Abstract
Background
Cancer risk varies geographically, and migrants are influenced by different risk factors before, during and after migration. Increased migration from non-Western countries to the Nordic countries calls for a better understanding of the migrants’ cancer risk and the change in risk patterns over time. The aim of this study was to compare the incidence and mortality of breast, colorectal and lung cancer between non-Western immigrant and the native female population in Denmark, Finland, Iceland, and Norway.
Material and methods
Data from national registries were processed and pre-analysed in each country. Multivariate Poisson regression models were used to model the relative differences in incidence and mortality as rate ratios (RR). The country-specific estimates and summary statistics were pooled together using a random effects model.
Results
Non-Western immigrant women had significantly lower breast (RR 0.71, 0.65–0.78), colorectal (RR 0.72, 0.57–0.92) and lung (RR 0.55, 0.42–0.72) cancer incidence rates than native women, and the risk of these cancers among immigrant women increased with duration of residence. Differences were parallel in breast, colorectal and lung cancer mortality (RR 0.64, 0.55–0.74; RR 0.66, 0.48–0.92; RR 0.51, 0.34–0.79). Among immigrant women, higher education increased the risk for breast cancer and decreased it for lung cancer.
Conclusion
The results significantly complement and add to the previous findings of cancer burden and cancer burden transition among migrants and provide evidence of a prolonged cancer risk advantage among non-Western immigrant women. However, the findings show an increasing risk of lifestyle-related cancers with increasing duration of residence in the host country. Further studies are needed to discover underlying reasons for this phenomenon.
Similar content being viewed by others
Background
Breast, colorectal and lung cancer are the three most common cancers and leading causes of cancer death in women in the Nordic countries [1, 2]. Incidence of these diet and lifestyle driven cancers is lowest in non-Western regions, such as Sub-Saharan Africa and Central Asia. A transition to a Western lifestyle has, however, increased both the incidence and mortality of breast, colorectal and lung cancers also in these low-risk areas [1, 3, 4].
The number of migrant women with a non-Western background has increased considerably in the Nordic countries and elsewhere in Europe in recent decades. Whilst the overall cancer incidence and mortality among these women are still lower compared with the native female population, the risk profiles may vary depending on cancer site and the individual’s age, country of origin and destination, socioeconomic position, period of immigration and duration of residence in the host country [5,6,7,8,9]. Migrants moving to Western countries might experience declining risks of communicable diseases but face a high risk of chronic disease associated with adoption of unhealthy lifestyles. Vulnerability to obesity due to adoption of a more sedentary lifestyle and increased intake of energy dense foods [10] increase the risk for breast and colorectal cancer [11]. Increased migration calls for a better understanding of migrants’ cancer risk and the change in risk patterns over time.
In the Nordic countries, immigrant populations differ by periods of arrival, country of origin and marital status [12]. There is also a different share of migrant workers, students and refugees in these populations. In Finland, most immigrants have moved from the neighbouring countries, Russia and Estonia at working-age either in the 1990s or after. Labour migration in the 2000s, particularly from Poland, has influenced the structure of the immigrant population in Iceland the most. In contrast, Norway and Denmark have a longer history as host countries with established groups of immigrant workers recruited, from countries such as Turkey and Pakistan in the 1970s.
We studied the incidence and mortality of breast, colorectal and lung cancer among non-Western immigrant women and compared these to corresponding figures in the native female population in Denmark, Finland, Iceland, and Norway. Additional analyses take into account duration of residence, age at immigration, and level of education.
Methods
Data sources
We utilised the individual data in the Danish, Finnish, Icelandic and Norwegian national registries to form the study population. In each country, data on country of birth, residential history and education level were retrieved from the population registry or the statistical office; first primary cases in breast (C50), colorectal (C18–20), and lung (C33–34) cancers from the cancer registry; and cause of death from the statistical office, the health data authority, or the cancer registry. These data were linked with each other using an individual code which exists for all residents. Figure 1 shows the flow of data collection in different countries.
