Abstract
Background
Women with inherited mutations in the BRCA1 or BRCA2 genes have increased lifetime risks for developing breast and/or ovarian cancer and may develop these cancers around the age of 30 years. Therefore, prevention of breast and ovarian cancer in these women may need to start relatively early in life. In this study we systematically evaluate the long-term effectiveness and cost effectiveness of different prevention strategies for breast and ovarian cancer in women with BRCA-1/2 mutation in Germany.
Methods
A decision-analytic Markov model simulating lifetime breast and ovarian cancer development in BRCA-1/2 carriers was developed. Different strategies including intensified surveillance (IS), prophylactic bilateral mastectomy (PBM), and prophylactic bilateral salpingo-oophorectomy (PBSO) alone or in combination at different ages were evaluated. German clinical, epidemiological, and economic (in 2022 Euro) data were used. Outcomes included cancer incidences, mortality, life years (LYs), quality-adjusted life years (QALYs), and discounted incremental cost-effectiveness ratios (ICER). We adopted the German health-care system perspective and discounted costs and health effects with 3% annually.
Results
All intervention strategies are more effective and less costly than IS alone. Prevention with PBM plus PBSO at age 30 maximizes life expectancy with 6.3 LYs gained, whereas PBM at age 30 with delayed PBSO at age 35 improves quality of life with 11.1 QALYs gained, when compared to IS alone. A further delay of PBSO was associated with lower effectiveness. Both strategies are cost effective with ICERs significantly below 10,000 EUR/LYG or QALY.
Conclusion
Based on our results, PBM at age 30 plus PBSO between age 30 and 40 prolongs life and is cost effective in women with BRCA-1/2 mutations in Germany. Serial preventive surgeries with delayed PBSO potentially improve quality of life for women. However, delaying PBM and/or PBSO further may lead to increased mortality and reduced QALYs.
Similar content being viewed by others
Introduction
Women who have inherited mutations in the BRCA1 or BRCA2 genes (BRCA-1/2) have substantially elevated lifetime risks for developing breast (80–90% lifetime risk) and/or ovarian cancer (18–40% lifetime risk) [1]. BRCA-1/2 mutation carriers develop breast and ovarian cancer on average 20 years earlier than non-mutation carriers [2].
In Germany, various options for early detection and prevention of breast and ovarian cancer are available for mutation carriers [3]. Currently recommended is intensified surveillance (IS) of the breast, which includes palpation, ultrasound, mammography and magnet resonance imaging (MRI) [3]. Another option is risk-reducing surgery such as prophylactic bilateral mastectomy (PBM) and/or prophylactic bilateral salpingo-oophorectomy (PBSO). PBM is estimated to decrease the risk for developing breast cancer by over 95% [4, 5]. PBSO is estimated to reduce the risk for ovarian cancer by over 90% [6, 7] and the risk for breast cancer by 45% [8].
Despite their potential benefits, all of these options may have negative consequences for the women, and it remains unclear which combination or sequence of preventive interventions at which age may be optimal. In order to make an informed and evidence-based decision on the optimal option, all consequences (i.e., benefits, harms and costs) have to be weighed against each other. Decision-analytic models are commonly used to handle a decision problem of such complex nature using explicit and quantitative methods to identify the optimal options based on utilitarianism [9,10,11].
Thus, the objective of this study was to develop and apply a decision-analytic model for the evaluation of the long-term effectiveness and cost-effectiveness of different strategies to prevent breast and ovarian cancer in women with BRCA-1/2 mutations in Germany.
Methods
Decision-analytic model
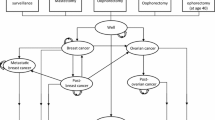
A decision-analytic Markov state-transition model simulating the natural history of breast and ovarian cancer in women with BRCA-1/2 gene mutations over a lifelong time horizon was developed (Fig. 1). In a state-transition model a decision problem is conceptualized in terms of a set of (health) states and transitions among these states [12]. A state-transition model was chosen because the natural history of disease can be well described by health states and transitions over time [12]. Since this decision problem can be represented with a manageable number of health states incorporating all characteristics relevant to the decision problem, including the relevant history, a cohort simulation was chosen [12]. In this model, a hypothetical cohort of women moves in annual cycles through different health states over a lifetime (starting at birth) based on stage-specific breast or ovarian cancer occurrence, cancer detection and treatment history (Fig. 1).
