Abstract
Background
To compare the oncological outcomes of patients with FIGO 2018 stage IIIC cervical cancer (CC) involving different local tumor factors who underwent abdominal radical hysterectomy (ARH), neoadjuvant chemotherapy and radical surgery (NACT), or radical chemoradiotherapy (R-CT).
Methods
Based on tumor staging, patients with stage IIIC were divided into T1, T2a, T2b, and T3 groups. Kaplan–Meier and Cox proportional hazards regression analysis were used to compare their overall survival (OS) and disease-free survival (DFS) of 5 years.
Results
We included 4,086 patients (1,117, 1,019, 869, and 1,081 in the T1, T2a, T2b, and T3 groups, respectively). In the T1 group, NACT was correlated with a decrease in OS (hazard ratio [HR] = 1.631, 95% confidence interval [CI]: 1.150–2.315, P = 0.006) and DFS (HR = 1.665, 95% CI: 1.255–2.182, P < 0.001) than ARH. ARH and NACT were not correlated with OS (P = 0.226 and P = 0.921) or DFS (P = 0.343 and P = 0.535) than R-CT. In the T2a group, NACT was correlated with a decrease in OS (HR = 1.454, 95% CI: 1.057–2.000, P = 0.021) and DFS (HR = 1.529, 95% CI: 1.185–1.974, P = 0.001) than ARH. ARH and NACT were not correlated with OS (P = 0.736 and P = 0.267) or DFS (P = 0.714 and P = 0.087) than R-CT. In the T2b group, NACT was correlated with a decrease in DFS (HR = 1.847, 95% CI: 1.347–2.532, P < 0.001) than R-CT nevertheless was not correlated with OS (P = 0.146); ARH was not correlated with OS (P = 0.056) and DFS (P = 0.676). In the T3 group, the OS rates of ARH (n = 10), NACT (n = 18), and R-CT (n = 1053) were 67.5%, 53.1%, and 64.7% (P = 0.941), and the DFS rates were 68.6%, 45.5%, and 61.1%, respectively (P = 0.761).
Conclusion
R-CT oncological outcomes were not entirely superior to those of NACT or ARH under different local tumor factors with stage IIIC. NACT is not suitable for stage T1, T2a, and T2b. Nevertheless ARH is potentially applicable to stage T1, T2a, T2b and T3. The results of stage T3 require confirmation through further research due to disparity in case numbers in each subgroup.
Similar content being viewed by others
Background
CC is the predominant genital-tract malignancy in women. Tumor staging reflects both prognosis and survival, and is crucial for the management of tumor and treatment guidance. International Federation of Gynecology and Obstetrics (FIGO) staging is widely used for CC. In 2018, FIGO released a new staging process [1], which was revised in 2019 [2]. It was mainly updated to add stage IIIC, referring to involvement of the pelvic lymph nodes(LNs) and/or para-aortic LNs regardless of tumor size and extent of spread. Certain scholars dispute stage IIIC prognosis, which is solely based on the lymph-node-metastasis stage, without considering the local invasion scope, tumor size, and other factors [3]. The “2022 NCCN Cervical Cancer Clinical Practice Guidelines (1st Edition)” recommend R-CT for stage IIIC [4]; therefore, patients with relatively localized tumors are denied surgery.
Although FIGO staging is recommended by most medical guidelines, the European Society of Gynecologic Oncology, European Society of Radiotherapy and Oncology, and European Society of Pathology guidelines recommend tumor-node-metastasis (TNM) staging. Therefore, certain scholars have raised the following question: “Using T-staging, are the oncological outcomes of different treatments in patients with FIGO 2018 stage IIIC consistent?” To date, only a small number of studies have focused on this stage, using small sample sizes and few stratified comparisons. Therefore, based on the 1538 project database, our study screened FIGO 2018 stage IIIC cases using T-staging stratification, that is, according to T1, T2a, T2b, and T3 stratification (corresponding to FIGO 2009 stages I, IIA, IIB, and III cases with LN metastasis), to investigate the survival outcomes of ARH, NACT, and R-CT for the selection of appropriate treatment strategies for FIGO 2018 stage IIIC CC.