Study population
Our study population consisted of all women registered as residents in 1986–2019 in Denmark, 1973–2017 in Finland, 1986–2020 in Iceland, and 1990–2015 in Norway. The country-specific follow-up times varied depending on data availability, and the beginning and coverage of registration. In each country, non-Western immigrants were defined as women born outside the Nordic countries, Western and Southern Europe, Northern America, Australia, and New Zealand. We excluded from the study population all women who emigrated within one year after immigration (2.5% of the immigrant women) and women with missing history of residence or clearly incorrect dates (0.1%) (Please see Additional file 1 for data details).
Statistical analysis
All non-Western women were followed from the date of immigration until death, emigration, age of 95, or end of the year of the study period, whichever occurred first. The date of immigration was defined as the start of the first period of residence in the Nordic country in question, and the date of emigration as the start of the first period of residence elsewhere.
Aggregate data on the number of incident cancers, cancer deaths and person-years at risk were formed by 10-year age group and 10-year calendar period for immigrant and native women. For immigrant women, the data were also stratified by region of birth (Central and South Asia, East Asia and Pacific, Latin America and Caribbean, Middle East and North Africa, Russia and Eastern Europe, Sub-Saharan Africa; please see Additional file 1 for region details), age at immigration (0–19, 20–29, 30–39, 40 + years), duration of residence (1–9, 10–19, 20 + years), and education level at the end of follow-up (primary 0–9 years or missing, secondary 9–12 years, tertiary 12 + years).
Multivariate Poisson regression models were used to model the relative differences in incidence and mortality as rate ratios (RR) with 95% confidence intervals in the above-listed categories and subgroups. RRs were adjusted by attained age, calendar year and region of birth. First, we compared the incidence and mortality of breast, colorectal, and lung cancer between the non-Western immigrant women and the native female population. The analyses of impact of immigration age, duration of residence, and education level were performed only among migrants.
All statistical analyses were performed using the R program version 4.0.2. To ensure compliance with the EU General Data Protection Regulation (GDPR) individual data were processed and pre-analysed separately in each Nordic country, with standard R scripts developed by the Finnish Cancer Registry. After these pre-analyses, the country-specific estimates and summary statistics were sent to Finland where the estimates were pooled together using a random effects model [13].
Results
There were altogether 766,033 non-Western immigrant women in the study population who contributed with 3.7% of the total of 190 million person-years at risk. Women born in Russia/the Former Soviet Union or Eastern Europe accounted for the largest group of all non-Western female immigrants (Fig. 2). However, the proportion of women migrating from the region was far larger in Finland (56%) and Iceland (69%) than in Denmark (35%) and Norway (38%), whereas the share of immigrant women from Middle East and North Africa was larger in Denmark (23%) than in the other countries (Finland 10%; Iceland 3%; Norway 12%). Furthermore, differences by individual country of birth existed within the regions, e.g., Russian population was biggest in Finland, and Polish women were in the majority in Denmark, Iceland and Norway.
Altogether there were 463,542 primary cancers in the study population (Table 1). Among the non-Western women, there were markedly more breast cancers than colorectal and lung cancers. Breast cancer was also the leading cause of cancer death.
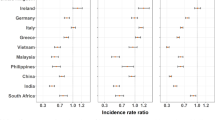
The pooled Nordic results showed both lower incidence and lower mortality among the non-Western immigrant women compared to the native female population (Fig. 3). This was true for all studied cancers. Immigrant women had a 45% lower (RR 0.55, 0.42–0.72) lung cancer risk, a 29% lower (RR 0.71, 0.65–0.78) breast cancer risk and a 28% lower (RR 0.72, 0.57–0.92) colorectal cancer risk than native women. The differences in mortality were parallel. Between the country-specific results, the findings were most similar in breast cancer incidence. In other cancers, there were notable differences. Incidence and mortality were significantly lower among the migrants also when we looked at the pooled results by region of birth (Additional file 2). Differences in results between regions and by cancer existed.
