Abstract
Background
Current radiotherapy guidelines and consensus statements uniformly recommend elective region irradiation (ERI) as the standard strategy for nasopharyngeal carcinoma (NPC). However, given the scarcity of skip-metastasis, the improved assessment accuracy of nodal involvement, and the striking advancements in chemotherapy for NPC, a one-fits-all delineation scheme for clinical target volumes of the nodal region (CTVn) may not be appropriate anymore, and modifications of the CTVn delineation strategy may be warranted. Involved site irradiation (ISI) covering merely the initially involved nodal site and potential extranodal extension has been confirmed to be as effective as ERI with decreased radiation-related toxicities in some malignancies, but has not yet been investigated in NPC. This study aims to compare the regional control, survival outcomes, radiation-related toxicities, and quality of life (QoL) of ISI with conventional ERI in NPC patients with a limited nodal burden.
Methods
ISRT-NPC is a prospective, multicenter, open-label, noninferiority, phase III randomized controlled trial. A total of 414 patients will be randomly assigned in a 1:1 ratio to receive ISI or ERI. Randomization will be stratified by institution scale and N stage. Generally, in the ISI group, the high-risk CTV1 (dose: 60 Gy) includes a 1-cm expansion of the positive LN as well as the VIIa and the retrostyloid space above the bilateral transverse process of the atlantoaxial spine (C1), regardless of N status. The low-risk CTV2 (dose: 50 Gy) covers the cervical nodal region with a 3-cm caudal expansion below the transverse process of C1 for N0 disease and a 3-cm expansion below the positive LN for positive LNs.
Discussion
The results of this trial are expected to confirm that ISI is a non-inferior strategy to ERI in stage I-III patients with low LN burden, enabling the minimization of treatment-related toxicity and improvement of long-term QoL without compromising regional control.
Trial registration
ClinicalTrails.gov, NCT05145660. Registered December 6, 2021.
Similar content being viewed by others
Background
Nasopharyngeal carcinoma (NPC) is one of the most common head and neck malignancies. Southeast Asia is the top epidemic region for NPC, with an age-standardized incidence rate of five (per 100,000 population) [1]. Given the complicated anatomical location and high radiosensitivity of NPCs, radiotherapy is the mainstay of treatment for these tumors. The National Comprehensive Cancer Network (NCCN) guidelines recommend radiotherapy alone for stage I NPC and concurrent chemoradiotherapy (CCRT) with or without induction chemotherapy as the standard treatment for stage II-IVA NPC [2].
Intensity-modulated radiotherapy (IMRT) is well-accepted as the preferred radiotherapy technique in treating NPC. In addition, advancements in chemotherapy, such as the regimen optimization of induction chemotherapy and consolidation metronomic chemotherapy, have been extensively investigated and validated to improve the outcome of NPCs, achieving 5-year overall survival (OS) and local-regional control (LC) rates exceeding 90% for stage II-III NPC [3]. These extraordinary survival rates have yielded a population that will experience not only long-term survival but also marked treatment-related sequelae [4, 5]. Therefore, de-escalation of the therapeutic strategy is required for considerably curable malignancies, with the aim of reducing treatment-related toxicity while maintaining excellent cure rates.
Current guidelines recommend at least 70 Gy of radiotherapy to the gross tumor volume (GTV), namely the primary lesion at the nasopharynx as well as involved lymph nodes (LNs) [2]. The clinical target volumes for the nodal region (CTVn) that have been outlined to cover potential sub-clinically involved areas are generally divided into high- (CTVn1, 60–70 Gy), intermediate- (CTVn2, 60 Gy) and low-risk regions (CTVn3, 50–54 Gy) [6]. The international guidelines uniformly recommend that the CTVn2 includes a higher risk of subclinical lesions in all patients with NPC [6]. Nonetheless, these recommendations are not risk-adjusted for nodal location and burden. The results from our institutional real-world study of 2025 patients revealed that the 5-year regional-recurrence-free survival (RRFS) significantly worsened with an increasing nodal burden (97.6%, 97.5%, 94.6% and 91.8% for N0-3 stages, respectively), which confirmed the significant heterogeneity in stage II-IVa patients [7]. Therefore, subjecting everyone to the same treatment modality may be an arbitrary approach. Furthermore, numerous previous studies have reported that most regional recurrences are observed within the nodal GTV or CTVn1 region with high doses and that the marginal or out-of-field failure rates were below 10% [8, 9]. Therefore, lower-intensity cervical irradiation may be justified for early- to mid-stage patients with a relatively low burden of LNs (LB-LN).
