Abstract
Background
We conducted a systematic review and meta-analysis to summarize the predictive and prognostic ability of the log odds of positive lymph nodes (LODDS) staging system and compare it with pathological N (pN) classification and the ratio-based lymph node system (rN) for the overall survival (OS) of gastric cancer (GC).
Methods
Through a systematic review till March 7, 2022, we identified population-based studies that reported the prognostic effects of LODDS in patients with GC. We compare the predictive effectiveness of the LODDS staging system with that of the rN and pN classification systems for the OS of GC.
Results
Twelve studies comprising 20,312 patients were included in this systematic review and meta-analysis. The results showed that LODDS1, LODDS2, LODDS3, and LODDS4 in GC patients were correlated with poor OS compared with LODDS0 (LODDS1 vs. LODDS0: HR = 1.62, 95% CI (1.42, 1.85); LODDS2 vs. LODDS0: HR = 2.47, 95% CI (2.02, 3.03); LODDS3 vs. LODDS0: HR = 3.15, 95% CI (2.50, 3.97); LODDS4 vs. LODDS0: HR = 4.55, 95% CI (3.29, 6.29)). Additionally, significant differences in survival were observed among patients with different LODDS classifications (all P-values were < 0.001) with the same rN and pN classifications. Meanwhile, for patients with different pN or rN classifications with the same LODDS classification, prognosis was highly similar.
Conclusion
The findings show that LODDS is correlated with the prognosis of GC patients and is superior to the pN and rN classifications for prognostic assessment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC), a common highly recurrent malignant tumor, is the fifth most prevalent tumor, and is the third most frequent primary cause of tumor-related death worldwide [1]. Multiple markers or methods predict the prognosis and therapeutic outcomes of patients with GC. Among these, nodal involvement has long been considered as one of the greatest maker for prognosis and metastasis [2, 3]. For this reason, the International Union Against Cancer (UICC) and the American Joint Committee on Cancer (AJCC) have adopted categories based on the number of metastatic lymph nodes (LNs) as the basis for the N stage of the tumor–node–metastasis (TNM) classification. The TNM staging system is the most widely used system for GC staging assessment. For optimal staging of GC, the latest edition recommends the examination of 16 or more LNs for nodal metastatic status determination [4]. Unfortunately, poor compliance was demonstrated with the recommendation: > 15 LNs were removed in only 29% of patients, and no nodes were removed at all in 9% [5,6,7]. Multiple studies have demonstrated that the accuracy of pathological N (pN) classification for patients’ prognostic assessment is affected by the number of examined LNs [6, 8]. Particularly, if the number of examined LNs is less than 16, N3b patients may be inappropriately classified as N3a because the cutoff for N3a/N3b is 15 metastatic LNs [9]. This phenomenon has been referred to as stage migration. To overcome the potential bias associated with the pN classification, other parameters have been proposed. Some authors have claimed that the ratio-based lymph node system (rN) can be an alternative for patients with fewer than 15 LNs examined, defined as the ratio of metastatic LNs to examined LNs [10,11,12]. Chen et al. showed that lymph node ratio (LNR) carries more information than the pN classification in patients with an inadequate node count [13]. However, the rN0 classification was congruent with the pN0 classification in prognostic assessments of node-negative patients with GC [12, 14]. For this reason, another novel solution, the log odds of positive lymph nodes (LODDS), was proposed recently [11]. LODDS, as a novel prognostic indicator, is defined as the log of the ratio between the number of positive nodes and the number of negative nodes and further discriminates patients with N0 GC [14, 15]. LODDS, first proposed in breast cancer in which it performed equally well as a prognostic indicator in node-positive and node-negative patients [16], was later generalized to several cancers, including GC [17,18,19,20,21,22]. The LODDS classification was superior in predicting the prognosis of GC patients with < 15 examined LNs and those with N0 status compared with the rN and pN classifications [11, 23,24,25]. However, the studies supporting this finding have several limitations, including a lack of large, multicenter surveillance studies and available inclusion criteria for patients and different predicative capabilities of LODDS in different studies.
We conducted a systematic review and meta-analysis to summarize the predictive and prognostic ability of the LODDS staging system and compare it with the rN and pN classification systems to address these limitations.