Illustration of the decision-analytic model. Health states representing the natural history of breast and ovarian cancer represented in the Markov model are shown as bubbles: No breast and ovarian cancer (well), undetected invasive breast cancer stage I to stage IV, diagnosed invasive breast cancer stage I to stage IV (pT1- pT4), breast cancer survivors 10 years after initial breast cancer diagnosis and treatment (breast cancer survivor), death from breast cancer (breast cancer death), undetected invasive ovarian cancer stage I to stage IV, diagnosed invasive ovarian cancer stage I to stage IV (FIGO states I–IV), ovarian cancer survivors 10 years after ovarian cancer diagnosis and treatment (ovarian cancer survivor), death from ovarian cancer (ovarian cancer death), and death from other causes (death other causes). Progression from one health state to the other is indicated with solid arrows and remaining in the same health state with curved arrows
Women may remain in the same health state, progress to another health state, may survive cancer or die from cancer or from other causes. We assumed that once detected with cancer, women are treated according to German treatment guidelines [3, 6]. Women initially diagnosed with cancer and treated, may die from ovarian or breast cancer as a function of stage-specific survival rates for each cancer. Women diagnosed with cancer and surviving ten years after initial diagnosis are moving to a cancer survivor health state with a similar mortality risk as the general female population [13]. Recurrent cancer is not modeled explicitly, it is assumed to be included for each cancer state based on survival data linked to initial stage at diagnosis. In all health states women may die from other causes than cancer with age- and gender-specific mortality.
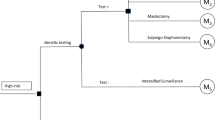
Compared strategies
Overall, we compared 16 different strategies for breast and ovarian cancer prevention in women with BRCA-1/2 gene mutations. Strategies were based on current German recommendations [3, 6]. These recommendations state that women with BRCA-1/2 gene mutations should receive IS for breast cancer as standard care [3] including half-yearly breast palpation plus ultrasound and yearly MRI for the breast plus mammography starting at age 30 [3]. For the detection of ovarian cancer, no IS is recommended [14,15,16]. The German guideline further suggests offering women PBM as of age 30 [3] and laparoscopic PBSO as of age 40 or when childbearing is completed [6]. We considered the following prevention strategies, which consisted of different single or combined PBM and/or PBSO strategies with different order and age at intervention, and assessed the effects for women who are participating and adhering to these strategies including intensified surveillance: (1) Standard care (IS for breast cancer), (2) PBM at age 30, (3) PBM at age 35, (4) PBM at age 40, (5) PBSO at age 30, (6) PBSO at age 35, (7) PBSO at age 40, (8) PBM plus PBSO at age 30, (9) PBM plus PBSO at age 35, (10) PBM plus PBSO at age 40, (11) PBM at age 30 plus PBSO at age 35, (12) PBM at age 30 plus PBSO at age 40, (13) PBM at age 35 plus PBSO at age 40, (14) PBM at age 35 plus PBSO at age 45, (15) PBM at age 40 plus PBSO at age 45, (16) PBSO at age 35 plus PBM at age 40.
Model parameters
Annual transition probabilities along with the effectiveness of different strategies used to populate the model are summarized in Table 1. Disease progression parameters of breast and ovarian cancer were calibrated in a systematic and hierarchical fashion to fit age-and BRCA-1/2-specific breast and ovarian cancer incidences, derived from literature [2], and stage distribution of breast and ovarian cancer from a German cancer registry (MCR - Munich Cancer Registry) [17, 18]. Details on calibration methods and data used for calibration are summarized in Additional File 1. Stage-specific annual breast and ovarian cancer mortality rates were based on original data from the MCR for the years 1998–2015 [17, 18]. Based on these data, five-year disease specific survival rates for breast cancer stage pT1, pT2, pT3 and pT4 were 99.3%, 87.6%, 59.5%, and 26.2%, respectively [17]; for ovarian cancer FIGO stage I–IV these rates were 87.1%, 70.4%, 35.0%, and 15.5%, respectively [18]. In the model, women could die from causes other than breast and ovarian cancer according to German age-specific all-cause mortality rates for females using German life tables from the German Federal Statistical Office (DESTATIS) [13]. Since all-cause mortality rates from German life tables include females dying of breast or ovarian cancer, background mortality was adjusted for age-specific cancer-related mortality. Age-and cancer-specific mortality rates were derived from the Robert Koch Institute (RKI) [19].
We used quality-of-life indices (“utilities”) to weight life years with the specific health-related quality of life women experience in different health states. Utilities range from 0 (i.e., reflecting death) to 1 (reflecting best possible health) [20]. Utilities were derived from literature and included stage-specific utility values for diagnosed cancer patients with BRCA-1/2 mutations and utility values after prophylactic surgery (Table 2). Utility for undetected cancer was approximated using data from literature (see Additional file 2). We assumed the utility of (healthy) mutation carriers to be reduced due to prophylactic surgery (Table 2) and we assumed the women’s health-related quality of life to increase after prophylactic surgery to regain the utility of the women’s preceding health state in the subsequent year. This was implemented through a relative disutility applied to the base utility of the current health state.
