Abstract
Background
Patients with locally advanced irresectable or clinically node positive urothelial cancer (UC) have a poor outcome. Currently, these patients can only be cured by receiving induction chemotherapy and, if an adequate radiological response is obtained, radical surgical resection. Long-term survival, however, strongly depends on the absence of residual tumor in the surgical resection specimen, i.e. a pathological complete response (pCR). The reported pCR rate following induction chemotherapy in locally advanced or clinically node-positive UC is 15%. The 5-year overall survival rate for patients achieving a pCR is 70–80% versus 20% for patients who have residual disease or nodal metastases. This clearly demonstrates the unmet need to improve clinical outcome of these patients. Recently, the JAVELIN Bladder 100 study demonstrated an overall survival benefit of sequential chemo-immunotherapy in patients with metastatic UC. The CHASIT study aims to translate these findings to the induction setting by assessing the efficacy and safety of sequential chemo-immunotherapy in patients with locally advanced or clinically node-positive UC. In addition, patient biomaterials are collected to investigate biological mechanisms of response and resistance to chemo-immunotherapy.
Methods
This multicenter, prospective phase II clinical trial includes patients with stage cT4NxM0 or cTxN1-N3M0 UC of the bladder, upper urinary tract or urethra. Patients who do not experience disease progression after 3 or 4 cycles of platinum-based chemotherapy are eligible for inclusion. Included patients receive 3 cycles of anti-PD-1 immunotherapy with avelumab followed by radical surgery. Primary endpoint is the pCR rate. It is hypothesized that sequential chemo-immunotherapy results in a pCR rate of ≥ 30%. To obtain a power of 80%, 64 patients are screened and 58 patients are included in the efficacy analysis. Secondary endpoints are toxicity, postoperative surgical complications, progression-free, cancer-specific and overall survival at 24 months.
Discussion
This is the first study to assess the potential benefit of sequential chemo-immunotherapy in patients with locally advanced or node positive UC. If the primary endpoint of the CHASIT study is met, i.e. a pCR rate of ≥ 30%, a randomized controlled trial is foreseen to compare this new treatment regimen to standard care.
Trial registration
NCT05600127 at Clinicaltrials gov, registered on 31/10/2022.
Similar content being viewed by others
Background
Urothelial cancer (UC) is the 10th most common cancer worldwide [1]. The current standard of care for patients with non-metastatic muscle invasive bladder cancer (MIBC) is cisplatin-based neoadjuvant chemotherapy followed by removal of the bladder (radical cystectomy, RC) and pelvic lymph node dissection (LND) [2]. Patients with muscle invasive, locally advanced irresectable or clinically node-positive disease (stage cT4NxM0 or cTxN1-N3M0) have a poor prognosis. Due to the extent of affected lymph nodes, patients are ineligible to undergo surgery. However, curative treatment by radical resection is still possible, given that patients respond adequately to treatment with induction chemotherapy. The absence of residual tumor in the surgical resection specimen is defined as a pathological complete response (pCR), which corresponds with a good prognosis. Patients who achieve a pCR have an excellent outcome with reported 5-yr overall survival rates of 70–80% [3]. Nevertheless, only 15% of locally advanced irresectable or clinically node-positive patients achieve a pCR [3]. In contrast, patients with residual muscle invasive disease (≥ ypT2) or nodal metastases (> ypN0) have 5-yr overall survival rates of 15–20% [4]. Therefore, there is a clear unmet need to increase the pCR rate and improve outcome of patients with locally advanced or clinically node-positive MIBC.
Pre-operative immunotherapy for MIBC is currently investigated in several clinical trials, either as monotherapy or concurrent with chemotherapy [5,6,7,8,9]. Neoadjuvant monotherapy with three cycles pembrolizumab in patients with stage cT2-3bN0M0 UC (n = 50) resulted in a pCR of 42%, whereas after neoadjuvant treatment with atezolizumab, 31% of cT2-4N0M0 patients (n = 95) achieved a pCR [5, 6]. Trials which administer both chemotherapy and immunotherapy usually do this concurrently instead of sequentially. In the AURA trial, patients were enrolled in distinct cohorts according to their cisplatin eligibility. The cisplatin eligible patients were treated with avelumab combined with cisplatin-gemcitabine or ddMVAC. Treatment with cisplatin-gemcitabine resulted in a pCR rate of 32% (n = 9) compared to 43% (n = 12) after ddMVAC [10]. In the cisplatin ineligible cohort, patients received either paclitaxel-gemcitabine and avelumab or avelumab alone. After treatment with paclitaxel-gemcitabine and avelumab, 7% of patients (n = 2) achieved pCR, in contrast to 25% (n = 7) after treatment with avelumab only [11]. However, these trials merely focus on the neoadjuvant setting and include patients with localized and lymph-node negative MIBC only.