Methods
Data collection
Chinese Cervical Cancer Clinical (Four-C) study is a multi-center, retrospective cohort study, including 63,926 patients with various stages of CC who were hospitalized in 47 Chinese hospitals during 2004–2018. This study was approved by the Ethics Committee of Nanfang Hospital, Southern Medical University (ethical number: NFEC-2017–135, Clinical trial Registration Number: CHiCTR1800017778). We collect the following data by reviewing electronic medical records: general clinical data, preoperative laboratory test results, preoperative pathological results, relevant surgical data, preoperative adjuvant treatment data, postoperative adjuvant therapy data, postoperative pathological results, and follow-up data. We phoned to follow up and obtained messages of survival, recurrence, and complications, if failed, we obtained information from inpatient and outpatient medical records. All original data were reviewed and validated by two independent gynecologists to ensure the accuracy. The details of case collection in the Four-C study database is as shown in our previous studies [5,6,7]. In accordance with the journal’s guidelines, we will provide our data for the reproducibility of this study in other centers if such is requested.
Inclusion and exclusion criteria
TNM-staging tumor factors [8] were used to divide IIIC-stage patients into four groups: T1, T2a, T2b, and T3.
The following criteria were for inclusion: age ≥ 18 years; CC diagnosed by cervical biopsy; squamous cell carcinoma(SCC), adenocarcinoma(AC), or adenosquamous cell carcinoma(ASC) based on histology; FIGO 2018 stage IIIC (patients who underwent radiation therapy defined lymph node status depended on CT, MRI or/and PET-CT before treatment, and those who underwent radical surgery defined lymph node status depended on pathological examinations after surgery); ARH-group patients who underwent radical surgery (Q-M type-B or type-C radical hysterectomy pelvic lymphadenectomy ± para-aortic lymphadenectomy); NACT-group patients who underwent neoadjuvant therapy and radical surgery (Q-M type-B or type-C radical hysterectomy pelvic lymphadenectomy ± para-aortic lymphadenectomy); R-CT-group patients who underwent radiation therapy(RT),and RT dose ≥ 45 Gy; with follow-up outcomes.
The following criteria were for exclusion: gestation, cervical stump cancer, CC complicated with other malignant tumors, loss to follow-up, and failure to satisfy the inclusion criteria.
Outcome measurement
The main outcome measures were overall survival (OS) and disease-free survival (DFS) in all subgroups of FIGO 2018 stage IIIC, with the cut-off point 5 years after treatment. The concept of OS is the final time from diagnosis to effective follow-up or death from any cause, moreover the concept of DFS is the final time from diagnosis to effective follow-up, recurrence or death.
Statistics
No data were missing among the included cases. Mean ± standard deviation (x ± s) was used to represent continuous data, furthermore percentage (%) was used to represent counting data. Fisher's exact test or Chi-square test were used to compare categorical variables. Kaplan–Meier curves were used to describe changes in survival outcomes. Cox proportional risk regression models were used to adjust variables and evaluate the HRs and 95% CI of stratification for 5-year OS and DFS. SPSS 26.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analyses, and statistical significance was set at P < 0.05.
Results
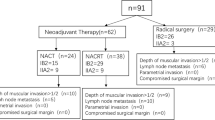
Based on the inclusion criteria, 4,086 CC cases (including 1,117, 1,019, 869, and 1,081 cases in the T1, T2a, T2b, and T3 groups, respectively) were included. Data screening process is shown in Fig. 1.
Clinicopathological characteristics of each group
Clinicopathological characteristics of each group are shown in Table 1. Clear distinctions were shown among the median age of the patients using the three treatments in the T1, T2a, T2b, and T3 groups (P < 0.05 for all). No sharp distinctions were observed in the proportion of histologic types between the T1 and T2a groups (P > 0.05 for all); however, a statistically significant distinction was shown between the T2b and T3 groups (P < 0.001 for all).