German direct medical cost data were derived from published literature including costs for IS, surgical procedures, medications, and other treatment procedures [21]. All costs were converted to the target year (2022) using the Gross Domestic Product deflator index (GDPD values) [22]. All women received continued intensified breast cancer surveillance at an average cost of €608 per year [21]. We assumed that women undergoing PBM (with or without PBSO) incur only half of these costs as MRI is excluded from surveillance in these cases. Estimates of prophylactic surgical costs were based on the published literature [21].
For drug costs, we separated non-advanced (FIGO I + II) from advanced (FIGO III + IV) ovarian cancer and non-metastatic (stage pT1-3) from metastatic breast cancer (stage pT4). Overall, proportions of women with non-metastatic breast cancer receiving adjuvant radiotherapy, chemotherapy, and endocrine therapy were based on data from the German Consortium for Hereditary Breast and Ovarian Cancer database derived by Müller et al. 2018 [21]. For women with metastatic breast cancer cost data were obtained from literature [21]. The chemotherapeutic regimens most frequently prescribed in Germany were assumed to be equally distributed among women. Breast cancer drug costs were estimated for specific cancer type subgroups. Both breast and ovarian cancer therapy costs for initial treatment (i.e., surgical, chemotherapy, medication, and other treatment costs) were implemented as one-time costs at cancer diagnosis. Women were assumed to receive follow-up treatment for ten and five years after initial treatment for breast and ovarian cancer, respectively [3, 6]. Annual costs for follow-up treatment include IS as well as treatment of recurrent cancer. Recurrent cancer was assumed to be treated at the same costs as initial cancer treatment. Recurrent cancer was assumed to be included for each cancer state based on survival data linked to initial stage at diagnosis. Costs of palliative care were considered for all women dying from metastatic breast or ovarian cancer. Aggregated one-time initial treatment and palliative care costs as well as aggregated annual follow-up costs (in 2022€) are summarized in Table 3.
Statistical analyses
Base-case analysis
We performed a deterministic cohort simulation over a lifelong time horizon starting the evaluation at 30 years to predict the following outcomes: reduction in breast and ovarian cancer incidence (in %) and cancer-specific mortality (in %), undiscounted life expectancy (in life years (LYs)), undiscounted quality-adjusted life years (QALYs), total lifetime costs, discounted incremental cost-effectiveness ratios (ICER) expressed in Euros (€) per life-year gained (LYG) and discounted incremental cost-utility ratios (ICUR) in Euros (€) per QALY gained. As a point of reference, a willingness-to-pay threshold (WTP) of 90,000 €/LYG or QALY was assumed [23]. We adopted the German health-care system perspective and discounted costs and health effects by 3% annually in the cost-effectiveness analyses [24]. Strategies are considered dominated if they provide less health benefit at higher costs when compared to any other strategy. As dominated strategies should not be considered by decision makers, they were eliminated from the calculation of cost-effectiveness ratios. Furthermore, extended dominance was applied to eliminate strategies, for which costs and benefits are dominated by a mix of two other alternatives [25]. We used an efficiency frontier approach to assess and visualize the trade-off between benefits and costs [24, 26]. This approach excludes strategies that have a smaller benefit and greater cost than any other (combination of) strategy due to dominance. The curve connecting all non-dominated strategies is called the efficiency frontier. Comparisons are made in a stepwise fashion comparing each strategy with the next effective strategy on the efficiency frontier using ICERs and ICURs. The model was programmed in TreeAge Pro Version 2023 [27].
Sensitivity analyses
Deterministic one-way sensitivity analyses were performed on utilities, intervention effect measures, costs and the discount rate to estimate the uncertainty surrounding model assumptions and input parameters and to assess the robustness of the results [28]. Utilities and effect measures were varied between a 20% increase and reduction, the discount rate was varied from 1 to 10% and costs were varied between 50% and 200% of the base case value. Percentages in relation to base case value were used for sensitivity analyses as there were no predefined ranges of values for the different model parameters available from literature.
Model validation
The model was validated on four levels: [1] technical verification for face validity, [2] internal validation (e.g., debugging, consistency and plausibility checks), [3] cross-model validation and [4] external validation with historical data [29].
All methods were performed in accordance with relevant guidelines which are referred to throughout the methods section.
Results
Model validation
Internal validation showed that the model predictions were consistent with epidemiological data used in the model. In cross-model and external validations, the model compared well with other published models and to historical BRCA-1/2 specific data not used to populate the model. The model-predicted risk for developing breast cancer until the age of 70 years was 68%, and thus comparable to observed data for BRCA-1/2 mutation carriers provided by the literature ranging between 57% [2] and 84% [30, 31]. The model-predicted ovarian cancer risk until the age of 70 years was 21%, which compares, although slightly lower, still well with observed data from one study [2] with 28% (CI: 11–36%). For both cancer types, the model predicted a risk for developing cancer of 90% until the age of 70 years, which is similar to an estimate of 92% by Easton et al. [30] and Ford et al. [31]
Clinical effectiveness
All intervention strategies were more effective than IS. Over a lifetime, for the different evaluated prevention strategies, the decision analysis resulted in a relative risk reduction for developing breast cancer between 23.0 and 85.3% and for dying from breast cancer between 25.9 and 86.8% when compared to standard care (i.e., IS for breast cancer). Compared to standard care, the predicted life-time relative risk reduction for ovarian cancer ranged from 8.6 to 81.8% and the predicted reduction in ovarian cancer mortality ranged from 12.9 to 83.3%. For both cancer types, these benefits were highest for women undergoing PBM plus PBSO at age 30.