In patients with locally advanced irresectable or node-positive UC, treatment with ipilimumab and nivolumab resulted in a pCR of 46% at interim analysis [12]. The Javelin Bladder 100 trial reported promising results with the sequential treatment of chemo- and immunotherapy. Patients with locally advanced or metastatic MIBC who had at least stable disease after platinum-based systemic chemotherapy, were treated with avelumab maintenance therapy [13]. This resulted in prolonged median overall survival and progression free survival compared to best supportive care after 2 years follow-up (23.8 vs. 15.0 months and 5.5 vs. 2.1 months, respectively) [14]. The present study aims to translate these beneficial outcomes of sequential chemo-immunotherapy to patients with locally advanced or node-positive UC. This study is the first to assess sequential chemo-immunotherapy in the induction and non-metastatic setting of UC.
The CHASIT study aims to improve the pCR rate and clinical outcome in patients with locally advanced irresectable or node-positive UC of the bladder, upper urinary tract or urethra. Patients with at least stable disease after treatment with platinum-based induction chemotherapy will receive 3 cycles of avelumab, an anti-PD-L1 antibody, followed by radical surgery. We hypothesize that sequential chemo-immunotherapy is more effective in obtaining pCR compared to induction chemotherapy alone, which results in improved clinical outcome.
Methods
Clinical trial design
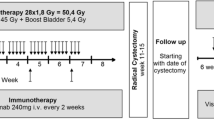
The CHASIT trial is a prospective, multicenter, non-randomized single arm phase II clinical trial. The following Dutch hospitals are participating: Erasmus Medical Center in Rotterdam, Amphia hospital in Breda, Radboud University Medical Center in Nijmegen and Jeroen Bosch hospital in Den Bosch. Patients with at least stable disease after treatment with platinum-based chemotherapy (gemcitabine + cisplatin, gemcitabine + carboplatin or (dd)MVAC) are eligible for inclusion and will be treated with avelumab. After avelumab treatment, response evaluation is performed with radiological imaging. In the absence of disease progression, patients are scheduled for surgical excision of the primary tumor and lymph nodes (Fig. 1). The study has been approved by the Medical Ethical Committee of the Erasmus University Medical Centre (MEC 2022 − 0257) on 10/10/2022, and has been registered at Clinicaltrials.gov (NCT05600127) on 31/10/2022.
Objectives and endpoints
The main objective of this study is to improve the pathological response rate with sequential chemo-immunotherapy. The primary endpoint is the pCR rate, defined as the proportion of patients without residual disease in the surgical resection specimen (ypT0N0, carcinoma in situ is allowed) in the intention-to-treat analysis. Secondary end points are progression-free, cancer-specific and overall survival at 24 months, calculated from the time of first administration of avelumab, safety and tolerability of avelumab, surgical complications within 30 and 90 days from the date of surgery, the rate of pathological non-invasive residual UC in the resection specimen (< ypT2N0) and the proportion of patients in whom radical surgery is delayed > 8 weeks after the last administration of avelumab due to immune-related toxicity. All adverse events are graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE version 5.0) [15]. Surgical complications are graded according to Clavien-Dindo classification system for surgical complications [16].
Patient population
Eligible patients must have histologically confirmed UC of the bladder, upper urinary tract or urethra, clinical stage cT4NxM0 or cTxN1-N3 M0, and had at least stable disease after a minimum of 3 or a maximum of 4 cycles of platinum-based induction chemotherapy. A maximum of 50% divergent differentiation of histological subtypes is permitted. Clinical staging is based upon bimanual examination under anesthesia, computerized tomography (CT) with or without Positron Emission Tomography (PET) scan. Resection of the disease with curative intent should be possible and is assessed at a multidisciplinary board meeting. Patients must be fit and willing to undergo surgery. WHO performance status 0–2 and adequate bone marrow, renal and liver function are required. Further details on inclusion and exclusion criteria can be found in Appendix 1.