As a part of IMRT, elective region irradiation (ERI), defined as prophylactic irradiation of the entire region of involvement plus at least one subsequent level, has become the standard strategy since the two-dimensional (2D) era [10]. However, ERI inevitably leads to acute and late toxicities closely related to quality of life (QoL), including persistent xerostomia, subcutaneous fibrosis, and mouth-opening difficulties [4, 5, 11, 12]. Furthermore, the radiation-related adverse effects on the lymphatic system reduce lymphocyte counts, impair the preservation of immune function, and even promote tumor growth by limiting the adaptive immune response [13]. Additionally, a combination of radiotherapy and chemotherapy exacerbates treatment-related toxicities, which remains an unaddressed point of concern in the IMRT era [3].
In comparison with ERI, involved site irradiation (ISI), a modified modality performed using cervical irradiation covering merely the initially involved nodal site and possible extranodal extension (ENE), has been confirmed to be quite effective in hematologic malignancies with low radiation-related toxicities [14, 15]. Nevertheless, to the best of our knowledge, no study has reported the validity of ISI in NPC. Given the scarcity of skip node metastases in NPC and the increased sensitivity of diagnostic imaging modalities in detecting occult involved LNs, the introduction of ISI for NPC treatment can be considered to be justifiable [16, 17].
Consequently, this study will apply the ISI strategy in early to mid-stage NPC and compare its regional control rates, survival outcomes, radiation-related toxicities, and QoL with the conventional ERI approach. We hypothesize that ISI with appropriate volume reduction of CTVn can reduce treatment-related toxicities and better preserve immune function without compromising regional control.
Methods
Study design
This study is designed as a multicenter, noninferiority, subject-blinded, randomized, phase III trial. A total of 414 patients harboring stage I-III NPC with LB-LN (detailed definition is presented in “Inclusion criteria” section) from eight tertiary hospitals in China will be randomized (1:1) to the ISI or ERI groups. The flow chart and study design schedule are presented in Fig. 1; Table 1, respectively.
Flow chart showing the overview of this trial. CTV1, high-risk clinical target volume for the nodal region; CTV2, low-risk clinical target volume for the nodal region; C1, the atlantoaxial spine; ENE, extranodal extension; GTV, Gross tumor volume; Gy, gray; LN, lymph node; MAD, maximum diameter; QoL, quality of life; RPLN, retropharyngeal lymph node
Primary endpoint
The 3-year RRFS (Regional-recurrence-free survival, which is measured from the registration to the documented regional recurrence) will be compared between the ISI and ERI groups.
Secondary endpoint
The effects of ISI on 3-year OS (measured from registration to documented death from any cause or last follow-up), distant metastasis-free survival (DMFS, measured from registration to documented distant metastasis or death from any cause), and progression-free survival (PFS, measured from registration to documented locoregional recurrence or distant metastasis or death from any cause); early (from the start of radiotherapy until the 1 month after radiotherapy) and late toxicities; and general and late toxicity-related QoL will be evaluated.
Inclusion criteria
-
(1)
Age between 18 and 75 years;
-
(2)
Karnofsky performance status (KPS) score ≥ 70;
-
(3)
Pathologically confirmed World Health Organization (WHO) type II-III NPC;
-
(4)
TNM stage I-III (T1-3N0-2M0) according to the 8th American Joint Committee on Cancer / Union for International Cancer Control (AJCC/UICC) staging system with a maximum diameter (MAD) of cervical involved LNs ≤ 3 cm [18] and without high-grade ENE [19, 20], namely LB-LN;
-
(5)
Available baseline nasopharynx and neck computed tomography (CT) or magnetic resonance imaging (MRI) (strongly advocated) data (including functional MRI sequences) and measurable tumor lesions;
-
(6)
All procedures for defining the tumor burden completed within 4 weeks of registration;
-
(7)
Survival expectancy of at least 6 months;
-
(8)
Normal marrow and organ function: hemoglobin ≥ 120 g/L, WBCs ≥ 4 × 109 /L, platelets ≥ 100 × 109 /L; liver and kidney function-related indicators within 1.5*the normal upper limit;
-
(9)
Patient willingness to comply with the protocol;
-
(10)
Patient willingness and ability to provide an informed consent form.