For the different prevention strategies, the average gain in undiscounted life expectancy and quality-adjusted life expectancy was 2.0–6.3 LY and 3.6–11.1 QALYs, respectively, when compared to standard care.
Compared to standard of care, PBM alone at age 30 yielded 2.8 LYG (4.9 QALYs) gained and PBSO alone at age 30 yielded 2.5 LYG (4.4 QALYs) gained. In contrast, a combined PBM plus PBSO at age 30 yielded 6.3 LYG and was the most effective strategy in terms of life expectancy. PBM at age 30 and delayed PBSO at age 35 yielded 11.1 QALYs and was the most effective strategy in terms of QALYs. Delaying the first surgery and/or delaying the second surgery by more than 5 years resulted in a reduction in life expectancy and QALYs. Detailed results on benefits in terms of life-years and QALYs gained for each evaluated strategy are presented in Fig. 2.
Clinical effectiveness in undiscounted incremental life years (LYs) and undiscounted incremental quality-adjusted life years (QALYs) compared to standard care (intensified surveillance for breast cancer). incr.: incremental; LYs: Life years; PBM: Prophylactic bilateral mastectomy; PBSO: Prophylactic bilateral salpingo-oophorectomy; QALYs: quality-adjusted life years; y: years of age
Cost effectiveness
All intervention strategies were more effective and less costly than IS alone. Combined strategies with PBM at age 30 (i.e., PBM at age 30 plus delayed PBSO) dominated all other strategies. A serial combination of PBM at age 30 and delayed PBSO at age 40 was the least costly strategy at total discounted costs of €8,760 and more effective than standard care (intensified surveillance), thus being the reference strategy (Fig. 3A, B). Considering life expectancy only, moving from PBM at age 30 plus PBSO at age 40 to the next more effective strategy PBM at age 30 plus PBSO at age 35 yielded an ICER of 2,912 €/LYG. Moving from PBM at age 30 plus PBSO at age 35 to PBM plus PBSO at age 30 yielded an ICER of 9,100 €/LYG (Fig. 3A). Considering women’s quality of life in terms of QALYs gained, PBM plus PBSO at age 30 was dominated by strategies with delayed PBSO (Fig. 3B). Among the non-dominated strategies, PBM at age 30 plus PBSO at age 40 was the least costly strategy. Moving from this strategy to the next more effective strategy PBM at age 30 plus PBSO at age 35 yielded an ICUR of 761 €/QALY gained (Fig. 3B).
Cost-effectiveness plane of different preventive strategies:(A) discounted total life-time costs (in €) versus effectiveness expressed in discounted total life years (LYs) and (B) discounted total life-time costs (in €) versus discounted total quality-adjusted life years (QALYs). The respective stepwise ICER (in €/LY) and ICUR (in €/QALY) on the efficiency frontier (blue line) are shown in boxes. Reference strategy: PBM at age 30 and PBSO at age 40 (S12)
PBM: Prophylactic bilateral mastectomy; PBSO: Prophylactic bilateral salpingo-oophorectomy; y: years of age
Sensitivity analyses
In all sensitivity analyses, model results were robust against variations in costs, utilities, and effect measures with a stable rank order of evaluated strategies. Model results were sensitive regarding variations in the annual discount rate only. For a low discount rate (1%) the strategy PBM at age 30 plus a PBSO at age 40 was dominated by PBM at age 30 plus PBSO at age 35. With an annual discount rate of 10% several strategies became undominated with standard care being the new reference strategy. Results of the sensitivity analyses are summarized in additional file 3 (Table S2 A: Summary of sensitivity analysis results: Incremental cost-effectiveness ratios compared to the next non-dominated strategy, Table S2 B: Summary of Sensitivity analysis results: incremental cost – utility ratios compared to the next non-dominated strategy).
Discussion
Our model-based results suggest that PBM plus PBSO at age 30 is highly effective regarding life years, but serial PBM at age 30 with delayed PBSO between age 35 and 40 is most effective considering QALYs for women with BRCA-1/2 mutations in Germany. Also, PBM at age 30 followed by delayed PBSO at age 35 or 40 can be considered highly cost effective when applying a recently published model-based WTP threshold for Germany [23]. Thus, our findings support current recommendations of the German guidelines regarding age and type of prophylactic surgery [3, 6].