Comparison of oncological outcomes of the three treatment methods in each group
Comparison of oncological outcomes of the three treatment methods in the T1 group
In the T1 group, 1,117 cases were divided into R-CT (n = 58), ARH (n = 838), and NACT (n = 221) subgroups. Kaplan–Meier analysis revealed statistically sharp distinctions in 5-year OS (76.5% vs. 81.7% vs. 75.3%, P = 0.015) and DFS ( 73.6% vs.74.3% vs. 60.1%, P = 0.001) among the three subgroups (Fig. 2A, B). Cox proportional hazards regression analysis revealed NACT was not correlated with 5-year OS (HR = 1.040, 95% CI: 0.481–2.249, P = 0.921) or DFS (HR = 1.227, 95% CI: 0.642–2.344, P = 0.535) than R-CT. Furthermore, ARH was not correlated with 5-year OS (HR = 0.637, 95% CI: 0.307–1.322, P = 0.226) and 5-year DFS (HR = 0.741, 95% CI: 0.400–1.375, P = 0.343) than R-CT (Table 2). However, NACT was correlated with a decrease in OS (HR = 1.631, 95% CI: 1.150–2.315, P = 0.006) and DFS (HR = 1.665, 95% CI: 1.255–2.182, P < 0.001) than ARH (Table 3). AC was associated with a decrease in 5-year OS (HR = 1.734, 95% CI: 1.092–2.573, P = 0.020) and 5-year DFS (HR = 1.786, 95% CI: 1.241–2.570, P = 0.002) than SCC; nonetheless, ASC was not correlated with 5-year OS and DFS (P > 0.05 for all). Moreover, age was not correlated with 5-year OS and DFS (P > 0.05 for all) (Tables 2 and 3).
Comparison of oncological outcomes of the three treatments in the T2a group
In the T2a group, 1,019 cases were divided into R-CT (n = 157), ARH (n = 608), and NACT (n = 254) subgroups. Kaplan–Meier analysis revealed statistically sharp distinctions in 5-year DFS (69.1% vs. 64.9% vs. 54.5%, P = 0.005) among the three subgroups; however, there was no distinctions in 5-year OS ( 76.3% vs. 75.8% vs. 69.5%, P = 0.079) (Fig. 2C, D). Cox proportional hazards regression analysis revealed NACT was not correlated with 5-year OS (HR = 1.339,95% CI:0.800–2.243, P = 0.267) or DFS (HR = 1.425,95% CI:0.950–2.139, P = 0.087) than R-CT. Furthermore, ARH was not correlated with 5-year OS (HR = 0.921, 95% CI: 0.572–1.484, P = 0.736) and 5-year DFS (HR = 0.932, 95% CI: 0.640–1.357, P = 0.714) than R-CT (Table 2). Nevertheless, NACT was correlated with a decrease in OS (HR = 1.454, 95% CI: 1.057–2.000, P = 0.021) and DFS (HR = 1.529, 95% CI: 1.185–1.974, P = 0.001) than ARH (Table 3). ASC was associated with a decrease in 5-year OS (HR = 3.182, 95% CI: 1.803–5.617, P < 0.001) and 5-year DFS (HR = 2.210, 95% CI: 1.288–3.792, P = 0.004) than SCC; nonetheless, AC was not correlated with 5-year OS and DFS (P > 0.05 for all). Moreover, age was not associated with 5-year OS and DFS (P > 0.05 for all) (Tables 2 and 3).
Comparison of the oncological outcomes of the three treatments in the T2b group
In the T2b group, 869 cases were divided into R-CT (n = 645), ARH (n = 73), and NACT (n = 151) subgroups. Kaplan–Meier analysis revealed statistically sharp distinctions in 5-year OS (73.9% vs. 86.3% vs. 68.5%, P = 0.011) and DFS (72.6% vs. 64.0% vs. 47.6%, P < 0.001) among the three subgroups (Fig. 2E, F). Cox proportional hazards regression analysis revealed NACT correlated with a decrease in 5-year DFS (HR = 1.847, 95% CI: 1.347–2.532, P < 0.001) than R-CT; however, there was no correlation with OS (HR = 1.353, 95% CI: 0.901–2.032, P = 0.146). ARH was not correlated with 5-year OS (HR = 0.490, 95% CI: 0.236–1.017, P = 0.056) and DFS (HR = 1.101, 95% CI 0.702–1.725, P = 0.676). AC was correlated with a decrease in 5-year OS (HR = 1.999, 95% CI: 1.092–3.660, P = 0.025) and DFS (HR = 2.098, 95% CI: 1.298–3.392, P = 0.003) than SCC. However, ASC was not correlated with 5-year OS and DFS (P > 0.05 for all). Furthermore, age was not correlated with 5-year OS and DFS (P > 0.05 for all) (Table 4).