Exclusion criteria
-
(1)
MAD of cervical metastatic LNs > 3 cm [18];
-
(2)
High-grade ENE of cervical LNs (including matted nodes and LNs infiltrating the adjacent muscle, parotid gland, vessels or skin) [19, 20];
-
(3)
AJCC T4 or N3 stage;
-
(4)
History of other malignancies (except for stage I non-melanotic skin cancer or in-situ cervical cancer);
-
(5)
Pregnant or lactating women or women of childbearing age without contraception;
-
(6)
Concurrent enrollment in another interventional clinical trial;
-
(7)
Uncontrolled comorbidities that may reduce compliance with the trial, such as myocardial infarction, arrhythmia, cerebrovascular disease, ulcer disease, psychiatric disease, and uncontrollable diabetes;
-
(8)
Unwillingness to comply with procedures and requirements as per the study protocol regularly.
Randomization
An eligibility checklist and patient consent must be completed and obtained before randomization. Eligible patients will be allocated randomly to the ISI and ERI groups on a 1:1 basis using a computer-generated randomization scheme with a block size of four. Randomization will be stratified by institution scale (large vs small) and N stage (N0 vs N1 vs N2).
Pre-treatment evaluation (baseline)
The enrolled patients are required to complete the following examinations within 4 weeks before registration:
-
(1)
Thorough medical history and physical examination: evaluation of KPS score, weight, height, vital signs, and physical examination of the nasopharynx, cervical LNs, and nervous system.
-
(2)
Laboratory tests: blood routine examinations, biochemistry evaluations, urine and stool routine analysis, circular lymphocyte phenotyping, and measurement of Epstein–Barr virus (EBV) DNA concentration.
-
(3)
Radiological imaging examinations: fiberoptic nasopharyngoscopy and tumor biopsy, MRI and CT contrast imaging of the nasopharynx and neck, cervical ultrasound, chest CT, abdominal CT/ultrasound, emission computed tomography (ECT) bone scans, and positron emission tomography (PET)/CT (optimal; performed at the discretion of the attending physician).
-
(4)
Baseline documentation: electrocardiogram, pulmonary function, and QoL assessment.
Radiotherapy
Simulation: To ensure consistency in the multicenter setting, the localization and immobilization procedures on CT and MRI will be based on the Chinese Consensus Guidelines for Radiotherapy in NPC (version 2020) [21]. In brief, CT and MRI scans will be performed in the supine position with a thermoplastic mask at the head, neck, and shoulder. The scans will be captured in 3-mm slices from the head to 3 cm below the sternoclavicular joint.
-
1.
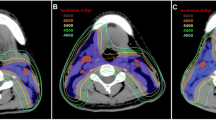
Target volume delineation: The same principles will be shared between the two arms regarding the delineation of GTVnx, GTVnd, and CTV for the primary tumor and organs at risk (OAR). Detailed definition of GTVnd is in accordance with the international guidelines [6]. Suspicious LN is defined as the one with MAD between 5mm and 10 mm as well as ambiguous radiological features. With regard to the CTV for LNs, patients will be randomized to follow the respective strategies of ISI and ERI groups (Table 2). Figure 2 A-C shows the delineation examples of ISI and ERI.
Fig. 2 A Comparison of CTV delineation between the ISI and ERI groups in patients with N0 disease (Upper panel: ERI; Lower panel: ISI). CTV, clinical target volume; ERI, elective region irradiation; ISI, involved site irradiation. B Comparison of CTV delineation between the ISI and ERI groups in patients with ipsilateral positive LNs in level II extending to level III (Upper panel: ERI; Lower panel: ISI). CTV, clinical target volume; ERI, elective region irradiation; ISI, involved site irradiation; LN, lymph node. C Comparison of CTV delineation between the ISI and ERI groups in patients with bilateral positive LNs (Upper panel: ERI; Lower panel: ISI). CTV, clinical target volume; ERI, elective region irradiation; ISI, involved site irradiation; LN, lymph node
-
3.
Dose prescription and suggested dose constraints of OARs: As per the latest ASCO/CSCO and NCCN guidelines, dose prescription is shown in Table 3. Dose constraints of OARs are based on those recommended in the 2020 international guideline on dose prioritization for NPC [22].