Methods
Data sources
We searched PubMed, Medline, Embase, Web of Science and the Cochrane Library for relevant studies from inception to March 7, 2022, which formed the basis for evidence used to conduct the meta-analysis. The following keywords were used: “log odds of positive lymph nodes”, “LODDS”, and “gastric cancer”. We used the following strategy: (((((((((gastric neoplasms[MeSH Terms]) OR (stomach neoplasms)) OR (stomach carcinoma)) OR (gastric tumor)) OR (gastric carcinoma)) OR (stomach cancer)) OR (stomach tumor)) OR (gastric neoplasms)) OR (GC)) AND ((log odds of positive lymph nodes) OR (LODDS)). We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting systematic reviews [26] and registered our review on Prospero (https://www.crd.york.ac.uk/prospero/). The PROSPERO registration number is CRD42021274996.
Inclusion and exclusion criteria
We included studies that enrolled ≥ 100 patients with GC (diagnosed with the gold standard test) who were classified by the LODDS, rN, and pN classification systems of the AJCC and followed for at least five years. We included studies that reported at least one of the outcomes of interest or studies wherein the outcome could be calculated according to data extracted from the published data. We excluded conference abstracts, reviews, case reports, ongoing trials, open-label trials, comments, letters, meeting records, and studies that enrolled patients with a total sample size of less than 100 patients, as studies with very small samples are more prone to bias and contribute little information to pooled analyses. We excluded articles that shared a study population with another article and those that did not provide crucial information needed for detailed stratification.
Study selection
Using a standardized form, two reviewers independently screened the titles and abstracts identified by the search, and the full text was obtained to check eligibility. When a disagreement arose and they were unable to reach a consensus, an adjudicator was consulted to resolve discrepancies.
Data extraction
Using a standardized form, two reviewers worked independently to perform duplicate data abstraction in each eligible study. When a disagreement arose and they were unable to reach a consensus, an adjudicator helped to resolve disagreements. We collected information regarding study characteristics (including year of publication, single-center/multicenter study, clinical study design, country of patients, and patient year), patient characteristics (including patient number, tumor stage, patient age, population, neoadjuvant therapy, number of harvested LNs, and number of metastatic LNs), and all patient-important outcomes (overall survival (OS)).
Outcomes and quality assessment
Prognostic values (OS) were used to compare the different LODDS groups. We compared the predictive and prognostic abilities of the LODDS staging system with those of the rN and pN classification systems.
Using the Newcastle–Ottawa Scale (NOS) [27], two investigators, independently and in duplicate, assessed the quality of the included articles, including study group selection, comparability of groups and outcome of interest. The full score was 9 points, and a score of 1–4 points indicated low-quality, while a score of 5–9 points indicated high-quality.
Data analysis and statistical methods
The data were analyzed using Stata statistical software version 16.0 (Stata Corporation, College Station, TX) and SPSS version 22 (IBM Corporation).
To statistically assess the prognostic effects of LODDS, we extracted the hazard ratio (HR) and 95% confidence interval (CI) of 5-year OS from the included studies. If not directly provided in the original literature, the estimated HR and 95% CI were used to assess prognostic effects based on the method described by Tierney et al. [28], and an HR greater than 1 suggested a higher risk of disease progression or death in the patients. A random-effects model (REM) was used to pool the data. All statistical values were combined with 95% CIs and P values for two-sided testing at α = 0.05. To assess the existence of heterogeneity among studies, we tested OS for heterogeneity across cohorts using Cochran’s Q statistic and used I2 to measure the extent of heterogeneity [29]. For the I2 statistic, heterogeneity was defined as low (25%–50%), moderate (50%–75%) or high (> 75%) [30]. For the Q statistic, P ≤ 0.1 was considered to indicate significant heterogeneity. In addition, subgroup analyses were performed based on the differences in baseline characteristics and risk factors in the data retrieved. Then, we also conducted a sensitivity analysis in which each study was removed in turn to evaluate the undue influence of the study on the overall summary estimates including Duval and Tweedie’s trim-and-fill method [31], and Galbraith plots [32]. Publication bias was investigated with qualitative and quantitative methods, including funnel plots and Egger’s test [33]. The groups in each pN and rN stage were regrouped in accordance with LODDS, and the OS differences within groups and between groups were analyzed using chi-squared tests to compare the homogeneity of the three staging methods. P values for the pooled results were two-sided, and the significance level was 0.05.