Comparison of the oncological outcomes of the three treatments in the T3 group
In the T3 group, 1,081 cases were divided into R-CT (n = 1,053), ARH (n = 10), and NACT (n = 18) subgroups. Kaplan–Meier analysis revealed no statistically sharp distinctions in 5-year OS (64.7% vs. 67.5% vs. 53.1%, P = 0.941) and DFS (61.1% vs. 68.6% vs. 45.5%, P = 0.761) among the three subgroups (Fig. 2G, H). Cox proportional hazards regression analysis revealed NACT was not correlated with 5-year OS (HR = 0.780, 95% CI: 0.284–2.139, P = 0.629) and DFS (HR = 1.075, 95% CI: 0.499–2.316, P = 0.853) compared with R-CT. ARH was not correlated with 5-year OS (HR = 0.879, 95% CI: 0.281–2.746, P = 0.824) and DFS (HR = 0.747, 95% CI: 0.239–2.328, P = 0.614). ASC was correlated with a decrease in 5-year OS (HR = 2.733, 95% CI: 1.109–6.733, P = 0.029) than SCC; nevertheless, it was not correlated with 5-year DFS (HR = 2.156, 95% CI: 0.946–4.916, P = 0.068), and AC was not correlated with 5-year OS and DFS (P > 0.05 for all). In addition, age was not correlated with 5-year OS and DFS (P > 0.05 for all) (Table 4).
Discussion
Summary of main results
A key change to the FIGO 2018 staging system was LN metastasis inclusion, which indicates its importance in tumor progression and prognosis. Thus, this group is treated differently, which is helpful for clinical research and medical intervention. However, the 2018 FIGO Cervical Cancer guidelines have no stratified treatment recommendations for these patients. Since the release of the 2018 FIGO staging system, a wave of research on new staging treatment strategies and prognosis has emerged. Based on the 1538 project database, this study conducted a real-world study on FIGO 2018 stage IIIC CC using T-staging to investigate the oncological outcomes of ARH, NACT, and R-CT. The results demonstrate that different treatments affect the oncological outcomes of patients with T1, T2a, T2b, and T3 CC in FIGO 2018 stage IIIC. Mortality and recurrence/death risks were higher in NACT than in ARH in the T1 and T2a group, while recurrence/death risk was higher in NACT than in R-CT in the T2b group. Among the T1, T2a, T2b, and T3 groups, no statistically significant differences in death and recurrence/death risks were noted between ARH and R-CT. ARH may be an alternative initial treatment for patients with FIGO 2018 stage IIIC CC. NACT is not recommended for stage T1, T2a and T2b. The number of cases in the three subgroups of the T3 stage varied greatly, therefore further research is required to confirm the results.
Results in the context of published literature
TNM-staging
Valid staging systems are characterized by intragroup homogeneity, that is, same-staged patients essentially exhibit minimal prognostic differences. Previous related studies suggested that although LN metastasis is an important prognostic factor for patients with CC, including all patients with LN metastasis in the same stage leads to high patient heterogeneity [9, 10]. Certain studies found that CC prognosis was related to T-staging [11, 12]; these results may be related to stage IIIC patient heterogeneity. In a retrospective study by Matsuo K et al. [9], 733 patients with stage IIIC1 were divided into T1, T2, and T3 groups based on T-staging. T3b had a lower survival rate, revealing a significant OS difference based on T-staging among stage IIIC1 patients; however, this study exclusively included stage IIIC1 patients. Evidently, the prognosis of stage IIIC patients is also affected by local tumor factors, with a significantly different OS rate. The vast heterogeneity among these patients not only affects prognostic prediction but also clinical decision-making. Treating stage IIIC patients, with sole consideration of LN status, without stratifying local tumor factors and extent of spread, may be inappropriate. Therefore, our study stratified stage IIIC cases using T-staging and compared the oncological outcomes of ARH, NACT and R-CT.
Treatment strategies
CC treatment should be implemented in a planned and sequential stage-based manner, adjusting according to surgical results and post-radiotherapy tumor regression. Due to the advantages of preserving ovarian function, tissue elasticity, and reproductive function, surgical-treatment is increasing [13]. RT is predominant for advanced cervical cancer. Chemotherapy is used as an adjuvant therapy for RT sensitization.