Chemotherapy
Concurrent chemotherapy
For stage III patients, concurrent cisplatin (100 mg/m², d1-3, Q3w, maximum to three cycles) will be administrated with radiotherapy. For stage II patients, the use of concurrent chemotherapy will be determined by the discretion of physicians. T2N0-1 patients with all nodes < 3 cm, no level IV or Vb nodes involvement, no extranodal extension [ENE] and pretreatment Epstein-Barr virus [EBV] DNA < 500 copies/mL may be considered as candidates of RT alone. Stage I patients will receive radiotherapy alone.
Induction and consolidation chemotherapy
The application of induction chemotherapy and consolidation chemotherapy is dependent on the physician’s discretion.
Follow-up
During the treatment course, all enrolled patients will be examined and treatment-related adverse events will be carefully documented weekly. Patients will be followed up at least every three months in the first two years, six months in the third to fifth year, and then yearly. The detailed schedule and examination items are shown in Table 1.
Assessment of recurrence
In patients showing signs of local recurrence during follow-up, MRI, CT, PET-CT or ultrasound-guided fine-needle aspiration cytology (US-FNAC) should be performed to confirm recurrence, as necessary. The planning CT and diagnostic imaging modalities will be compared side-by-side to determine the exact site of recurrence and its relative location to the ISI region.
Assessment of toxicity and QoL
Acute treatment-related toxicities will be graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. The Radiation Therapy Oncology Group (RTOG) and European Organization for Research and Treatment of Cancer (EORTC) late radiation morbidity scoring schemes will be used to assess late radiation toxic effects. The EORTC QLQ-C30 and EORTC QLQ-H&N35 questionnaires will be applied to evaluate the general QoL. The Groningen Radiation Therapy Induced Xerostomia questionnaire (GRIX) will be used to assess the QoL associated with serostomia. The MD Anderson Dysphagia Inventory (MDADI) composite score and Swallowing Quality of Life Questionnaire (SWAL-QoL) will be used to evaluate swallowing capacity and dysphagia-related QoL.
Sample size calculation
The primary endpoint is RRFS. Based on previous reports, the 3-year failure free regional control rate was assumed to be 96% in both groups [23]. According to expert consensus, data from institutional experiences, and published literature, a 6% difference was set as the noninferiority margin [3, 7, 24]. To obtain a power of 85% and a one-sided α value of 2.5%, a total of 414 patients would be enrolled with a dropout or loss of follow-up rate of 5%.
Statistical analysis
The case distribution between the ISI and ERI groups in each center will be described. Compliance will be assessed according to the case report form to compare implementation between the two groups. Total drop-out rates and adverse event drop-out rates will be compared between the two groups using Fisher’s exact test. Baseline comparisons will be performed by using t-tests or non-parametric tests for continuous data. Chi-square test will be used to compare categorical data. Both intention-to-treat (ITT) and per-protocol (PP) analysis will be used to assess the efficacy of ISI. Safety analysis will be performed in the safety population. Time-to-event data will be censored in the absence of observations of regional failure at the date of last follow-up or loss to follow-up. The Kaplan–Meier method will be used to estimate the survival rates. Differences between treatment groups will be assessed using log-rank tests. Interaction analysis for RRFS will be undertaken to assess whether differential effects were present between ISI and ERI in predefined subgroups. Adverse events will be listed and analyzed using the chi-square test or Fisher’s exact test. Serious adverse events should be listed in detail and compared between groups. The principal investigator and the protocol committee will perform the analysis. A Data Monitoring Committee (DMC) will be set up to oversee the trial and to decide whether the trial should be stopped.
Ethics
The study protocol has been approved by the ethics committee of the Cancer Hospital, Chinese Academy of Medical Sciences (22/107-3308). The study has been registered in ClinicalTrails.gov (NCT05145660).
Trial status
The Recruitment started in August 2022 and is currently ongoing.
Discussion
The target volume delineation consensus for NPC still recommends uniform CTVn borders across the different N categories [6, 25]. Moreover, the current recommendations are based on the anatomic landmarks easily identifiable during surgery, such as the hyoid bone and cricoid cartilage [26]. However, the actual lymphatic drainage should have not been restricted by the anatomical structures; theoretically, it is more likely to be affected by the size of the LN as well as the distance from the foci of the tumor deposit. Therefore, the subclinical target of the nodal region in NPC remains a matter of debate for the radiation oncologists.