The reduced costs for strategies with PBM at age 30 can be explained by a reduction in costs for intensified surveillance after surgical removal of the breasts; a MRI is no longer needed in these women. Thus, the earlier a bilateral mastectomy is conducted, the lower are the costs for the respective strategy. Moreover, cancer risk is reduced drastically at an early age, resulting in lower cancer treatment costs for the remaining lifetime. Regarding QALYs, only serial strategies were not dominated. This suggests that when taking into account health-related quality of life, an early PBSO may not be the best option for a woman. For woman’s life an early PBSO implies that women are put into artificial menopause and lose their ability to have children at a relatively young age. Importantly, our decision analyses show that IS for breast cancer alone was the most costly and the least effective strategy. Naturally, this “strategy” includes women who have not yet decided for or against prophylactic surgery. In order to prevent cancer effectively and in time, a serial prophylactic surgery should be offered and discussed in a patient-shared decision-making setting as an option to such women along with explicitly communicating the potential losses associated with deferring the decision.
While there are some studies that have evaluated the cost-effectiveness of genetic testing, screening and/or prevention in women with elevated risk for breast and/or ovarian cancer, for example, Ashkenazi Jewish women [32] and women with BCRA-1/2 mutation [33, 34] (for a recent review see Sroczynski et al. 2020 [35]), there are currently, only two other studies that have evaluated the cost-effectiveness of different prevention strategies in BRCA-1/2 mutation carriers for the German health care context [21]. While Müller et al. 2019 [36] focus on the cost-effectiveness of genetic testing for identifying BRCA-1/2 mutation carriers followed by different prevention strategies compared to no genetic testing, Müller et al. 2018 [21] evaluate similar strategies as our study. In contrast to Müller et al. 2018 [21], our results suggest a serial strategy of PBM at 30 years followed by delayed PBSO to be cost effective, whereas in Müller et al. 2018 [21] this strategy was dominated by a non-serial combination of PBM and PBSO at age 30. The strength of our analysis is that we included a wider age range of serial combinations of PBM and PBSO. A consideration of a wider range of serial combinations is crucial as it firstly reflects the current recommendations of the German guidelines and secondly and most importantly the findings of this study show that a delayed PBSO not only suggests having a positive impact on the quality of life of women but also to be a cost-effective option for women. In contrast to Müller et al. 2018 [21], our model considers different cancer stages in more detail and therefore accounts for differences in stage-specific survival rates, utilities, and costs. In addition, modeling undetected and detected cancer and calibrating to the reported age- and stage-specific incidences, allows for detailed clinical analysis including risk and mortality reduction.
Clearly, a decision for undergoing prophylactic surgery may have a huge impact on the woman’s quality of life. This is reflected in the results of our decision analysis. As suggested by our results, the time point of the second prophylactic surgery might have an influence on the quality of life of the remaining lifetime of women. In general, however, a decision on whether to undergo surgery or not in the first place depends on a woman’s individual characteristics such as her familial and personal situation, whether her family planning is completed as well as her individual utilities, level of anxiety and risk attitude. Both prophylactic surgery and IS have positive and negative short- and long-term consequences for women. In our model, we implemented short-term consequences of the surgery itself by quality-of-life reduction (disutility) due to surgery and assumed women to recover from surgery in the following years. Potential long-lasting consequences are not implemented in our model, as their time scale and intensity are unknown. The strategy IS is less invasive than prophylactic surgery, but the least effective option based on our results.
From a health care or decision maker’s perspective, a new medical intervention should have an additional benefit compared to the current standard; with an acceptable benefit-harm relation and cost-effectiveness relation. According to the results of our decision-analytic modeling study, the best option for BRCA-1/2 mutation carriers is a PBM at the age of 30 followed by delayed PBSO between age 35 and 40, as this is both effective with an acceptable benefit-harm relation considering QALYs and cost effective compared to other strategies.
Strengths of our study include the fact that we developed and applied a validated decision-analytic model, which systematically synthesizes current evidence and state of knowledge of breast and ovarian cancer prevention in German women with BRCA-1/2 mutations. Compared to existing models, we included a wider range of serial strategies, explicitly modelled cancer stage, and distinguished detected from undetected cancer. We validated the model against observed epidemiological data from German cancer registries to make it applicable to the German health care context. Finally, our model is flexible and can be adapted and used to answer future research questions of similar kind.