Results
Study characteristics
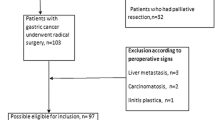
Based on the search strategy mentioned above, the original search yielded 209 records. Finally, we included 12 unique studies [11, 12, 14, 15, 23,24,25, 34,35,36,37,38] published between 2010 and 2021 with 20,312 patients according to the eligibility criteria. The flowchart of the search and selection process is shown as a PRISMA flowchart in Fig. 1.
Tables 1 and 2 summarizes the main characteristics of the studies included in the meta-analysis. Because of the different hierarchical levels of LODDS in the included studies, we re-stratified the groups in the meta-analysis. Eleven studies were retrospective, and one study used a prospectively maintained database. Among these studies, eight studies were from China [11, 12, 14, 23, 24, 35, 37, 38], and one study each was from Hungary [25], Korea [34], America [15], and Italy [36]. Among the included studies, only five studies [11, 24, 25, 35, 36] included patients without neoadjuvant therapy, three studies [15, 23, 37] did not limit the number of patients who received or did not receive neoadjuvant therapy, and the remaining studies [12, 14, 34, 38] did not report the relevant information. Four studies [11, 12, 14, 38] compared the predictive and prognostic abilities of the LODDS staging system with those of the rN or pN classification systems.
Study analysis
LODDS and OS in GC
We analyzed OS in different LODDS categories according to the data from the included articles. The results of the pooled analysis are summarized in Table 3.
When pooling the HR for OS, LODDS1, LODDS2, LODDS3, and LODDS4 in GC patients were correlated with poor OS compared with LODDS0 (Fig. 2). Patients with LODDS1 had inferior OS compared with those with LODDS0 (HR = 1.62, 95% CI (1.42, 1.85)), and the heterogeneity was significant (I2 statistic = 63.5%, P heterogeneity = 0.003). The pooled results indicated that LODDS2 GC patients had a worse OS (HR = 2.47, 95% CI (2.02, 3.03)) than LODDS0 GC patients. There was significant heterogeneity (I2 statistic = 86.2%, P heterogeneity < 0.001). Compared with LODDS0 GC patients, LODDS3 GC patients had a worse OS (HR = 3.15, 95% CI (2.50, 3.97)), and the heterogeneity was significant (I2 statistic = 92.1%, P heterogeneity < 0.001). The results of the pooled analysis using the REM showed that LODDS4 GC patients were also associated with poorer OS (HR = 4.55, 95% CI (3.29, 6.29)) than LODDS0 GC patients, and between-study heterogeneity was significant (I2 statistic = 96.6%, P heterogeneity < 0.001). Overall, as the LODDS grade increases, the prognosis of patients with GC becomes increasingly worse.
Estimated HR summary for OS. a LODDS1 vs. LODDS0, b LODDS2 vs. LODDS0, c LODDS3 vs. LODDS0, d LODDS4 vs. LODDS0. HR > 1 indicates more disease progression or deaths in the patients. Data were pooled using a random-effects model (REM). All statistical values were combined with 95% CIs and two-sided P-values, the threshold of which was set to 0.05. Abbreviations: HR, hazard ratio; OS, overall survival; LODDS, log odds of positive lymph nodes
Subgroup analysis
To analyze the potential sources of between-study heterogeneity, we performed subgroup analysis according to differences in the variables, including the publication year, country, patient number and whether patients received neoadjuvant therapy. The results are summarized in Table 3. Consistent with the above results, LODDS1, LODDS2, LODDS3, and LODDS4 GC patients had a worse OS than LODDS0 GC patients in all subsets. After subgroup analysis, it was found that the heterogeneity was mainly from whether patients received neoadjuvant therapy. In patients without neoadjuvant therapy, the result of the pooled analysis using the REM showed that LODDS1, LODDS2, LODDS3, and LODDS4 GC patients were also associated with poor OS (LODDS1 vs. LODDS0: HR = 1.85, 95% CI (1.64, 2.07); LODDS2 vs. LODDS0: HR = 3.04, 95% CI (2.58, 3.58); LODDS3 vs. LODDS0: HR = 4.03, 95% CI (3.66, 4.43); LODDS4 vs. LODDS0: HR = 6.36, 95% CI (4.50, 8.99)) compared with LODDS0 GC patients. The heterogeneity of OS decreased to a nonsignificant level except for patients in the LODDS4 vs. LODDS0 group (LODDS1 vs. LODDS0: I2 statistic = 0.0%, P heterogeneity = 0.381; LODDS2 vs. LODDS0: I2 statistic = 29.2%, P heterogeneity 0.227; LODDS3 vs. LODDS0: I2 statistic = 0.0%, P heterogeneity = 0.975; LODDS4 vs. LODDS0: I2 statistic = 81.3%, P heterogeneity < 0.001).