Adjusted rate ratio (RR) in cancer incidence and mortality. Breast, colorectal and lung cancer incidence and mortality among non-Western immigrant women compared to native women. (Adjusted by attained age, calendar year and region of birth. I2 is the heterogeneity statistic and tau2 is the variance of the effect size parameters across the studies)
Table 2 shows the number of cancer cases and deaths as well as the adjusted rate ratio in cancer incidence and mortality among the non-Western women by duration of residence, age at immigration and education level. The risk of studied cancers increased with duration of residence in the host country. The increase was significant in breast and lung cancers: the longer the residence, the higher the incidence and mortality. For example, non-Western women living in the Nordic countries for 20 or more years had a 56% higher breast cancer mortality compared with women who had resided in these countries 9 years or less. By immigration age, significant differences in the incidence and mortality rates were found only for breast cancer mortality. Furthermore, there were significant differences in the breast and lung cancer incidence by education level. Breast cancer incidence increased with education level. The mortality in breast cancer, on the contrary, was highest among women with fewest years of education. Both incidence and mortality in lung cancer were lowest among women with the highest education level.
Discussion
Our study is a collaborative registry study between Denmark, Finland, Iceland, and Norway, and, to our knowledge, the first analysis of cancer incidence and mortality among non-Western immigrant women combining registry data from different Nordic countries. Our results show that women born in non-Western countries have significantly lower breast, colorectal and lung cancer incidence and mortality rates than native women. Additionally, the risk of these cancers and cancer deaths among immigrant women increase with duration of residence.
Our findings are in line with existing European and Nordic studies showing differences in risk of these cancers between non-Western migrant and native women [7,8,9, 14,15,16]. The differences likely stem from lifestyle and hormonal risk factors (pregnancy history, duration of breastfeeding, hormonal contraceptive use, and hormone-replacement therapy) [10, 11, 17,18,19]. Smoking is the strongest lifestyle-related risk factor causing almost 90% of lung cancers and also increasing the risk of colorectal cancer [20]. Colorectal and breast cancer are also attributable to other preventable risk factors, such as alcohol intake, obesity, Western diet and physical inactivity.
Prior studies from the Nordic countries have found immigrants attending organized breast cancer screening less actively than native women [21,22,23,24]. The lower attendance rates might explain the lower breast cancer incidence as cases may remain undiagnosed in the follow-up period. However, the mortality rates are also lower among the immigrant women and could be even lower with more active screening participation. Hence, differences in breast cancer screening participation cannot explain the difference in incidence and mortality.
Immigrants may emigrate when they develop poor health or expect to die soon, but their deaths are not registered in the statistics of the country of residence. However, recent study results from Sweden do not support the overall existence of such a phenomenon, since no systematic differences were observed in health among immigrants who had emigrated compared with those who remained in the country [25].
Incidence and mortality in studied cancers differed by the immigrants’ region of birth, which may partly be explained by the differences in background cancer risk in the countries of birth [1, 26]. Our results differed also by the Nordic host countries. This, in turn, may partly be explained by the fact that incidence and mortality rates among the general female population differ in each host country [2]. For example, the age-standardised rates (/100 000) for female colorectal cancer incidence and mortality in Norway were 36.0 and 10.4 in 2015–2019, respectively, whereas in Finland they were lower, 22.2 and 6.6, respectively (please see Additional file 3 for more details). Due to different immigration patterns and age structures in the host countries, immigrant groups differ by country. This affects the composition of the study population data and might also have caused the heterogeneity in country-specific estimates.
Among the immigrant women, adaptation to new environments and exposure to risk factors may be possible explanations for the observed changes in incidence and mortality by the duration of residence. Comparing results to previous Northern European studies is, however, difficult due to the variation in the study settings. Previous studies have showed either no differences or both lower and higher rates [9, 15, 27, 28]. This may be partly due to short follow-up times used in the studies. Education level was inversely related to breast and lung cancer incidence and mortality among the non-Western women, with higher education increasing risk for breast cancer and decreasing it for lung cancer. These results are in line with earlier findings from Sweden [29] and recent Finnish cancer statistics [30]. With lung cancer in particular, the difference in incidence and mortality between education levels is highlighted. The differences observed by education level in breast cancer might also be a consequence of the higher screening attendance among women with higher levels of education. Overall, migrant women with lower education may remain at risk of social isolation and lack the knowledge of available health care services partly due to inadequate health literacy [31, 32].
Collecting data for migrant health requires consensus on data collection and similar definitions. Our study benefits from strong cultural and social connections between the Nordic countries and the existence of similar institutions – the Nordic welfare model and national population-based registries – which provide a unique basis for research combining data from different countries in the region.