Radical surgical treatment can remove metastatic pelvic LN to minish burthen of tumor and determine LN status, thus guiding postoperative supplementary treatment selection. Avoiding excessive treatment is important. Currently, radical surgery and pelvic lymphadenectomy are preferred for early CC. A study of FIGO 2009 IB1 and IIA1 CC by Wu et al. [14] suggested that no significant oncological-outcome difference existed between ARH and R-CT. Landoni’s study of 19/343 IB1 and IIA1 CC cases also concurred that ARH and R-CT had similar effects. Our study agreed with these findings. However, a study on FIGO 2009 IB1-IIA2 CC [15] concluded that ARH oncological outcomes were superior to those of R-CT; nevertheless, it exclusively included SCC cases. A seven-study meta-analysis by Yan et al. [16] revealed that ARH had obvious advantages over chemoradiotherapy for IB2-IIA CC. Jang et al. [17] conducted a study on FIGO 2009 IB1-IIA CC and found that the oncological prognosis of ARH was significantly superior to that of concurrent chemoradiotherapy (CCRT). Bansal et al. [18] analyzed 4,885 cases of FIGO 2009 IB1-IIA CC and found that when the tumor diameter is < 6 cm, ARH potentially benefits patient survival more than R-CT; when it is > 6 cm, the two are equivalent. For FIGO 2018 stage IIIC, the oncological prognosis of R-CT does not prevail over that of ARH, considering that R-CT has a series of complications, such as weakened ovarian function [19], vaginal constriction, and dry intercourse [20], which seriously affect patients’ quality of life. Hence, ARH is recommended as an initial treatment.
NACT is the predominant pre-operative adjuvant therapy for CC, while FIGO guidelines recommend it for clinical trials or areas where radiotherapy equipment is lacking. NCCN guidelines only recommend it for small-cell neuroendocrine carcinoma of the cervix. At present, whether NACT can improve the prognosis of patients with CC remains controversial [21, 22]. In the T1 and T2a group of ours, NACT was associated with a decrease in 5-year OS and 5-year DFS than ARH. Meantime, relevant studies found worse prognosis or no difference between post-NACT surgery and radical radiotherapy for patients with CC. A single-center, phase III, randomized controlled trial by Gupta et al. [23] found that for FIGO 2009 stage IB2-IIB cervical SCC, the group undergoing post-NACT surgery had a statistically difference in 5-year DFS compared with the CCRT group; however no sharp distinction in 5-year OS. In the T2b group of ours, NACT was correlated with a decrease in 5-year DFS than R-CT; nevertheless, it was not correlated with 5-year OS. Duenas-Gonzalez et al. [24] did not detect any difference in response and viability between post-chemotherapy surgery and standard cisplatin-based chemoradiotherapy. Similar results were obtained in the T1, T2a and T3 groups in ours, and no statistical distinction in 5-year OS and DFS was noted between NACT and R-CT. Evidently, in FIGO 2018 stage IIIC, patients in the T1 and T2a groups potentially benefited more from ARH than NACT; meanwhile, in the T2b group, NACT was not efficaciously advantageous. Therefore, NACT should be used with caution in stage T1,T2a and T2b.
Previous studies predominantly used FIGO 2009 staging, included cases with positive and negative LN, and did not exclude patients treated with laparoscopic surgery. Differences in adjuvant-treatment regimens and treatment courses also existed. Several studies advocate that LN metastasis is an important factor affecting CC prognosis [25,26,27,28]. Results of LACC study in 2018 [29] suggested that laparoscopic surgery had adverse oncology outcomes in patients with CC. Therefore, ours only included LN-positive cases and was limited to laparotomy-treated patients, thus potentially justifying the inconsistencies between this and previous studies.
Strengths and weaknesses
Ours is a large-scale research based on FIGO 2018 stage IIIC CC cases. Notably, it innovatively stratified patients basing on local tumor conditions to compare the prognosis of different treatment methods. Notwithstanding, this study also had some shortcomings. First, this was a real-world, retrospective analysis, resulting in unbalanced data between groups. Second, the numbers of cases among the T3 subgroup were significantly different, thus potentially affecting the results’ reliability. Third, there is a limitation on a distinction between staging modalities. Patients who underwent radical surgery in the ARH and NACT groups defined LN status depended on pathological examinations after surgery; nevertheless, those who underwent radical chemoradiotherapy in the R-CT group defined lymph node status depended on CT or/and MRI before surgery. There is a difference in false positive rates between the two methods. Fourth, no further stratification based on N stage was conducted in this study due to insufficient information on the status of para-aortic lymph nodes.