To explore the potential sources of heterogeneity in the LODDS4 vs. LODDS0 group, we also used Galbraith plots and Duval and Tweedie’s trim-and-fill method to further explore the source of heterogeneity in OS, and the results showed that the training set of the study by Jian-Hui C et al. [35] might have mainly contributed substantial heterogeneity to OS (Fig. 3a). After omitting this study, the pooled HR was not obviously affected (HR = 5.13, 95% CI (4.46, 5.68); Fig. 3b), but the heterogeneity for OS dropped to a nonsignificant level (from I2 statistic = 81.3%, P heterogeneity < 0.001 to I2 statistic = 2.2%, P heterogeneity = 0.381; Fig. 3c).
Process of exploring the potential sources of heterogeneity on OS. a galbraith plot for OS. b Forest plot for OS after Jian-Hui C et al. (2016) is omitted. c change of heterogeneity before and after Ogawa S et al. (2016) is omitted. Weights are from random-effects analysis. P value for heterogeneity. Abbreviations: HR, hazard ratio; OS, disease-free survival; SE, standard error
Publication bias
Publication bias was assessed by funnel plots and Egger’s test. Formal evaluation using Egger’s test failed to identify significant publication bias in the analyses of LODDS1 vs. LODDS0 (p = 0.608), LODDS2 vs. LODDS0 (p = 0.799), LODDS3 vs. LODDS0 (p = 0.943), and LODDS4 vs. LODDS0 (p = 0.216) for OS. The results with P values for Egger’s test are listed in Table 3. In addition, we used funnel plots to detect publication bias, as shown in Figure S1. All of the funnel plots of the included articles showed a symmetrical distribution. Thus, no significant publication bias was found in the meta-analyses of OS.
Comparison of the homogeneity of prognostic assessments
Four studies [11, 12, 14, 38] compared the predictive and prognostic abilities of the LODDS staging system with those of the rN or pN classification systems. OS rates were compared among different pN and rN classifications when stratified by the LODDS classification and among different LODDS classifications when stratified by the pN or rN classification. The data from all these studies were therefore pooled, as shown in Table 4. Thus, for patients in each of the pN classifications, significant differences in survival were consistently observed among patients with different LODDS classifications. And, for patients in each of the rN classifications, significant differences in survival were consistently observed among patients with different LODDS classifications. Meanwhile, for patients in each LODDS classification, prognosis was highly homologous for those with different pN or rN classifications. These results indicated that the LODDS classification might be superior to the pN and rN classifications for prognostic assessment.
Discussion
Despite recent advances in the treatment of patients with GC, the OS is far from satisfactory. The accuracy of staging systems for patients with GC is important for predicting long-term survival, guiding treatment, and identifying patients for clinical trials. Because of the importance of nodal involvement in assessing prognosis and defining the management of patients with GC [2, 3], there has been intense interest in defining an optimal LN staging system. In fact, numerous different parameters have been proposed to stratify the long-term prognosis of patients with GC according to the status of LN metastasis [39, 40]. The UICC/AJCC staging systems for GC stratify the nodal staging, namely pN stage, according to the number of positive LNs. The major flaw of the pN classification is that it only considers the number of metastatic LNs, but ignores the influence of the number of examined LNs, which may lead to stage migration [13, 41,42,43,44]. To overcome the potential bias associated with the pN classification, other parameters have been proposed by analyzing both the number of examined LNs and the number of metastatic LNs. The rN is a simple method for nodal staging that is less influenced by the number of examined LNs than the pN classification. Some authors have claimed that the rN classification can be an alternative to the pN classification for patients with fewer than 15 LNs examined [45,46,47], and the rN classification can minimize the phenomenon of stage migration [48, 49]. However, the rN0 classification was congruent with the pN0 classification in prognostic assessments of node-negative patients with GC [12, 14], and a minimum number of LNs still need to be examined to ensure accuracy for prognostic assessment. LODDS, a novel prognostic LN-related index that considers the effects of the numbers of both positive LNs and negative LNs, was developed to improve the accuracy of prognostic assessment, particularly in patients with N0 status or with < 15 total harvested LNs [50]. The LODDS classification is also considered superior to the rN and pN classifications because it can be used to study LN involvement in patients at all classification levels [11, 51,52,53].