A fundamental strength of our study was the combining of large individual-level data from valid and comprehensive registers from four Nordic countries, over several decades. Our study provides new information on the long-term changes in cancer incidence and mortality post-migration in the studied area. Moreover, the way in which the analyses were performed in co-operation was beneficial. Before pooling the results, the data and the variable definitions were structured, defined and verified in the same way. Uniform analyses were done systematically with the same scripts in every participating country.
Immigrant women are still relatively few and young in the countries studied here. Therefore, only the small number of cancers were observed in different subgroups, and some of the results may be sporadic. Another limitation is that we used large geographic regions including various countries and health care practices, and women with different ethnic backgrounds, which may affect the observed results. Moreover, information on potential contributing factors, such as reason for migration, marital status and origin of spouses were not available. We did not account for return migration and excluded women at their first emigration. This, together with minor registration imperfection regarding the data on residing history and causes of death, is, however, unlikely to affect the results.
Conclusions
Registry-based longitudinal research among migrants is still scarce. This study offers evidence of a prolonged breast, colorectal and lung cancer risk advantage among non-Western immigrant women in the Nordic countries. However, our findings show that the risk of lifestyle-related cancers increased with increasing duration of residence in the host country. Further studies with different designs are needed to discover underlying reasons for this phenomenon.
Availability of data and materials
Country-specific summary data are available from the corresponding author upon reasonable request. Due to data protection regulations, the register data are not openly shared.
Abbreviations
- CI:
-
Confidence interval
- GDPR:
-
EU General Data Protection Regulation
- NCU:
-
Nordic Cancer Union
- RR:
-
Rate ratio
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Breast, colorectal and lung cancer incidence and mortality rates in the Nordic countries. NORDCAN database 2.0. Association of the Nordic Cancer Registries. https://nordcan.iarc.fr/en/dataviz/tables. Accessed 12 Oct 2022.
Bray F. Transitions in human development and the global cancer burden. In: World Cancer Report 2014. WHO Press. 2014. https://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2014. Accessed 12 Oct 2022.
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–91. https://doi.org/10.1136/gutjnl-2015-310912.
Arnold M, Razum O, Coebergh J-W. Cancer risk diversity in non-western migrants to Europe: an overview of the literature. Eur J Cancer. 2010;46:2647–59. https://doi.org/10.1016/j.ejca.2010.07.050.
Beiki O, Allebeck P, Nordqvist T, Moradi T. Cervical, endometrial and ovarian cancers among immigrants in Sweden: Importance of age at migration and duration of residence. Eur J Cancer. 2009;45:107–18. https://doi.org/10.1016/j.ejca.2008.08.017.
Hjerkind KV, Larsen IK, Aaserud S, Møller B, Ursin G. Cancer incidence in non-immigrants and immigrants in Norway. Acta Oncol. 2020;59:1275–83. https://doi.org/10.1080/0284186X.2020.1817549.
Mousavi SM, Sundquist K, Hemminki K. Morbidity and mortality in gynecological cancers among first- and second-generation immigrants in Sweden. Int J Cancer. 2012;131:497–504. https://doi.org/10.1002/ijc.26395.
Norredam M, Krasnik A, Pipper C, Keiding N. Cancer incidence among 1st generation migrants compared to native Danes – A retrospective cohort study. Eur J Cancer. 2007;43:2717–21. https://doi.org/10.1016/j.ejca.2007.09.017.
Labree LJW, van de Mheen H, Rutten FFH, Foets M. Differences in overweight and obesity among children from migrant and native origin: a systematic review of the European literature. Obes Rev. 2011;12:e535–47. https://doi.org/10.1111/j.1467-789X.2010.00839.x.
Soerjomataram I, Shield K, Marant-Micallef C, Vignat J, Hill C, Rogel A, Menvielle G, Dossus L, Ormsby JN, Rehm J, Rushton L, Vineis P, Parkin M, Bray F. Cancers related to lifestyle and environmental factors in France in 2015. Eur J Cancer. 2018;105:103–13. https://doi.org/10.1016/j.ejca.2018.09.009.