As all modeling studies, our study rests on assumption and has several limitations. The vast majority of the limitations are due to the lack of available data. First, the decision model does not consider heterogeneity of the population with respect to different BRCA mutation types, as BRCA-mutation type specific epidemiological data were not available for all required parts of the model. Literature reports for women with an inherited BRCA1 mutation a lifetime risk for breast cancer of 65–80% and 37–62% lifetime risk for developing ovarian cancer, while it reports for BRCA2 mutation carriers a lifetime risk of 45–85% for breast cancer and 11–23% for ovarian cancer [37]. Second, we assumed that stage-specific survival rates do not differ between mutation carriers and non-carriers, as stage-specific treatment procedures are the same. However, there is some evidence that cancer biology is different in BRCA1 carriers compared to non-carriers, with BCRA1 carriers having lower [38] or higher [39] and BRCA2 carriers having higher [40] survival rates, which would result in an over- or under-estimation of the ICER, respectively. But evidence on this topic is still very scarce. Stage-specific survival rates for breast and ovarian cancer in mutation carriers would be necessary to populate the model. Third, we assumed stage distributions for breast and ovarian cancer to be the same as in non-mutation carriers. However, mutation carriers develop cancer at an earlier age [2] compared to non-carriers. Whether this also affects the stage distribution is unknown. Fourth, since information on the proportion, frequency, and costs of stage-specific treatment options of breast and ovarian cancer in Germany is scarce, we assumed breast cancer in stage 1–3 to be treated similarly at similar costs and distinguished these from metastatic breast cancer only. Due to the lack of detailed costs data for ovarian cancer management we distinguished non-advanced from advanced ovarian cancer only. Although treatment resources likely differ between different cancer stages in sensitivity analyses results were shown to be robust. Fifth, we used utility estimates that are based on two different methods (standard gamble [SG] and time trade-off [TTO]) because not all utility estimates were available based on TTO only. Sixth, as deterministic sensitivity analyses show robust results over a wide range of relevant clinical and economic model parameter variation, we refrained from conducting a probabilistic sensitivity analysis. In addition, particularly in this field, there is a lack of evidence on the joint distribution of many of the dependent model parameters. Seventh, in most of the serial strategies, we implemented PBM to be followed by PBSO (in only one strategy PBSO is followed by delayed PBM). This is because the breast cancer risk is higher than the ovarian cancer risk, and therefore, PBM can also provide a greater benefit. Also, a delayed PBSO is reasonable because women undergoing PBSO are put into artificial menopause. This has a major additional impact on women’s quality of life in general, with earlier surgery impacting a women quality of life even more severely. Eighth, although women’s preferences, for instance regarding family planning, are expected to play an important role, they are not considered explicitly in this model. Future studies should integrate women’s preferences in comprehensive patient-shared decision making.
Conclusion
In conclusion, based on the results from our decision analysis, a combination of prophylactic surgeries is an effective and cost-effective cancer prevention option from a German health care perspective. Prophylactic surgery drastically reduces cancer risk but is also associated with more harms due to short-term invasive surgery complications and long-term unintended psychological effects in women. A delayed PBSO after a PBM may improve women’s quality of life and be a cost-effective prevention strategy. We suggest that efforts should be directed to inform women carefully and thoroughly with BRCA-1/2 mutations about their options to prevent breast and ovarian cancer. Our findings could potentially be used as an orientation for clinical experts and decision makers in Germany to guide further improvements in the management strategies for BRCA-1/2 mutation carriers and breast and/or ovarian cancer prevention.
Data Availability
All data used or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- IS:
-
Intensified surveillance
- DESTATIS:
-
German federal statistical office
- GDP:
-
Gross Domestic Product
- ICER:
-
Incremental cost-effectiveness ratio
- ICUR:
-
Incremental cost-utility ratio
- LY:
-
Life years
- LYG:
-
Life-year gained
- MCR:
-
Munich Cancer Registry
- MRI:
-
Magnet resonance imaging
- PBM:
-
Prophylactic bilateral mastectomy
- PBSO:
-
Prophylactic bilateral oophorectomy
- QALY:
-
Quality-adjusted life year
- RKI:
-
Robert Koch Institute
- SG:
-
Standard gamble
- TTO:
-
Time trade-off
- WHO:
-
World Health Organization
- WTP:
-
Willingness-to-pay threshold
References
Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin oncology: official J Am Soc Clin Oncol. 2007;25(11):1329–33.
Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–30.
Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft DK., AWMF). S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Mammakarzinoms http://www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom/ 2019 [Version [Version 4.2].2]
Lostumbo L, Carbine NE, Wallace J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database Syst Rev. 2010(11).
Meijers-Heijboer H, van Geel B, van Putten WL, Henzen-Logmans SC, Seynaeve C, Menke-Pluymers MB, et al. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2001;345(3):159–64.
Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft DK., AWMF). S3-Leitlinie Diagnostik, Therapie und Nachsorge maligner Ovarialtumoren https://www.leitlinienprogramm-onkologie.de/leitlinien/ovarialkarzinom/ 2019 [Version [Version 4.01].01]
Rebbeck TR, Lynch HT, Neuhausen SL, Narod SA, van’t Veer L, Garber JE, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346(21):1616–22.
Li X, You R, Wang X, Liu C, Xu Z, Zhou J, et al. Effectiveness of prophylactic Surgeries in BRCA1 or BRCA2 mutation carriers: a Meta-analysis and systematic review. Clin cancer research: official J Am Association Cancer Res. 2016;22(15):3971–81.
Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling Good Research Practices—Overview:a report of the ISPOR-SMDM modeling Good Research Practices Task Force–1. Med Decis Making. 2012;32(5):667–77.
Siebert U. When should decision-analytic modeling be used in the economic evaluation of health care? Eur J Health Econ. 2003;4(3):143–50.
Weinstein MC, Stason WB. Foundations of cost-effectiveness analysis for health and medical practices. N Engl J Med. 1977;296(13):716–21.
Siebert U, Alagoz O, Bayoumi AM, Jahn B, Owens DK, Cohen DJ, et al. State-transition modeling: a report of the ISPOR-SMDM modeling Good Research Practices Task Force–3. Value in Health. 2012;15(6):812–20.
DESTATIS. Life table: Germany, years, sex, completed age https://www.destatis.de: © Statistisches Bundesamt 2017 [updated 29 March; cited 2017. 2017:[Available from: www.destatis.de.
Buys SS, Partridge E, Black A, Johnson CC, Lamerato L, Isaacs C, et al. Effect of screening on ovarian cancer mortality: the prostate, lung, colorectal and ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305(22):2295–303.
Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. The Lancet. 2016;387(10022):945–56.
van der Velde NM, Mourits MJ, Arts HJ, de Vries J, Leegte BK, Dijkhuis G, et al. Time to stop ovarian cancer screening in BRCA1/2 mutation carriers? Int J Cancer. 2009;124(4):919–23.
Munich Cancer Registry (MCR). Auswertung Mammakarzinom Epidemiologische Daten, Typ AE www.tumorregister-muenchen.de 2017 [updated 4.01.2017. Available from: www.tumorregister-muenchen.de
Munich Cancer Registry (MCR). Auswertung Ovarialkarzinom Epidemiologische Daten, Type AE www.tumorregister-muenchen.de 2017 [updated 3.06.2016. Available from: www.tumorregister-muenchen.de
Robert Koch Institute ZfK. Datenbankabfrage: Mortalität, Rohe Rate pro 100.000 Einwohner in Deutschland Brust und Ovar http://www.krebsdaten.de/ 2017 [Available from: http://www.krebsdaten.de/.
Torrance GW. Utility approach to measuring health-related quality of life. J Chronic Dis. 1987;40(6):593–600.
Muller D, Danner M, Rhiem K, Stollenwerk B, Engel C, Rasche L, et al. Cost-effectiveness of different strategies to prevent breast and ovarian cancer in german women with a BRCA 1 or 2 mutation. Eur J Health Econ. 2018;19(3):341–53.
International Monetary Fund. World Economic Outlook Database https://www.imf.org/en/Publications/WEO/weo-database/2022/April/download-entire-database2022
Gandjour AA, Model-Based. Estimate of the cost-effectiveness threshold in Germany. Applied Health Economics and Health Policy; 2023.
Methods IQWiGG. Version 6.1 of 24 January 2022. https://www.iqwig.de/methoden/general-methods_version-6-1.pdf: Institute for Quality and Efficiency in Health Care (IQWiG); 2022.
Hunink MGM, Weinstein MC, Wittenberg E, Drummond MF, Pliskin JS, Wong JB, et al. Decision making in Health and Medicine: integrating evidence and values. 2 ed. Cambridge: Cambridge University Press; 2014.
Sroczynski G, Esteban E, Widschwendter A, Oberaigner W, Borena W, von Laer D, et al. Reducing overtreatment associated with overdiagnosis in cervical cancer screening—A model-based benefit–harm analysis for Austria. Int J Cancer. 2020;147(4):1131–42.
TreeAge Pro. 2023 R1.0. TreeAge Software. 2023 ed. Williamstown, MA: http://www.treeage.com
Briggs AH, Weinstein MC, Fenwick EAL, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM modeling Good Research Practices Task Force Working Group–6. Med Decis Making. 2012;32(5):722–32.
Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. Model transparency and validation: a report of the ISPOR-SMDM modeling Good Research Practices Task Force-7. Med Decis making: Int J Soc Med Decis Mak. 2012;32(5):733–43.
Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium American journal of human genetics. 1995;56(1):265–71.
Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium American journal of human genetics. 1998;62(3):676–89.
Manchanda R, Patel S, Antoniou AC, Levy-Lahad E, Turnbull C, Evans DG, et al. Cost-effectiveness of population based BRCA testing with varying Ashkenazi jewish ancestry. Am J Obstet Gynecol. 2017;217(5):578.e1-.e12.
Grann VR, Patel PR, Jacobson JS, Warner E, Heitjan DF, Ashby-Thompson M, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat. 2011;125(3):837–47.