This is the first meta-analysis that focused on the crucial roles of LODDS in predicting the prognosis of patients with GC. As a result, our study is more informative than any previous study. Our meta-analysis of twelve articles including 20,312 GC patients indicated that LODDS1, LODDS2, LODDS3, and LODDS4 patients have poor OS compared with LODDS0 patients, which shows that LODDS has prognostic value in patients with GC. When pooling the HR for OS, LODDS1, LODDS2, LODDS3, and LODDS4 in GC patients was correlated with poor OS compared with LODDS0 (LODDS1 vs. LODDS0: HR = 1.62, 95% CI (1.42, 1.85), I2 statistic = 63.5%, P heterogeneity = 0.003; LODDS2 vs. LODDS0: HR = 2.47, 95% CI (2.02, 3.03), I2 statistic = 86.2%, P heterogeneity < 0.001; LODDS3 vs. LODDS0: HR = 3.15, 95% CI (2.50, 3.97), I2 statistic = 92.1%, P heterogeneity < 0.001; LODDS4 vs. LODDS0: HR = 4.55, 95% CI (3.29, 6.29), I2 statistic = 96.6%, P heterogeneity < 0.001). As the LODDS grade increases, the prognosis of patients with GC becomes correspondingly worse. However, we discovered high levels of heterogeneity in all groups. To analyze the potential sources of between-study heterogeneity, we performed subgroup analysis according to differences in the variables, including the publication year, country, patient number and whether patients received neoadjuvant therapy. After subgroup analysis, it was found that the heterogeneity was mainly from whether patients received neoadjuvant therapy. Additionally, we found that patients without neoadjuvant therapy had poorer OS than patients with neoadjuvant therapy in the same group. Therefore, the results suggest that whether patients receive neoadjuvant therapy is one of the most important factors affecting the prognostic effectiveness of LODDS in patients with GC. Although we performed a subgroup analysis according to whether patients received neoadjuvant therapy, significant heterogeneity was still found in the pooled analysis of OS in the LODDS4 vs. LODDS0 group. To explore the potential sources of heterogeneity, we also used Galbraith plots and Duval and Tweedie’s trim-and-fill method to further explore the source of heterogeneity in OS, and the results showed that the training set of the study by Jian-Hui C et al. [35] might have mainly contributed substantial heterogeneity to OS.
Additionally, we assessed differences in survival among patients in different LODDS classifications for each of the pN or rN classifications. We summarized the results from four studies [11, 12, 14, 38] that compared the predictive and prognostic abilities of the LODDS staging system with those of the rN or pN classification systems. For patients in each of the rN and pN classifications, significant differences in survival were consistently observed among patients in different LODDS classifications. Meanwhile, for patients in each LODDS classification, prognosis was highly similar between patients with different pN or rN classifications (see Table 4). Thus, we considered that the superiority of the LODDS classification to the rN and pN classifications was mainly because of its potential to discriminate patients with the same ratio of node metastasis but different survival.
However, several limitations of the current meta-analysis should be emphasized. First, as several studies did not report HRs, HRs in this meta-analysis were estimated based on the method described by Tierney et al. [28]. Second, due to the lack of relevant information on the anatomic nodal group locations, we did not analyze the effect of the anatomic nodal group locations on the prognostic effectiveness of LODDS in patients with GC. Third, the optimal cutoff value of LODDS is not concluded. One critical problem is lack of consensus on the optimal cutoff value of LODDS when using it in the clinic and in experiments. Thus, LODDS is not yet used clinically on a large scale. Fourth, we were unable to apply the AUC, C-index, or AIC values to determine which of the LODDS, rN or pN classifications is superior because we did not have access to detailed patient information. Despite these limitations, this is the first meta-analysis focusing on the crucial roles of LODDS in predicting the prognosis of patients with GC. The results suggest that LODDS can predict the survival of GC patients. Moreover, it may be a novel prognostic predictor and a more accurate and sensitive stratification tool for use in clinical studies.