Implications for practice and future research
In conclusion, for patients with FIGO 2018 stage IIIC CC, different treatment strategies impact oncological outcomes. When selecting a treatment strategy for these patients, T-staging is required. Patients with stages T1, T2a, T2b, and T3 can select ARH for initial treatment. Because of the huge differences in the number of cases among the T3 subgroups, our results require confirmation through further research. NACT is not recommended for patients with stage T1, T2a and T2b. Evidently, the recommended treatment methods for patients with stage IIIC CC in the guidelines are debatable, and more prospective studies are warranted.
Conclusions
R-CT oncological outcomes were not entirely superior to those of NACT or ARH under different local tumor factors with stage IIIC. NACT is not suitable for stage T1, T2a and T2b. Nevertheless ARH is potentially applicable to stage T1, T2a, T2b and T3. Stage T3 results require confirmation through further research due to disparity in case numbers in each subgroup.
Availability of data and materials
The datasets used and/or analyzed for the current study are available from the corresponding author upon reasonable request.
Abbreviations
- CC:
-
Cervical cancer
- FIGO:
-
International Federation of Gynecology and Obstetrics
- NCCN:
-
National Comprehensive Cancer Network
- ARH:
-
Abdominal radical hysterectomy
- TNM:
-
Tumor-node-metastasis
- NACT:
-
Neoadjuvant chemotherapy
- R-CT:
-
Radical chemoradiotherapy
- SCC:
-
Squamous cell carcinoma
- ASC:
-
Adenosquamous cell carcinoma
- OS:
-
Overall survival
- DFS:
-
Disease-free survival
- AC:
-
Adenocarcinoma
References
Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. Int J Gynecol Obstet. 2018;143:22–36.
Merz J, Bossart M, Bamberg F, Eisenblaetter M. Revised FIGO staging for cervical cancer - a new role for MRI. RoFo Fortschritte auf dem Gebiet der Rontgenstrahlen und der Bildgeb Verfahren. 2020;192:937–44.
Salvo G, Odetto D, Pareja R, Frumovitz M, Ramirez PT. Revised 2018 International Federation of Gynecology and Obstetrics (FIGO) cervical cancer staging: a review of gaps and questions that remain. Int J Gynecol Cancer. 2020;30:873–8.
2022 NCCN clinical practice guideline sinoncology,cervical cancer. version1. National Comprehensive Cancer Network. 2022. https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf.
Li P, Liu P, Yang Y, Wang L, Liu J, Bin X, et al. Hazard ratio analysis of laparoscopic radical hysterectomy for IA1 With LVSI-IIA2 Cervical cancer: identifying the possible contraindications of laparoscopic surgery for cervical cancer. Front Oncol. 2020;10:1002.
Chen C, Wang W, Liu P, Li P, Wang L, Jin S, et al. Survival after abdominal Q-M Type B versus C2 radical hysterectomy for early-stage cervical cancer. Cancer Manag Res. 2019;11:10909–19.
Chen C, Duan H, Zhang W, Zhao H, Wang L, Kang S, et al. Comparison of survival outcomes with or without Para-aortic lymphadenectomy in surgical patients with stage IB1-IIA2 cervical cancer in China from 2004 to 2016. BMC Cancer. 2021;21:1–11.
Olawaiye AB, Baker TP, Washington MK, Mutch DG. The new (Version 9) American Joint Committee on Cancer tumor, node, metastasis staging for cervical cancer. CA Cancer J Clin. 2021;0:1–12.
Matsuo K, Machida H, Mandelbaum RS, Konishi I, Mikami M. Validation of the 2018 FIGO cervical cancer staging system. Gynecol Oncol. 2019;152:87–93.
Grigsby PW, Massad LS, Mutch DG, Powell MA, Thaker PH, McCourt C, et al. FIGO 2018 staging criteria for cervical cancer: Impact on stage migration and survival. Gynecol Oncol. 2020;157:639–43.
Wright JD, Matsuo K, Huang Y, Tergas AI, Hou JY, Khoury-Collado F, et al. Prognostic Performance of the 2018 International Federation of Gynecology and Obstetrics Cervical Cancer Staging Guidelines. Obstet Gynecol. 2019;134:49–57.
Tomizawa K, Kaminuma T, Murata K, Noda S-E, Irie D, Kumazawa T, et al. FIGO 2018 staging for cervical cancer: influence on stage distribution and outcomes in the 3D-image-guided brachytherapy Era. Cancers (Basel). 2020;12:1–10.