Extensive studies on CTVn volume reduction have mostly focused on omitting elective contralateral or lower-neck irradiation and have confirmed the safety, feasibility, and improved long-term QoL of this approach [24, 27,28,29]. Chen et al. conducted a prospective study of 212 patients with clinical N0-1 NPC and demonstrated the safety and efficacy of omitting level IV and Vb [27]. The study by Tang et al. consisting of 546 NPC patients with unilateral neck LN metastases reiterated the feasibility of contralateral lower-neck-sparing IMRT [30]. Similar results were reported in a study by Gao et al., who limited the nodal clinical target volume (CTV) to level II, III, and Va in 410 patients with cN0 NPC [31]. Notwithstanding these lower-neck sparing efforts, substantial late toxicities were still reported, such as persistent xerostomia, subcutaneous fibrosis, open mouth difficulty, decreased QoL, and radiation-related damage to the lymphatic system [4, 5, 11, 12, 32]. A recent randomized phase III noninferiority study demonstrated that elective upper-neck irradiation (UNI) of the uninvolved neck provided similar regional control and results in less radiation toxicity than standard whole-neck irradiation (WNI) in patients with N0-N1 NPC [24]. Nevertheless, the suitability of the above approach for patients with ipsilateral N2-3 disease and non-endemic populations requires further investigation. Moreover, these studies still depended heavily on the anatomical definition of the neck region without considering the specific site of LN. Therefore, much effort is required to further optimize the nodal CTV delineation strategy in NPC.
ISI, which is characterized by coverage of only the initially involved nodal sites and high-risk region, has been shown to reduce treatment-related toxicity without compromising high regional control rates in various hematological malignancies [14, 15]. This approach has not been tested in NPC due to concerns regarding the possibility of missing suspected metastatic LN. Nevertheless, ISI may be a qualified alternative to ERI in NPC based on the following considerations. First, skip involvement of cervical nodes in NPC is scarce [16]. Second, the majority of regional failures in cases of NPC mainly occur in high-dose areas within the irradiation field, suggesting that failure may be attributable to primary resistance to radiotherapy rather than inadequate target volume coverage [8, 9]. Third, the increased sensitivity of imaging modalities has made clinically inappreciable small nodal metastases detectable and resulted in a very low incidence of occult nodal metastases [17]. All of the abovementioned conditions allow for the hypothesis of further reduction of the volume of neck irradiation in low-risk NPC.
The most accurate delineation of the CTV is based on true extension of subclinical disease. However, complete pathologic examination of the lymphoid adipose tissue is impossible in NPC. Therefore, to determine the potential spread distance of LNs alongside the neck, we will investigate the potential drainage distance by measuring the distance of one positive lymph node surrounded by another positive lymph node. By marking the centers of the two most caudally located LNs in 73 patients with more than two involved cervical LNs, two coordinates ([X1, Y1, Z1] and [X2, Y2, Z2]) were obtained. The |Z1-Z2| value (caudal-cranial direction) was calculated, and the value of 2.96 cm was set as the potential caudal expansion distance by covering 95% of the patients (unpublished data). Additionally, according to the CTV delineation consensus for N + patients with head and neck cancer, CTV3 (elective dose CTV) was recommended to include nodal areas at least 2 cm cranial and caudal to GTV-N [33]. On the basis of these considerations, 3 cm below the GTVnd was set as the extended border for CTV2 [33].
Currently, there is no consensus regarding the microscopic extent of the disease or the CTV margin that should surround the involved gross node, i.e., determination of the best margin to find between CTVn1 and CTVn2 away from the long-established practice remains unknown. This can be attributed to the paucity of surgical pathology data regarding the exact area of extracapsular tumor infiltration. On the basis of common recommendations in current guidelines and considering the critical findings that no microscopic tumor extension beyond 10 mm has been observed among LNs involved in head and neck cancers (none-NPC), coupled with the common practice of the major centers in this trial, we will expand the GTVn by 10 mm to cover the CTVn1 [34]. In addition, since ENE is widely reported to be a more powerful determinant of poor survival outcomes, patients with high grade of ENE will not be included in this study [35]. Although no significant differences were observed between nodal size and ENE extension distance in head and neck cancers, MAD plays a critical prognostic role in NPC, and an MAD of 3 cm is widely used as an essential negative determinant of worse RRFS [18]. Herein, we will carefully evaluate the size and ENE status of nodes to determine enrollment and will exclude patients with MAD > 3 cm or high-grade ENE to balance potential confounding factors.