Conclusion
Our systematic review demonstrated that LODDS is correlated with the prognosis of GC patients and more accurately predicts the survival of GC patients than previous methods. As the LODDS grade increases, the prognosis of patients with GC becomes correspondingly worse. Additionally, we found that patients without neoadjuvant therapy had poorer OS than patients with neoadjuvant therapy in the same LODDS group. Therefore, the results suggest that whether patients receive neoadjuvant therapy is one of the strongest factors affecting the prognostic effectiveness of LODDS. Moreover, the LODDS classification is superior to the pN and rN classifications for prognostic assessment. Incorporating LODDS into the staging system of GC will enable clinicians to predict the prognosis of patients more accurately. Further high-quality, large-scale, international, well-designed multicenter prospective studies are needed to obtain the optimal cutoff point of LODDS and to find a simple and repeatable way to calculate LODDS values to facilitate the utilization of LODDS in the clinic.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- GC:
-
Gastric cancer
- LODDS:
-
Log odds of positive lymph nodes
- CI:
-
Confidence interval
- OS:
-
Overall survival
- UICC:
-
Union for International Cancer Control
- AJCC:
-
American Joint Committee on Cancer
- LN:
-
Lymph node
- LNR:
-
Lymph node ratio
- TNM:
-
Tumor–node–metastasis
- NOS:
-
Newcastle–Ottawa scale
- REM:
-
Random-effects model
- HR:
-
Hazard ratio
- HNC:
-
Head and neck cancer
- pN:
-
Pathological N
- rN:
-
Ratio-based lymph node
References
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Siewert J, Böttcher K, Stein H, et al. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228(4):449–61.
Wu C, Hsieh M, Lo S, et al. Relation of number of positive lymph nodes to the prognosis of patients with primary gastric adenocarcinoma. Gut. 1996;38(4):525–7.
Sano T, Coit D, Kim H, et al. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer. 2017;20(2):217–25.
Coburn N, Swallow C, Kiss A, et al. Significant regional variation in adequacy of lymph node assessment and survival in gastric cancer. Cancer. 2006;107(9):2143–51.
Schwarz R, Smith D. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Ann Surg Oncol. 2007;14(2):317–28.
Smith D, Schwarz R, Schwarz R. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23(28):7114–24.
Deng J, Liu J, Wang W, et al. Validation of clinical significance of examined lymph node count for accurate prognostic evaluation of gastric cancer for the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system. Chin J Cancer Res. 2018;30(5):477–91.
Kim CY, Yang DH. Adjustment of N stages of gastric cancer by the ratio between the metastatic and examined lymph nodes. Ann Surg Oncol. 2009;16(7):1868–74.
Coimbra F, Costa W, Montagnini A, et al. The interaction between N-category and N-ratio as a new tool to improve lymph node metastasis staging in gastric cancer: results of a single cancer center in Brazil. Eur J Surg Oncol. 2011;37(1):47–54.
Gu P, Deng J, Sun Z, et al. Superiority of log odds of positive lymph nodes (LODDS) for prognostic prediction after gastric cancer surgery: a multi-institutional analysis of 7620 patients in China. Surg Today. 2021;51(1):101–10.
Xu J, Bian YH, Jin X, et al. Prognostic assessment of different metastatic lymph node staging methods for gastric cancer after D2 resection. World J Gastroenterol. 2013;19(12):1975–83.
Wang J, Dang P, Raut C, et al. Comparison of a lymph node ratio-based staging system with the 7th AJCC system for gastric cancer: analysis of 18,043 patients from the SEER database. Ann Surg. 2012;255(3):478–85.
Sun Z, Xu Y, Li DM, et al. Log odds of positive lymph nodes: a novel prognostic indicator superior to the number-based and the ratio-based N category for gastric cancer patients with R0 resection. Cancer. 2010;116(11):2571–80.
Spolverato G, Ejaz A, Kim Y, et al. Prognostic performance of different lymph node staging systems after curative intent resection for gastric adenocarcinoma. Ann Surg. 2015;262(6):991–8.
Vinh-Hung V, Verschraegen C, Promish D, et al. Ratios of involved nodes in early breast cancer. Breast Cancer Res. 2004;6(6):R680-688.
Shi Z, Xiao ZQ, Li LJ, et al. Application of nomogram containing log odds of metastatic lymph node in gallbladder cancer patients. Ann Transl Med. 2020;8(10):655.