Procedures
Screening and response evaluation before study entry
Induction chemotherapy should have been platinum-based (gemcitabine + cisplatin (gemcitabine 1000 mg/m2, day 1 and day 8, cisplatin 70 mg/m2, day 1, 3 week interval) or gemcitabine + carboplatin (gemcitabine 1000 mg/m2, day 1 and day 8, carboplatin AUC 4.5, day 1, 3 week interval) or (dd)MVAC (methotrexate 30 mg/m2, day 1, vinblastine 3 mg/m2, day 2, doxorubicin 30 mg/m2, day 2, and cisplatin 70 mg/m2, day 2, 2 week interval, with G-CSF), no other regimens are allowed. After 3 cycles of chemotherapy, treatment response is assessed radiologically by thoracoabdominal (PET-)CT-scan. Response evaluation is based on RECIST v1.1 criteria [17]. Patients are discussed at a multidisciplinary tumor board meeting. Patients who have at least stable disease and are eligible for study inclusion. Patients are screened for eligibility and undergo physical examination and laboratory tests at the outpatient department. They are asked to provide written informed consent for study participation. If eligible, subjects are scheduled for sequential treatment with avelumab and radical surgery thereafter.
Treatment with avelumab
Patients receive a maximum of 3 cycles avelumab every 2 weeks, provided that toxicity is acceptable. Avelumab is supplied by Merck (CrossRef Funder ID: https://doi.org/10.13039/100009945), as part of an alliance between Merck and Pfizer. The first administration should be within 2–4 weeks after day 21 of the last cycle of chemotherapy. Prior to each cycle, blood and urine is examined. Avelumab is given intravenously in 1 h at a dosage of 800 mg. Pre-medication is mandatory and is performed as per manufacturer’s recommendation to mitigate infusion-related reactions. Adverse events related to immunotherapy are to be managed according to local guidelines. Dose reduction for toxicity management is not permitted. In case of persisting toxicity, the next cycle of avelumab administration may be omitted until toxicity is resolved or decreased to at least Grade 1 in severity according to the NCI-CTCAE criteria [15]. A maximum dose interruption of 2 weeks is allowed. Toxicity management guidelines are provided and include actions such as decreasing the infusion rate, symptomatic treatment of symptoms or discontinuation of avelumab treatment, depending on the severity of toxicity.
Radical surgery
After the last cycle of avelumab, patients are re-staged by thoracoabdominal CT. If there are no signs of disease progression as per RECIST v1.1 criteria [17], patients are scheduled for radical surgery. Surgery must be performed within 4 to 8 weeks after day 14 of the last cycle of avelumab. Surgical procedures are dependent upon the location of the primary tumor: in case of UC of the bladder, radical surgery consists of an anterior exenteration (female) or radical cystoprostatectomy (male), in case of UC of the upper urinary tract a radical nephro-ureterectomy or a radical urethrectomy in case of UC of the urethra. All surgical procedures are combined with an extended LND: pelvic LND in case of UC of the bladder, retroperitoneal LND in case of UC of the upper urinary tract and inguinal LND for UC of the urethra. Surgery is performed by a urologist and the surgical technique is at the discretion of the treating physician and may include open, laparoscopic or robot-assisted surgery.
Pathological review
All formalin-fixed, paraffin-embedded (FFPE) tissue specimens are reviewed centrally by an experienced uropathologist (GvL). The presence of residual cancer cells is assessed, as well as tumor grade (WHO 1973 and 2004/2016), the presence and proportion of divergent differentiation and urothelial subtypes, lymphovascular invasion, concomitant CIS, surgical margins status, the number of resected lymph nodes and lymph node metastasis, as well as the presence of extranodal extension.
Follow-up
Patients who continued to surgery after completion of ≥ 1 cycle of avelumab will have regular follow-up visits every 3 months until 2 years after study inclusion, including blood withdrawal and radiological examination. In case of a recurrence or disease progression, a diagnostic confirmatory tissue biopsy is performed. In case of disease progression, all subsequent treatment modalities applied will be recorded until 2 years after surgery. In addition, included patients who did not receive avelumab will be followed as well as part of the intention-to-treat analysis.