In conclusion, the results of this trial are expected to confirm that ISI is a safe and effective strategy to reduce neck irradiation volume in comparison with ERI in stage I-III patients with low LN loads, minimizing treatment-related toxicity and improving long-term QoL without compromising regional control.
Availability of data and materials
The datasets used and analysed during the current study will be presented within the manuscript and the additional supporting files.
Abbreviations
- C1:
-
Atlantoaxial spine
- CCRT:
-
Concurrent chemoradiotherapy
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- CTV:
-
Clinical target volume
- DMFS:
-
Distant metastasis-free survival
- EBV:
-
Epstein-Barr virus
- ECT:
-
Emission computed tomography
- ENE:
-
Extranodal extension
- EORTC:
-
European Organization for Research and Treatment of Cancer
- ERI:
-
Elective region irradiation
- GRIX:
-
Groningen Radiation Therapy Induced Xerostomia questionnaire
- GTV:
-
Gross tumor volume
- IMRT:
-
Intensity-modulated radiotherapy
- ISI:
-
Involved site irradiation
- ITT:
-
Intention-to-treat
- KPS:
-
Karnofsky performance status
- LC:
-
Local-regional control
- LN:
-
Lymph nodes
- MAD:
-
Maximum diameter
- MDADI:
-
MD Anderson Dysphagia Inventory
- NCCN:
-
National Comprehensive Cancer Network
- NPC:
-
Nasopharyngeal carcinoma
- OAR:
-
Organs at risk
- OS:
-
Overall survival
- PET:
-
Positron emission tomography
- PFS:
-
Progression-free survival
- PP:
-
Per-protocol
- PTV:
-
Planning target volume
- QoL:
-
Quality of life
- RPLN:
-
Retropharyngeal lymph node
- RRFS:
-
Regional-recurrence-free survival
- RTOG:
-
Radiation Therapy Oncology Group
- UNI:
-
Upper-neck irradiation
- WHO:
-
World Health Organization
- WNI:
-
Whole-neck irradiation
References
Chen Y-P, Chan ATC, Le Q-T, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. The Lancet. 2019;394(10192):64–80.
NCCN Clinical Practice Guidelines in Oncology. Head and Neck Cancers. Version 2.2022. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed 26 April 2022.
Tang LL, Guo R, Zhang N, Deng B, Chen L, Cheng ZB, Huang J, Hu WH, Huang SH, Luo WJ, et al. Effect of Radiotherapy alone vs Radiotherapy with Concurrent Chemoradiotherapy on Survival without Disease Relapse in patients with low-risk nasopharyngeal carcinoma: a Randomized Clinical Trial. JAMA. 2022;328(8):728–36.
McDowell LJ, Rock K, Xu W, Chan B, Waldron J, Lu L, Ezzat S, Pothier D, Bernstein LJ, So N, et al. Long-term late toxicity, quality of life, and emotional distress in patients with nasopharyngeal carcinoma treated with intensity modulated Radiation Therapy. Int J Radiat Oncol Biol Phys. 2018;102(2):340–52.
Lin YH, Huang TL, Chien CY, Chen HC, Hsu HC, Huang EY, Wang CJ, Huang YJ, Wang YM, Huang CC, et al. Pretreatment prognostic factors of survival and late toxicities for patients with nasopharyngeal carcinoma treated by simultaneous integrated boost intensity-modulated radiotherapy. Radiat Oncol. 2018;13(1):45.
Lee AW, Ng WT, Pan JJ, Poh SS, Ahn YC, AlHussain H, Corry J, Grau C, Gregoire V, Harrington KJ, et al. International guideline for the delineation of the clinical target volumes (CTV) for nasopharyngeal carcinoma. Radiother Oncol. 2018;126(1):25–36.
Shiran S, Junlin Y. Treatment outcomes and prognostic factors for patients with nasopharyngeal carcinoma treated with intensity-modulated Radiotherapy. Phd. Chinese Academy of Medical Sciences and Peking Union Medical College; 2020.
Xue F, Hu C, He X. Long-term patterns of Regional failure for nasopharyngeal carcinoma following intensity-modulated Radiation Therapy. J Cancer. 2017;8(6):993–9.
Li S, Wang K, Hou Z, Yang J, Ren W, Gao S, Meng F, Wu P, Liu B, Liu J, et al. Use of Radiomics Combined with Machine Learning Method in the recurrence patterns after intensity-modulated Radiotherapy for nasopharyngeal carcinoma: a preliminary study. Front Oncol. 2018;8:648.