Modi KB, Sekhon R, Rawal S, et al. Analysis of role of lymph node density, negative lymph node and lodds on survival in cervical cancer. Int J Gynecol Obstet. 2018;143:426–7.
Dziedzic D, Rudzinski P, Orlowski T, et al. Log odds as a novel prognostic indicator superior to the number-based and ratio-based category for non-small cell lung cancer. J Thorac Oncol. 2017;12(1):S731.
Lin P. Prognostic performance of different lymph node staging systems for esophageal squamous cell carcinoma. Dis Esophagus. 2016;29:157A.
Smith DD, Nelson RA, Schwarz RE. A comparison of competing lymph node staging schemes in resectable gastric cancer. Ann Surg Oncol. 2010;17:S64.
Zhang QW, Zhang CH, Li XB, et al. Comparison of three lymph node staging schemes for predicting survival in patients with colorectal cancer: a large population database and Chinese multicenter validation. United European Gastroenterol J. 2018;6(8):A277.
Pan SW, Wang PL, Xing YN, et al. Retrieved lymph nodes from different anatomic groups in gastric cancer: a proposed optimal number, comparison with other nodal classification strategies and its impact on prognosis. Cancer Commun. 2019;39(1):49.
Cao HH, Tang ZQ, Yu Z, et al. Comparison of the 8th union for international cancer control lymph node staging system for gastric cancer with two other lymph node staging systems. Oncol Lett. 2019;17(1):1299–305.
Tóth D, Bíró A, Varga Z, et al. Comparison of different lymph node staging systems in prognosis of gastric cancer: a bi-institutional study from Hungary. Chin J Cancer Res. 2017;29(4):323–32.
Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Tierney J, Stewart L, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
Higgins J, Thompson S, Deeks J, et al. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003;327(7414):557–60.
Guyatt G, Oxman A, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence–inconsistency. J Clin Epidemiol. 2011;64(12):1294–302.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63.
Galbraith R. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med. 1988;7(8):889–94.
Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997;315(7109):629–34.
Lee JW, Ali B, Park CH, et al. Different lymph node staging systems in patients with gastric cancer from Korean. Medicine (United States). 2016;95(25):e3860.
Jian-Hui C, Shi-Rong C, Hui W, et al. Prognostic value of three different lymph node staging systems in the survival of patients with gastric cancer following D2 lymphadenectomy. Tumour Biol. 2016;37(8):11105–13.
Aurello P, Petrucciani N, Nigri GR, et al. Log odds of positive lymph nodes (LODDS): what are their role in the prognostic assessment of gastric adenocarcinoma? J Gastrointest Surg. 2014;18(7):1254–60.
Liu H, Deng J, Zhang R, et al. The RML of lymph node metastasis was superior to the LODDS forevaluating the prognosis of gastric cancer. Int J Surg. 2013;11(5):419–24.
Qiu MZ, Qiu HJ, Wang ZQ, et al. The tumor-log odds of positive lymph nodes-metastasis staging system, a promising new staging system for gastric cancer after D2 resection in China. PLoS One. 2012;7(2):e31736.
Gong Y, Pan S, Wang X, et al. A novel lymph node staging system for gastric cancer including modified Union for cancer Control/American Joint Committee on cancer and Japanese Gastric Cancer Association criteria. Eur J Surg Oncol. 2020;46:e27–32.
Che K, Wang Y, Wu N, et al. Prognostic nomograms based on three lymph node classification systems for resected gastric adenocarcinoma: a large population-based cohort study and external validation. Ann Surg Oncol. 2021;28:8937–49.
Daw NC, Chi YY, Kalapurakal JA, et al. Activity of vincristine and irinotecan in diffuse anaplastic Wilms tumor and therapy outcomes of stage II to IV disease: results of the Children’s Oncology Group AREN0321 Study. J Clin Oncol. 2020;38(14):1558–68.
Shen J, Kim S, Cheong J, et al. The impact of total retrieved lymph nodes on staging and survival of patients with pT3 gastric cancer. Cancer. 2007;110(4):745–51.
Bouvier A, Haas O, Piard F, et al. How many nodes must be examined to accurately stage gastric carcinomas? Results from a population based study. Cancer. 2002;94(11):2862–6.