Biomarker analysis
Exploratory studies are planned to investigate biological mechanisms of response and resistance to chemo-immunotherapy. Findings will be correlated to clinical outcome in order to identify predictive biomarkers that allow the selection of patients who benefit most likely from immunotherapy. Biopsy and surgical tumor specimens pre- and post-immunotherapy are collected, as well as in case of disease progression during follow-up. In addition, blood and urine samples are collected at baseline, during avelumab treatment, prior to surgery and during follow-up.
Statistical analysis
Historically, the pCR rate following induction chemotherapy in patients with cT4NxM0 or cTxN1-N3M0 UC is 15% [3]. We hypothesize that the pCR in the study is equal to or higher than 30%. A one sample proportion test is used to compare the historical rate to the study arm. Assuming an alpha of 2.5%, 53 patients are required to obtain a power of 80%. Assuming a dropout rate of 10% and a screening failure rate of 10%, 64 patients need to be screened.
An interim analysis to assess efficacy and safety is planned after inclusion of the first 40 patients (75% of the total accrual). If the pCR in the first 40 subjects is less than 15%, the study will terminate prematurely. A data safety monitoring committee (DSMB) was installed to ensure the safety of the participants and the validity and integrity of safety data generated from the study. The study is open for inclusion since December 2022 and the estimated final inclusion date is December 2024. All data will be prospectively collected.
Discussion
Maintenance therapy with avelumab has shown to be well-tolerated and beneficial for survival in metastatic MIBC patients who had at least stable disease after systemic chemotherapy [13, 14]. We aim to translate these findings to patients with locally advanced or clinically node-positive disease. This is the first trial to assess the efficacy of sequential chemo-immunotherapy in this group of patients. We aim to test the hypothesis that sequential treatment with chemo-immunotherapy is effective, safe and does not cause a delay in surgical treatment. In addition, collected biomaterials will provide a basis for future exploratory studies on possible biomarkers of response and resistance to chemo-immunotherapy. The findings of this trial will contribute to understanding, improving and predicting clinical outcome for patients with locally advanced or node-positive UC.
Chemotherapy in the neoadjuvant setting is effective to obtain a pathological complete response. Neoadjuvant treatment of cT2-T4aN0M0 patients with MVAC increased the pCR rate from 15% after cystectomy alone to 38% [18]. Neo-adjuvant treatment with ddMVAC resulted in higher pCR rates and longer 3-year progression-free survival compared to neoadjuvant cisplatin and gemcitabine treatment in cT2-T4a patients (42% vs. 36% and 66% vs. 56%, respectively) [19]. In cT3-T4a patients, 37% of patients (n = 1013) achieved complete pathological downstaging to non-muscle invasive disease (≤(y)pT1N0) after neoadjuvant chemotherapy (20). The CHASIT study, however, includes patients with more advanced disease, representing a different patient population than the neoadjuvant setting. Therefore we expect that pCR rates will be lower compared to the neoadjuvant setting. Literature on this specific and relatively rare population is limited. In a cohort of 52 patients with histologically proven cN1-3 disease, 29% of patients achieved pCR after treatment with MVAC (21). This high pCR rate in lymph node-positive patients can be explained due to the inclusion of 11 patients with cN1 disease: these patients formally belong to the neoadjuvant setting (instead of induction setting). They are expected to respond better to NAC compared to cN2-3 patients, which causes an elevated pCR rate. There was no pCR rate reported for the cN2-3 subgroup (21). A larger cohort of patients with cT1N1-3M0 or cT2-4aN0-3M0 disease (n = 304) was treated with various regimens of induction chemotherapy: MVAC, gemcitabine + cisplatin or other regimens (gemcitabine + carboplatin, other carboplatin based regimens and taxanes). After a median of 4 cycles administered, the reported pCR rate was 15% [3]. Due to the limited number of other studies available, this study was selected as a historical cohort. The CHASIT study aims to translate the findings of the Javelin Bladder 100 trial and therefore only patients with response or stable disease after treatment with platinum-based chemotherapy are eligible for inclusion. In the Javelin Bladder 100 trial, patients received 4 to 6 cycles of chemotherapy and switched thereafter to avelumab maintenance therapy. In the CHASIT study, the last 2 cycles of chemotherapy are replaced by 3 cycles of immunotherapy.