Grégoire V, Coche E, Cosnard G, Hamoir M, Reychler H. Selection and delineation of lymph node target volumes in head and neck conformal radiotherapy. Proposal for standardizing terminology and procedure based on the surgical experience. Radiother Oncol. 2000;56(2):135–50.
Zheng Y, Han F, Xiao W, Xiang Y, Lu L, Deng X, Cui N, Zhao C. Analysis of late toxicity in nasopharyngeal carcinoma patients treated with intensity modulated radiation therapy. Radiat Oncol. 2015;10:17.
Miao J, Wang L, Zhu M, Xiao W, Wu H, Di M, Huang Y, Huang S, Han F, Deng X, et al. Long-term survival and late toxicities of elderly nasopharyngeal carcinoma (NPC) patients treated by high-total- and fractionated-dose simultaneous modulated accelerated radiotherapy with or without chemotherapy. Oral Oncol. 2019;89:40–7.
Fransen MF, Schoonderwoerd M, Knopf P, Camps MG, Hawinkels LJ, Kneilling M, van Hall T, Ossendorp F. Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint therapy. JCI Insight. 2018;3(23):e124507.
Wirth A, Mikhaeel NG, Aleman BMP, Pinnix CC, Constine LS, Ricardi U, Illidge TM, Eich HT, Hoppe BS, Dabaja B, et al. Involved Site Radiation Therapy in Adult Lymphomas: an overview of International Lymphoma Radiation Oncology Group Guidelines. Int J Radiat Oncol Biol Phys. 2020;107(5):909–33.
Patel CG, Ng AK. Involved-site Radiation Therapy for Early-Stage NLPHL. Int J Radiat Oncol Biol Phys. 2020;107(1):21–2.
Wang X, Hu C, Ying H, He X, Zhu G, Kong L, Ding J. Patterns of lymph node metastasis from nasopharyngeal carcinoma based on the 2013 updated consensus guidelines for neck node levels. Radiother Oncol. 2015;115(1):41–5.
Lai V, Khong PL. Updates on MR imaging and 18F-FDG PET/CT imaging in nasopharyngeal carcinoma. Oral Oncol. 2014;50(6):539–48.
Huang CL, Chen Y, Guo R, Mao YP, Xu C, Tian L, Liu LZ, Lin AH, Sun Y, Ma J, et al. Prognostic value of MRI-determined cervical lymph node size in nasopharyngeal carcinoma. Cancer Med. 2020;9(19):7100–6.
Chin O, Yu E, O’Sullivan B, Su J, Tellier A, Siu L, Waldron J, Kim J, Hansen A, Hope A, et al. Prognostic importance of radiologic extranodal extension in nasopharyngeal carcinoma treated in a canadian cohort. Radiother Oncol. 2021;165:94–102.
Mao Y, Wang S, Lydiatt W, Shah JP, Colevas AD, Lee AWM, O’Sullivan B, Guo R, Luo W, Chen Y, et al. Unambiguous advanced radiologic extranodal extension determined by MRI predicts worse outcomes in nasopharyngeal carcinoma: potential improvement for future editions of N category systems. Radiother Oncol. 2021;157:114–21.
Radiotherapy Guidelines for Nasopharyngeal Carcinoma in China. (Version 2020). CHIN J CANCER PREV TREAT. 2021;28(03):167–77.
Lee AW, Ng WT, Pan JJ, Chiang CL, Poh SS, Choi HC, Ahn YC, AlHussain H, Corry J, Grau C, et al. International Guideline on Dose Prioritization and Acceptance Criteria in Radiation Therapy Planning for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2019;105(3):567–80.
Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, Sun Y, Lin AH, Liu MZ, Ma J. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80(3):661–8.
Tang L-L, Huang C-L, Zhang N, Jiang W, Wu Y-S, Huang SH, Mao Y-P, Liu Q, Li J-B, Liang S-Q, et al. Elective upper-neck versus whole-neck irradiation of the uninvolved neck in patients with nasopharyngeal carcinoma: an open-label, non-inferiority, multicentre, randomised phase 3 trial. Lancet Oncol. 2022;23(4):479–90.
Gregoire V, Levendag P, Ang KK, Bernier J, Braaksma M, Budach V, Chao C, Coche E, Cooper JS, Cosnard G, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC, RTOG consensus guidelines. Radiother Oncol. 2003;69(3):227–36.