Lee H, Yang H, Kim W, et al. Influence of the number of lymph nodes examined on staging of gastric cancer. Br J Surg. 2001;88(10):1408–12.
Hsieh M, Wang S, Wei C, et al. S1 versus Doublet Regimens as Adjuvant Chemotherapy in Patients with Advanced Gastric Cancer after Radical Surgery with D2 Dissection-A Propensity Score Matching Analysis. Cancers (Basel). 2020;12(9):2384.
Li J, Lin Y, Wang Y, et al. Prognostic nomogram based on the metastatic lymph node ratio for gastric neuroendocrine tumour: SEER database analysis. ESMO Open. 2020;5(2):e000632.
Rawicz-Pruszyński K, Ciseł B, Mlak R, et al. The role of the lymph node ratio in advanced gastric cancer after neoadjuvant chemotherapy. Cancers. 2019;11(12):1914.
Shin J, Kim H, Lee W, et al. The Stage Migration Should be Reconsidered in Stage IIIA Rectal Cancer: Based on Propensity Score Analysis. Clin Colorectal Cancer. 2021;20(4):e273–8.
Li MM, Zhao S, Eskander A, et al. Stage Migration and Survival Trends in Laryngeal Cancer. Ann Surg Oncol. 2021;28(12):7300-9.
Wang X, Bao M, Zhang C. Prognostic value of different lymph node staging methods for node-positive cardia gastric cancer: a register-based retrospective cohort study. BMJ Open. 2021;11(8):e050378.
Deng W, Xu T, Wang Y, et al. Log odds of positive lymph nodes may predict survival benefit in patients with node-positive non-small cell lung cancer. Lung Cancer (Amsterdam, Netherlands). 2018;122:60–6.
Occhionorelli S, Andreotti D, Vallese P, et al. Evaluation on prognostic efficacy of lymph nodes ratio (LNR) and log odds of positive lymph nodes (LODDS) in complicated colon cancer: the first study in emergency surgery. World J Surg Oncol. 2018;16(1):186.
Wang J, Li J, Chen R, et al. Survival effect of different lymph node staging methods on ovarian cancer: an analysis of 10 878 patients. Cancer Med. 2018;7(9):4315–29.
Acknowledgements
Not applicable.
Funding
This project was supported by Project supported by the National Natural Science Foundation of China (No. 82073210), the Key Research and Development Program of Shaanxi Province of China (No. 2019ZDLSF01-02–01) and Xjijng Zhutui Project (No. XJZT18ML06). The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
Yiding Li: conceived and designed the study, selected studies, performed outcome analysis, wrote the paper, reviewed and edited the manuscript. Guiling Wu: conceived and designed the study, extracted data, performed outcome analysis, wrote the paper. Yujie Zhang: extracted data. Wanli Yang: searched literatures. Xiaoqian Wang: searched literatures. Lili Duan: selected studies. Liaoran Niu: selected studies. Junfeng Chen: extracted data. Jinqiang Liu: extracted data. Wei Zhou: reviewed and edited the manuscript. Weili Han: extracted data. Jing Wang: extracted data. Helun Zhong: extracted data. Gang Ji: extracted data. Liu Hong: reviewed and edited the manuscript. Daiming Fan: reviewed and edited the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Assessment of publication bias using Funnel plot analysis. a-b Funnel plot analysis of studies on OS ((a) LODDS1 vs. LODDS0, (b) LODDS2 vs. LODDS0, (c) LODDS1 vs. LODDS0, (d) LODDS2 vs. LODDS0); e–h Funnel plot analysis of studies which included the patients without neoadjuvant therapy on OS ((e) LODDS1 vs. LODDS0, (f) LODDS2 vs. LODDS0), (g) LODDS1 vs. LODDS0, (h) LODDS2 vs. LODDS0). Publication bias was not found in the meta-analyses of OS. All of the funnel plots of the included articles showed a symmetrical distribution. Thus, no significant publication bias was found in the meta-analyses of OS. Abbreviations: HR, hazard ratio; OS, overall survival; LODDS, log odds of positive lymph nodes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Y., Wu, G., Liu, J. et al. Log odds of positive lymph nodes as a novel prognostic predictor for gastric cancer: a systematic review and meta-analysis. BMC Cancer 23, 523 (2023). https://doi.org/10.1186/s12885-023-10805-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-10805-6