology, formal analysis, investigation, writing – original draft, writing – review and editing. A.K: methodology, writing – review and editing, supervision. C.U: methodology, supervision. J.J-J: supervision, funding acquisition. A.M: methodology. C.L: methodology, writing – review and editing. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publications
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wade-Mcbane, K., King, A., Urch, C. et al. Prehabilitation in the lung cancer pathway: a scoping review. BMC Cancer 23, 747 (2023). https://doi.org/10.1186/s12885-023-11254-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11254-x