Pettersen SV, Østby L. Scandinavian comparative statistics on integration. Immigrants in Norway, Sweden and Denmark. Samfunnsspeilet. 2013;5. https://www.ssb.no/en/befolkning/artikler-og-publikasjoner/_attachment/204333?_ts=1497ab864. Accessed 10 Sept 2022.
Brockwell SE, Gordon IR. A comparison of statistical methods for meta-analysis. Stat Med. 2001;20:825–40. https://doi.org/10.1002/sim.650.
Arnold M, Aarts MJ, Siesling S, Van der Aa M, Visser O, Coebergh J-W. Diverging breast and stomach cancer incidence and survival in migrants in The Netherlands, 1996–2009. Acta Oncol. 2012;52:1195–201. https://doi.org/10.3109/0284186X.2012.742962.
Van Hemelrijck WMJ, Rosskamp M, De Schutter H, Verdoodt F, Vanthomme K. Cancer risk among individuals of migrant origin in Belgium during the 2000s – Evidence of migration as a ‘cancer risk transition’? Soc Sci Med. 2021;269:113591. https://doi.org/10.1016/j.socscimed.2020.113591.
Visser O, van Leeuwen FE. Cancer risk in first generation migrants in North-Holland/Flevoland, The Netherlands, 1995–2004. Eur J Cancer. 2007;43:901–8. https://doi.org/10.1016/j.ejca.2006.12.010.
Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125:171–80. https://doi.org/10.1002/ijc.24343.
Barta JA, Powell CA, Wisnivesky JP. Global Epidemiology of Lung Cancer. Ann Glob Health. 2019;85(8):1–16. https://doi.org/10.5334/aogh.2419.
Gathani T, Ali R, Balkwill A, Green J, Reeves G, Beral V, Moser KA, Million Women Study Collaborators. Ethnic differences in breast cancer incidence in England are due to differences in known risk factors for the disease: prospective study. Br J Cancer. 2014;110:224–9. https://doi.org/10.1038/bjc.2013.632.
Katzke VA, Kaaks R, Kühn T. Lifestyle and cancer risk. Cancer J. 2015;21:104–10. https://doi.org/10.1097/PPO.0000000000000101.
Bhargava S, Tsuruda K, Moen K, Bukholm I, Hofvind S. Lower attendance rates in immigrant versus non-immigrant women in the Norwegian Breast Cancer Screening Programme. J Med Screen. 2018;25:155–61. https://doi.org/10.1177/0969141317733771.
Jensen LF, Pedersen AF, Andersen B, Vedsted P. Identifying specific non-attending groups in breast cancer screening–population-based registry study of participation and socio-demography. BMC Cancer. 2012;12:518. https://doi.org/10.1186/1471-2407-12-518.
Kristiansen M, Thorsted BL, Krasnik A, von Euler-Chelpin M. Participation in mammography screening among migrants and non-migrants in Denmark. Acta Oncol. 2012;51:28–36. https://doi.org/10.3109/0284186X.2011.626447.
Lagerlund M, Maxwell AE, Bastani R, Thurfjell E, Ekbom A, Lambe M. Sociodemographic predictors of non-attendance at invitational mammography screening – a population-based register study (Sweden). Cancer Causes Control. 2002;13:73–82. https://doi.org/10.1023/A:1013978421073.
Dunlavy A, Cederström A, Katikireddi SV, Rostila M, Juárez SP. Investigating the salmon bias effect among international immigrants in Sweden: a register-based open cohort study. Eur J Public Health. 2022;32:226–32. https://doi.org/10.1093/eurpub/ckab222.
Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, Gavin A, Visser O, Bray F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–87. https://doi.org/10.1016/j.ejca.2018.07.005.
Stirbu I, Kunst A, Vlems F, Visser O, Bos V, Deville W, Nijhuis H, Coebergh J. Cancer mortality rates among first and second generation migrants in the Netherlands: Convergence toward the rates of the native Dutch population. Int J Cancer. 2006;119:2665–72. https://doi.org/10.1002/ijc.22200.
Mousavi SM, Fallah M, Sundquist K, Hemminki K. Age- and time-dependent changes in cancer incidence among immigrants to Sweden: colorectal, lung, breast and prostate cancers. Int J Cancer. 2012;131:E122–8. https://doi.org/10.1002/ijc.27334.