Wijers OB, Levendag PC, Tan T, van Dieren EB, van Sörnsen de Koste J, van der Est H, Senan S, Nowak PJ. A simplified CT-based definition of the lymph node levels in the node negative neck. Radiother Oncol. 1999;52(1):35–42.
Chen JZ, Le QT, Han F, Lu LX, Huang SM, Lin CG, Deng XW, Cui NJ, Zhao C. Results of a phase 2 study examining the effects of omitting elective neck irradiation to nodal levels IV and vb in patients with N (0–1) nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2013;85(4):929–34.
Huang CL, Xu C, Zhang Y, Zhou GQ, Mao YP, Liu Q, Sun Y, Ma J, Tang LL. Feasibility of ipsilateral lower neck sparing irradiation for unilateral or bilateral neck node-negative nasopharyngeal carcinoma: systemic review and meta-analysis of 2, 521 patients. Radiat Oncol. 2018;13(1):141.
Cho WK, Oh D, Lee E, Kim TG, Lee H, Nam H, Noh JM, Ahn YC. Feasibility of selective Neck irradiation with lower Elective Radiation Dose in treating Nasopharynx Cancer Patients. Cancer Res Treat. 2019;51(2):603–10.
Tang LL, Tang XR, Li WF, Chen L, Tian L, Lin AH, Sun Y, Ma J. The feasibility of contralateral lower neck sparing intensity modulation radiated therapy for nasopharyngeal carcinoma patients with unilateral cervical lymph node involvement. Oral Oncol. 2017;69:68–73.
Gao Y, Zhu G, Lu J, Ying H, Kong L, Wu Y, Hu C. Is elective irradiation to the lower neck necessary for N0 nasopharyngeal carcinoma? Int J Radiat Oncol Biol Phys. 2010;77(5):1397–402.
Gou X, Duan B, Shi H, Qin L, Xiao J, Chen N. The relations of dosimetric parameters with long-term outcomes and late toxicities in advanced T-stage nasopharyngeal carcinoma with IMRT. Head Neck. 2020;42(1):85–92.
Hansen CR, Johansen J, Samsoe E, Andersen E, Petersen JBB, Jensen K, Andersen LJ, Sand HMB, Bertelsen AS, Grau C. Consequences of introducing geometric GTV to CTV margin expansion in DAHANCA contouring guidelines for head and neck radiotherapy. Radiother Oncol. 2018;126(1):43–7.
Caudell JJ, Meredith RF, Spencer SA, Keene KS, Dobelbower MC, Bonner JA. Margin on gross tumor volume and risk of local recurrence in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2010;76(1):164–8.
Lu T, Hu Y, Xiao Y, Guo Q, Huang SH, O’Sullivan B, Fang Y, Zong J, Chen Y, Lin S, et al. Prognostic value of radiologic extranodal extension and its potential role in future N classification for nasopharyngeal carcinoma. Oral Oncol. 2019;99:104438.
Acknowledgements
Not applicable.
Funding
This work was supported by the Beijing Hope Run Special Fund of Cancer. Foundation of China (LC2021L06). The funding source has no role in study design, data collection, analysis, interpretation, the writing of the manuscript, or the decision to submit the current study.
Author information
Authors and Affiliations
Contributions
Study concept and design: JLY and JBW. Drafting of the trial protocol: YL. Critical revision of the trial protocol for important intellectual content: JLY and JBW. Obtaining funding: JLY. Coordinating investigator: JLY, JBW, YQH, FL, DSH, ZJC, PGW, JGL, JYQ, JF and YXL. Study implementation: JLY, JBW, YQH, FL, DSH, ZJC, PGW, JGL, JYQ, JF and YXL. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Institutional review board approval was obtained for the ISRT-NPC trial from the ethics committee of the Cancer Hospital, Chinese Academy of Medical Sciences (reference number 22/107-3308). The ISRT-NPC trial is published under NCT05145660 on ClinicalTrials.gov. Written informed consent will be obtained from all participants included in the study prior to randomization.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Y., Han, Y., Liu, F. et al. Involved site radiation therapy in stage I-III nasopharyngeal carcinoma with limited lymph node burden (ISRT-NPC) or elective region irradiation: a study protocol for a multicenter non-inferiority randomized controlled phase III clinical trial. BMC Cancer 23, 724 (2023). https://doi.org/10.1186/s12885-023-11212-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11212-7