Li S, Hu T, Lv W, Zhou H, Li X, Yang R, et al. Changes in Prevalence and Clinical Characteristics of Cervical Cancer in the People’s Republic of China: a Study of 10,012 Cases From a Nationwide Working Group. Oncologist. 2013;18:1101–7.
Wu S-G, Zhang W-W, He Z-Y, Sun J-Y, Wang Y, Zhou J. Comparison of survival outcomes between radical hysterectomy and definitive radiochemotherapy in stage IB1 and IIA1 cervical cancer. Cancer Manag Res. 2017;9:813–9.
Landoni F, Colombo A, Milani R, Placa F, Zanagnolo V, Mangioni C. Randomized study between radical surgery and radiotherapy for the treatment of stage IB–IIA cervical cancer: 20-year update. J Gynecol Oncol. 2017;28(3):e34.
Yan RN, Zeng Z, Liu F, Zeng YY, He T, Xiang ZZ, et al. Primary radical hysterectomy vs chemoradiation for IB2-IIA cervical cancer: a systematic review and meta-analysis. Medicine (Baltimore). 2020;99:e18738.
Jang T-K, Shin S-J, Chung H, Kwon S-H, Cha S-D, Lee E, et al. A retrospective comparison of outcome in IB2 and IIA cervical cancer patients treated with primary concurrent chemoradiation versus radical hysterectomy with or without tailored adjuvant therapy. Obstet Gynecol Sci. 2017;60:549–57.
Bansal N, Herzog TJ, Shaw RE, Burke WM, Deutsch I, Wright JD. Primary therapy for early-stage cervical cancer: radical hysterectomy vs radiation. Am J Obstet Gynecol. 2009;201:485.e1-485.e9.
Hoekman EJ, Knoester D, Peters AAW, Jansen FW, de Kroon CD, Hilders CGJM. Ovarian survival after pelvic radiation: transposition until the age of 35 years. Arch Gynecol Obstet. 2018;298:1001–7.
Bergmark K, Åvall-Lundqvist E, Dickman PW, Henningsohn L, Steineck G. Vaginal changes and sexuality in women with a history of cervical cancer. N Engl J Med. 1999;340:1383–9.
Zhao H, He Y, Yang S-L, Zhao Q, Wu Y-M. Neoadjuvant chemotherapy with radical surgery vs radical surgery alone for cervical cancer: a systematic review and meta-analysis. Onco Targets Ther. 2019;12:1881–91.
Kim HS, Kim JY, Park NH, Kim K, Chung HH, Kim YB, et al. Matched-case comparison for the efficacy of neoadjuvant chemotherapy before surgery in FIGO stage IB1-IIA cervical cancer. Gynecol Oncol. 2010;119:217–24.
Gupta S, Maheshwari A, Parab P, Mahantshetty U, Hawaldar R, Sastri S, et al. Neoadjuvant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy in patients with stage IB2, IIA, or IIB squamous cervical cancer: a randomized controlled trial. J Clin Oncol. 2018;36:1548–55.
Duenas-Gonzalez A, Lopez-Graniel C, Gonzalez-Enciso A, Mohar A, Rivera L, Mota A, et al. Concomitant chemoradiation versus neoadjuvant chemotherapy in locally advanced cervical carcinoma: results from two consecutive phase II studies. Ann Oncol. 2002;13:1212–9.
Li X, Wei LC, Zhang Y, Zhao LN, Li WW, Ping LJ, et al. The prognosis and risk stratification based on pelvic lymph node characteristics in patients with locally advanced cervical squamous cell carcinoma treated with concurrent chemoradiotherapy. Int J Gynecol Cancer. 2016;26:1472–9.
Song S, Kim JY, Kim YJ, Yoo HJ, Kim SH, Kim SK, et al. The size of the metastatic lymph node is an independent prognostic factor for the patients with cervical cancer treated by definitive radiotherapy. Radiother Oncol. 2013;108:168–73.
Okazawa M, Mabuchi S, Isohashi F, Suzuki O, Ohta Y, Fujita M, et al. The prognostic significance of multiple pelvic node metastases in cervical cancer patients treated with radical hysterectomy plus adjuvant chemoradiotherapy. Int J Gynecol Cancer. 2012;22:490–7.
Hong KS, Ju W, Choi HJ, Kim JK, Kim MH, Cho KS. Differential diagnostic performance of magnetic resonance imaging in the detection of lymph node metastases according to the tumor size in early-stage cervical cancer patients. Int J Gynecol Cancer. 2010;20:841–6.
Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N Engl J Med. 2018;379:1895–904.
Acknowledgements
We thank Min Hao (The Second Hospital of Shanxi Medical University), Bin Ling (China-Japan Friendship Hospital), Lixin Sun and Hongwei Zhao (Shanxi Cancer Hospital), Jihong Liu and Lizhi Liang (Sun Yat-sen University Cancer Center), Lihong Lin and Yu Guo (Anyang Tumor Hospital), Li Wang (The Affiliated Tumor Hospital of Zhengzhou University), Weidong Zhao (Anhui Provincial Cancer Hospital), Yan Ni (The Yuncheng Central Hospital of Shanxi Province), Wentong Liang and Donglin Li (Guizhou Provincial People’s Hospital), Xuemei Zhan and Mingwei Li (Jiangmen Central Hospital), Weifeng Zhang (Ningbo Women & Children’s Hospital), Peiyan Du (The Affiliated Cancer Hospital and Institute of Guangzhou Medical University), Ziyu Fang (Liuzhou Workers’ Hospital), Rui Yang (Shenzhen Hospital of Peking University), Long Chen (Qingdao Municipal Hospital), Encheng Dai and Ruilei Liu (Linyi People’s Hospital), Yuanli He and Mubiao Liu (Zhujiang Hospital, Southern Medical University), Jilong Yao and Zhihua Liu (Shenzhen Maternity & Child Health Hospital), Xueqin Wang (The Fifth Affiliated Hospital of Southern Medical University), Anwei Lu (Maternal and Child Health Hospital of Guiyang Province), Shuangling Jin (Peace Hospital affiliated to Changzhi Medical College), Ben Ma (Guangzhou First People’s Hospital), Zhonghai Wang (Shenzhen Nanshan People’s Hospital), Lin Zhu (The Second Hospital of Shandong University), Hongxin Pan (The Third Affiliated Hospital of Shenzhen University), Qianyong Zhu (No. 153. Center Hospital of Liberation Army/Hospital No. 988 of the Chinese People’s Liberation Army Joint Support Force), Dingyuan Zeng and Zhong Lin (Maternal and Child Health Care Hospital of Liuzhou), Xiaohong Wang (Laiwu People’s Hospital/Jinan City People’s Hospital), and Bin Zhu (The Affiliated Yiwu Women and Children Hospital of Hangzhou Medical College) for their contribution during the data collection.
Funding
This study received funding from the National Science and Technology Support Program of China (Grant No. 2014BAI05B03), the National Natural Science Fund of Guangdong (Grant No. 2015A030311024), and the Science and Technology Plan of Guangzhou (Grant No. 158100075).
Author information
Authors and Affiliations
Contributions
All authors approved the final version of the study. Yanna Ye, Zhiqiang Li, Shan Kang and Yongxiu Yang contributed equally to the work. Yanna Ye conceived, designed and supervised the study, interpreted the data, and developed and revised the manuscript. Zhiqiang Li carried out the literature search, data collection, data analysis and interpretation and drafted and revised the manuscript. Shan Kang conceived and designed the study, interpreted the data, and developed and revised the manuscript. Yongxiu Yang carried out the literature search, data analysis and interpretation and figures and drafted and revised the manuscript. Bing Ling provided resources and contributed to writing the original draft. Jilong Yao and Pengfei Li provided resources and validation. Xueqin Wang and Shipeng Gong provided resources and software. Huijiang Fan provided resources and software. Yanxiang Kong and Yuye Cao carried out the data analysis and interpretation. Jinghe Lang conceived the study. Ping Liu and Chunlin Chen conceived, designed and supervised the study.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was accomplished following the ethical principles according to the Declaration of Helsinki 1964. This retrospective study was approved by the Ethics Committee of the Nanfang Hospital of Southern Medical University (approval number NFEC-2017–135 and clinical trial number CHiCTR1800017778; International Clinical Trials Registry Platform Search Port, https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR1800017778, registered at 14/08/2018), who deemed that written informed consent was not necessarily due to the retrospective nature of the research and concealment of patient information.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ye, Y., Li, Z., Kang, S. et al. Treatment of FIGO 2018 stage IIIC cervical cancer with different local tumor factors. BMC Cancer 23, 421 (2023). https://doi.org/10.1186/s12885-023-10801-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-10801-w