Beiki O, Hall P, Ekbom A, Moradi T. Breast cancer incidence and case fatality among 4.7 million women in relation to social and ethnic background: a population-based cohort study. Breast Cancer Res. 2012;14:R5. https://doi.org/10.1186/bcr3086.
Pitkäniemi J, Malila N, Tanskanen T, Degerlund H, Heikkinen S, Seppä K. Cancer in Finland 2019. Cancer Society of Finland Publication No. 98. Cancer Society of Finland, Helsinki. 2021. https://syoparekisteri.fi/assets/files/2021/07/Cancer_in_Finland_2019.pdf. Accessed 10 Sept 2022.
Gele AA, Pettersen KS, Torheim LE, et al. Health literacy: the missing link in improving the health of Somali immigrant women in Oslo. BMC Public Health. 2016;16:1134. https://doi.org/10.1186/s12889-016-3790-6.
Wångdahl J, Lytsy P, Mårtensson L, et al. Health literacy among refugees in Sweden – a cross-sectional study. BMC Public Health. 2014;14:1030. https://doi.org/10.1186/1471-2458-14-1030.
Acknowledgements
We thank Bo Søborg for his work in data formulation for Denmark.
Funding
This work was supported by the Nordic Cancer Union (NCU, case number R250-A14915). The funding source had no involvement in study design, data analysis, data interpretation, writing or submission of the article.
Author information
Authors and Affiliations
Contributions
ML*: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Visualization, Writing – original draft and editing. AL*: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – review & editing. SH: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. MN: Validation, Writing – review & editing. GU: Supervision. SC: Data curation, Formal analysis, Writing – review & editing. HS: Data curation, Formal analysis, Validation; Writing – review & editing. EH: Data curation. ST: Data curation, Formal Analysis, Visualization. IV: Writing – review & editing. SN: Validation, Writing – review & editing. TS: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing. *Joint first authorship, Lamminmäki and Leivonen contributed equally to this paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All phases of this study were conducted in accordance with relevant guidelines and regulations in each country. All administrative permissions to access the raw data used in the study were applied and granted in each country according to the European Union’s General Data Protection Regulation (article 30). In Denmark, the project was listed at the record of processing activities for research projects in the Central Denmark Region (J. No: 1–16-02–210-20). In Finland, the data were used in accordance with the Act on the National Institute of Health and Welfare (668/2008) and based on authorisations (VRK VRK/3059/2018–2 and THL/1081/6.02.00/2018) granted under the Act on Secondary Use of Health and Social Data (552/2019). In Iceland, the study was approved by The National Bioethics Committee of Iceland (VSN-20–204, VSN-20–204-V1, VSN-20–204-V2, VSN-20–204-V3), and in Norway, it was approved by the Regional Committee for Medical and Health Research Ethics South East A (reference code 17772). The analyses were performed using country-specific anonymised data. These summary data are available from the corresponding author upon reasonable request.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary table 1. Data details by country. Supplementary table 2. Regions of birth (non-Western immigrants) and country groupings.
Additional file 2:
Supplement figure 1. Adjusted breast cancer incidence and mortality rate ratios (RR) among non-Western immigrant women compared to native women. (Adjusted by attained age and calendar year. I² is the heterogeneity statistic and tau² is the variance of the effect size parameters across the studies). Supplement figure 2. Adjusted colorectal cancer incidence and mortality rate ratios (RR) among non-Western immigrant women compared to native women. (Adjusted by attained age and calendar year. I² is the heterogeneity statistic and tau² is the variance of the effect size parameters across the studies). Supplement figure 3. Adjusted lung cancer incidence and mortality rate ratios (RR) among non-Western immigrant women compared to native women. (Adjusted by attained age and calendar year. I² is the heterogeneity statistic and tau² is the variance of the effect size parameters across the studies).
Additional file 3.
Age−standardized (W) cancer incidence and mortality per 100 000 among female populations in Denmark, Finland, iceland and Norway in 2015–2019.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lamminmäki, M., Leivonen, A., Heinävaara, S. et al. A population-based cohort study on changes in breast, lung and colorectal cancer incidence and mortality among non-Western immigrant women. BMC Cancer 23, 665 (2023). https://doi.org/10.1186/s12885-023-11140-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11140-6