Norum J, Hagen AI, Maehle L, Apold J, Burn J, Moller P. Prophylactic bilateral salpingo-oophorectomy (PBSO) with or without prophylactic bilateral mastectomy (PBM) or no intervention in BRCA1 mutation carriers: a cost-effectiveness analysis. European journal of cancer (Oxford, England: 1990). 2008;44(7):963 – 71.
Sroczynski G, Gogollari A, Kuehne F, Hallsson LR, Widschwendter M, Pashayan N et al. A Systematic Review on Cost-effectiveness Studies Evaluating Ovarian Cancer Early Detection and Prevention Strategies. Cancer prevention research (Philadelphia, Pa). 2020;13(5):429 – 42.
Muller D, Danner M, Schmutzler R, Engel C, Wassermann K, Stollenwerk B, et al. Economic modeling of risk-adapted screen-and-treat strategies in women at high risk for breast or ovarian cancer. Eur J Health Econ. 2019;20(5):739–50.
Balmana J, Diez O, Castiglione M. BRCA in breast cancer: ESMO clinical recommendations. Annals of oncology: official journal of the European Society for Medical Oncology. 2009;20(Suppl 4):19–20.
Moller P, Borg A, Evans DG, Haites N, Reis MM, Vasen H, et al. Survival in prospectively ascertained familial breast cancer: analysis of a series stratified by tumour characteristics, BRCA mutations and oophorectomy. Int J Cancer. 2002;101(6):555–9.
Rubin SC, Benjamin I, Behbakht K, Takahashi H, Morgan MA, LiVolsi VA, et al. Clinical and pathological features of ovarian cancer in women with germ-line mutations of BRCA1. N Engl J Med. 1996;335(19):1413–6.
Bolton KL, Chenevix-Trench G, Goh C, Sadetzki S, Ramus SJ, Karlan BY, et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307(4):382–90.
Rebbeck TR, Friebel T, Lynch HT, Neuhausen SL, van ‘t Veer L, Garber JE, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin oncology: official J Am Soc Clin Oncol. 2004;22(6):1055–62.
Grann VR, Patel P, Bharthuar A, Jacobson JS, Warner E, Anderson K, et al. Breast cancer-related preferences among women with and without BRCA mutations. Breast Cancer Res Treat. 2010;119(1):177–84.
Schleinitz MD, DePalo D, Blume J, Stein M. Can differences in breast Cancer utilities explain disparities in breast Cancer Care? J Gen Intern Med. 2006;21(12):1253–60.
Havrilesky LJ, Broadwater G, Davis DM, Nolte KC, Barnett JC, Myers ER, et al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol. 2009;113(2):216–20.
Kearns B, Chilcott J, Whyte S, Preston L, Sadler S. Cost-effectiveness of screening for ovarian cancer amongst postmenopausal women: a model-based economic evaluation. BMC Med. 2016;14(1):200.
Acknowledgements
Not applicable.
Funding
The project FORECEE (4 C) has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement number 634570 and The Eve Appeal. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. All authors, external and internal, had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Contributions
Lára R. Hallsson: Conception and design, validation, model development, analysis and interpretation, formal analysis, investigation (acquisition of data), writing – original draft, writing – review & editing, visualization. Gaby Sroczynski: Conception and design, investigation (acquisition of data), model development, analysis and interpretation, writing – review & editing, supervision, project administration, funding acquisition. Jutta Engel: Resources, provision of data, writing – review & editing. Uwe Siebert: conception and design, model development, analysis and interpretation, writing – review & editing, supervision, project administration, funding acquisition. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved and registered at the Research Committee for Scientific Ethical Questions (RCSEQ) (Registration number 2157). The RCSEQ is an independent, institutional decision-making body of the Private University UMIT TIROL, Hall / Tyrol, and the Health University of Applied Sciences Tyrol (fhg), reviewing planned research projects at these institutions that do not fall within the jurisdiction of a statutory ethics committee (AMG, MPG, UG, ABGB, KaKuG iV TirKAG, etc.) and include special categories of personal data and / or vulnerable groups of persons. The RCSEQ reviews planned research projects for scientific-ethical criteria. The RCSEQ consists of nine members and is based on the cooperation agreement of October 4, 2018, concluded between UMIT TIROL and fhg, and the RCSEQ- Rules of Procedure, adopted on October 16, 2018 (https://www.umit-tirol.at/page.cfm?vpath=universitaet/organe/rcseq). The study is purely based on secondary data sources. No patient-level data were obtained. An informed consent from patients was not applicable. The requirement for informed consent was waived within an abridged procedure by the RCSEQ (Registration number 2157) because of the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jutta Engel, one of our highly estimated co-authors, deceased recently.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hallsson, L.R., Sroczynski, G., Engel, J. et al. Decision-analytic evaluation of the comparative effectiveness and cost-effectiveness of strategies to prevent breast and ovarian cancer in German women with BRCA-1/2 mutations. BMC Cancer 23, 590 (2023). https://doi.org/10.1186/s12885-023-10956-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-10956-6