Although ideally a randomized controlled trial (RCT) is the preferred study design, an RCT would necessitate a large number of subjects to be included, making it logistically and financially challenging to conduct this trial. This does not seem feasible on a national level. Therefore, we decided to conduct a single arm phase II study. If the CHASIT study shows that sequential chemo-immunotherapy leads to a pCR rate of ≥ 30%, an international randomized controlled trial is foreseen to compare this new treatment regimen to standard care.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available due to the fact that data sill have to be generated and the current manuscript contains the study protocol only. Once data collection has finished, data will be available from the corresponding author on reasonable request.
Abbreviations
- CT:
-
Computerized tomography
- DSMB:
-
Data safety monitoring board
- FFPE:
-
Formalin-fixed, paraffin-embedded
- MIBC:
-
Muscle-invasive bladder cancer
- pCR:
-
Pathological complete response
- LND:
-
Lymph node dissection
- PET:
-
Positron emission tomography
- RC:
-
Radical cystectomy
- RCT:
-
Randomized controlled trial
- UC:
-
Urothelial carcinoma
References
International WCRF, Bladder cancer statistics: World Cancer Research Fund International. ; 2020 [Available from: https://www.wcrf.org/cancer-trends/bladder-cancer-statistics/.
Witjes JA, Bruins HM, Carrión A, Cathomas R, Compérat EM, Efstathiou JA, et al. EAU Guidelines on muscle-invasive and metastatic bladder cancer. European Association of Urology; 2022.
Zargar-Shoshtari K, Zargar H, Lotan Y, Shah JB, van Rhijn BW, Daneshmand S, et al. A multi-institutional analysis of outcomes of patients with clinically node positive urothelial bladder cancer treated with induction chemotherapy and radical cystectomy. J Urol. 2016;195:53–9.
Meijer RP, Mertens LS, van Rhijn BW, Bex A, van der Poel HG, Meinhardt W, et al. Induction chemotherapy followed by surgery in node positive bladder cancer. Urology. 2014;83(1):134–9.
Necchi A, Anichini A, Raggi D, Briganti A, Massa S, Luciano R, et al. Pembrolizumab as Neoadjuvant Therapy before Radical Cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an Open-Label, Single-Arm, phase II study. J Clin Oncol. 2018;36(34):3353–60.
Powles T, Kockx M, Rodriguez-Vida A, Duran I, Crabb SJ, Van Der Heijden MS, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med. 2019;25(11):1706–14.
Galsky MD, Hoimes CJ, Necchi A, Shore N, Witjes JA, Steinberg G, et al. Perioperative pembrolizumab therapy in muscle-invasive bladder cancer: phase III KEYNOTE-866 and KEYNOTE-905/EV-303. Future Oncol. 2021;17(24):3137–50.
Martinez Chanza N, Soukane L, Barthelemy P, Carnot A, Gil T, Casert V, et al. Avelumab as neoadjuvant therapy in patients with urothelial non-metastatic muscle invasive bladder cancer: a multicenter, randomized, non-comparative, phase II study (oncodistinct 004 - AURA trial). BMC Cancer. 2021;21(1):1292–300.
Powles T, Meeks JJ, Galsky MD, Van Der Heijden MS, Nishiyama H, Al-Ahmadie HA et al. A phase III, randomized, open-label, multicenter, global study of efficacy and safety of durvalumab in combination with gemcitabine plus cisplatin for neoadjuvant treatment followed by durvalumab alone for adjuvant treatment in muscle-invasive bladder cancer (NIAGARA). 2021 Genitourinary Cancers Symposium: Journal of Clinical Oncology; 2021.
Martinez Chanza N. Avelumab as the basis of neoadjuvant chemotherapy regimen in platinum eligible and ineligible patients with non-metastatic muscle invasive bladder cancer: AURA trial. In: Klaassen ZM, MSc., editor. European Society for Medical Oncology (ESMO); Sep 16 - Sep 21, 2021; Lugano, Switzerland: UroToday; 2021.
Martinez Chanza N. Avelumab as the basis of neoadjuvant regimen in platinum-eligible and -ineligible patients with nonmetastatic muscle-invasive bladder cancer: AURA (Oncodistinct-004) trial. In: Wallis CJD, editor. American Society of Clinical Oncology (ASCO); June 3 - June 7, 2022. Chicago: UroToday; 2022.
van Dijk N, Gil-Jimenez A, Silina K, Hendricksen K, Smit LA, de Feijter JM, et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat Med. 2020;26(12):1839–44.
Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2020;383(13):1218–30.
Powles T, Park SH, Voog E, Caserta C, Pérez-Valderrama B, Gurney H et al. Avelumab first-line (1L) maintenance for advanced urothelial carcinoma (UC): Long-term follow-up results from the JAVELIN Bladder 100 trial. ASCO Genitourinary Cancers Symposium; February 16, 2022; Chicago: Journal of Clinical Oncology; 2022. p. 487.
Institute NC, Common Terminology Criteria for Adverse Events (CTCAE). : National Cancer Institute; 2022 [updated 04-19-2021. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Eisenhauer 1 EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66.
Pfister C, Gravis G, Fléchon A, Chevreau C, Mahameddi H, Laguerre B, Dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin or gemcitabine and cisplatin as perioperative chemotherapy forpatients with nonmetastatic muscle-invasive bladder cancer: results of the GETUG-AFU V05 VESPER Trial. J Clin Oncol., Hermans TJN, Voskuilen CS, Deelen M, Mertens LS, Horenblas S, Meijer RP, Superior efficacy of neoadjuvant chemotherapy and radical cystectomy in cT3-4aN0M0 compared to cT2N0M0 bladder cancer. Int J Cancer. 2019;144(6):1453 – 9. 21. Nieuwenhuijzen JA, Bex A, Meinhardt W, Kerst JM, Schornagel JH et al. H VANT, Neoadjuvant methotrexate, vinblastine, doxorubicin and cisplatin for histologically proven lymph node positive bladder cancer. J Urol. 2005;174(1):80 – 5.
Acknowledgements
In each participating center, the principal investigator will be responsible for recruitment, adherence to the study protocol and follow-up of the included patients. Members of the CHASIT study group:
Dr. A.G. van der Heijden, urologist, Radboud University Medical Center, Nijmegen.
Dr. N. Mehra, medical oncologist, Radboud University Medical Center, Nijmegen.
Dr. J.A.P. Leijte, urologist, Amphia Hospital, Breda.
Dr. H.M. Westgeest, medical oncologist, Amphia Hospital, Breda.
Dr. T.J. Smilde, medical oncologist, Jeroen Bosch Hospital, ‘s Hertogenbosch.
Dr. S. van der Meer, urologist, Jeroen Bosch Hospital, ‘s Hertogenbosch.
Funding
The CHASIT study is financially supported by Merck (CrossRef Funder ID: https://doi.org/10.13039/100009945), as part of an alliance between Merck and Pfizer. The funding body has been involved in the study design and selection of primary and secondary outcomes. The funding body has no role in data collection, data analysis nor data interpretation. Merck is the manufacturer of the study drug avelumab. Merck (CrossRef Funder ID: https://doi.org/10.13039/100009945) and Pfizer reviewed this manuscript for medical accuracy only before journal submission. The authors are fully responsible for the content of this manuscript, and the views and opinions described in the publication reflect solely those of the authors.
Author information
Authors and Affiliations
Contributions
VR participated in the study design, is the study coordinator and drafted the manuscript. YS participated in the study design and is study coordinator. DR, RW, TZ and JB initiated the trial and participated in the study design. GvL, TZ and JB critically revised and supervised drafting of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
JB performed consultancy work for BMS, Janssen, Astellas, Merck and Pfizer of which all financial rewards were attributed to the Erasmus Medical Center. In addition, JB was involved in research agreements for MSD and Janssen. TZ has been employed on project basis for Janssen Pharmaceuticals. The remaining authors have no competing interests to declare.
Ethics approval and consent to participate
The study has been approved by the Medical Ethical Committee of the Erasmus University Medical Centre (MEC 2022 − 0257) on 10/10/2022 and has been registered at Clinicaltrials.gov (NCT05600127) on 31/10/2022. The study will be conducted according to the principles of the Declaration of Helsinki (10th version, Portaleza, 2013) and will be in accordance with the Dutch Medical Research Involving Human Subjects Act (WMO). Informed consent to participate in the study is obtained from all participants. Individual patient data will not be published.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rutten, V.C., Salhi, Y., Robbrecht, G.J. et al. The CHASIT study: sequential chemo-immunotherapy in patients with locally advanced urothelial cancer – a non-randomized phase II clinical trial. BMC Cancer 23, 539 (2023). https://doi.org/10.1186/s12885-023-10963-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-10963-7