Abstract
Background
Breast cancer (BC) is a prevalent disease that harms women's health, and in-depth investigations of the pathogenesis, treatment, and prevention of BC are the focus of many research programs. Chidamide (CHI) is a histone deacetylase suppressor that depresses histone deacetylase functions, thereby influencing cell growth through an epigenetic mechanism. However, CHI effects upon BC are largely unknown. Present research aimed to confirm the possibility of using CHI combined with chemotherapy drug doxorubicin (DOX) to prevent chemotherapeutic BC resistance in vivo and in vitro.
Methods
In this study, CCK8 (a plate colony formation assay) was applied to detect cell proliferation. Flow cytometry detection showed the apoptotic cell death of both T47D and MCF-7 cells. Nude mouse xenografts were used to detect tumor growth and pulmonary metastasis. High-throughput sequencing was used to detect expression of different genes.
Results
Our data showed that CHI treatment reduced BC cell proliferation, tumor growth, and cell invasion. CHI treatments stimulated BC cell apoptosis by promoting ULK2-mediated autophagy and increasing MCF-7 cell sensitivity to DOX, resulting in decreased tumor growth.
Conclusion
Collectively, our results illustrated that CHI enhanced DOX cytotoxicity by promoting apoptosis and autophagy in BC cells, which advised that CHI could be a candidate drug for BC patient treatments.
Similar content being viewed by others
Background
Breast cancer (BC) is a life-threatening malignant cancer commonly diagnosed among females around the globe [1]. Despite developments in diagnosis and therapeutic strategies, the prognosis for BC patients is not promising because of high chemoresistance and metastasis frequency [2,3,4,5]. Thus, a better understanding of essential signaling pathways and new therapeutic target discovery are crucial to provide a better prognosis for BC patients.
Chidamide (CHI) was the first subtype-selective histone deacetylase inhibitor (HDACi) that China synthesized to develop independently, and it selectively inhibits HDAC10 in class IIb and HDAC3, HDAC2, and HDAC1 in class I [6,7,8,9]. CHI is applied to treat BC due to its comfortable administration, good curative effects, strong targeting, and few adverse reactions. In combined therapy, Jiang et al. [10] found that several oncogenic signaling pathways could be simultaneously targeted, which increases the probability of preventing drug resistance in difficult-to-cure advanced BC.
In the present investigation, we tested CHI efficacy using BC cell lines. CHI has a synergistic sensitization effect with chemotherapy drug doxorubicin (DOX), as it enhances DOX cytotoxicity by promoting autophagy and apoptosis in BC cells. Therefore, we also evaluated the potential of combining CHI with DOX to prevent BC chemotherapeutic resistance. Our results provided the experimental foundation for further clinical applications of CHI.
Methods
Ethics statement
Our group obtained BALB/c nude mice (aged 4 weeks with 15 ~ 20 g weight) from Shanghai SLAC Laboratory Animal Co., Ltd., Shanghai, China. Ethics Committee at Guangdong Provincial People's Hospital (KY2020-675–01) approved all procedures, which we carried out following ARRIVE guidelines. Surgical processes were performed with anesthesia to minimize suffering. Our group anesthetized mouse through intraperitoneal injection with 30 mg/kg of sodium pentobarbital.
Cell culture
Our team purchased human BC cell lines (T47D and MCF-7) from American Type Culture Collection (Manassas, VA, USA), which our team cultivated in Dulbecco’s Modified Eagle’s medium (Gibco, Grant Island, NY, USA). We supplied cell lines with 10% FBS in humidified atmosphere including 5% CO2 at 37 °C. Our team purchased CHI from Chipscreen Biosciences (Shenzhen, China). We dissolved CHI in DMSO and treated T47D along with MCF-7 cells via different CHI concentrations. We purchased DOX from Rhawn (Shanghai, China), which was dissolved in DMSO to generate different concentrations.
Next-generation and strand-specific RNA-Seq library
Our team gained total RNA from MCF-7 cells with or without CHI treatment. After agarose electrophoresis and Nanodrop inspection as well as quantification concerning total RNA samples, oligo (dT) magnetic beads (rRNA removal kit was utilized directly if RNA was prokaryotic or degraded) were employed for mRNA enrichment. All RNA sequencing libraries were prepared applying the kit, and the steps included RNA fragment inversion into the first-strand cDNA with random primers, additional dUTP for the synthesis regarding double-strand cDNA terminal repair, second-strand cDNA, addition of A, Illumina matching connector connections, and PCR amplification to prepare the final library. The library that constructed was inspected utilizing Agilent 2100 system (Santa Clara, CA, USA), quantified employing quantitative PCR, which we sequenced using Illumina NovaSeq 6000 sequencer (San Diego, CA, USA).
Cell viability analysis
Our lab utilized a cell counting kit (CCK8, Dojindo, Japan) to validate CHI or DOX effects on cell viability separately or integratively. We incubated cells in 10% CCK8 diluted in normal culture medium at 37 °C until visual color transformation happened. Our team measured proliferation rates at 1, 2, and 3 days post treatment. Our lab detected absorbance in each well utilizing microplate reader set at 450 nm.
Transwell migration assay
We assessed cell migration utilizing Costar Transwell cell culture inserts (Corning Incorporated, Corning, NY, USA) following manufacturer’s guidelines. After 1 d incubation, our team erased cells from transwell chamber upper surfaces using cotton swabs. We fixed these cells on the lower surfaces using methanol for 10 min, which we stained with crystal violet. We photographed and calculated the percentage of stained cells in five fields that were selected at random.
Plate colony formation assay
We used MCF-7 and T47D cells treated with CHI, DOX, or CHI + DOX at different concentrations to prepare count cell suspensions. We added 200 cells to wells of 6-well plates and further cultured them in an incubator for 2 weeks. We changed the cell culture medium every 3 days and observed the cell state and captured photographs using a fluorescence microscope prior to the experiment ending. The cells were cleaned twice utilizing phosphate buffered saline (PBS). Afterwards, 500 µL crystal violet solution (0.1%) were put to each well to stain the cells for 5 min. After washing the cells three times utilizing ddH2O, we photographed the cells under the microscope using a digital camera.
Annexin V staining
We used the Annexin V-FITC/PI Assay Kit (ImmunoWay, Plano, TX, USA) following manufacturer’s recommendations to assess apoptosis of the cells in each treatment. We washed the cells twice (1 × 105) via PBS and then suspended them in 100 μL of binding buffer, which we stained using 5 μL of Annexin V-FITC for 0.5 h in dark. We added 5 μL of propidium iodide for 5 min and then added binding buffer to bring the total volume to 250–300 μL. We measured fluorescence utilizing flow cytometer (BD, Franklin Lakes, NJ, USA). Quantitative values were calculated as the average Annexin V-positive cell percentiles of three independent experiments.
In vivo experiments
To establish the nude mice models of BC, 2 × 106 MCF-7 cells without Matrigel were injected into nude mice flank. 14 days after cell injection, we randomly divided tumor-bearing mice into 4 groups with 5 mice per subgroup: i) control group (normal saline); ii) DOX group (5 mg DOX/kg body weight); iii) CHI group (5 mg CHI/kg body weight); and iv) CHI + DOX group (5 mg CHI + 5 mg DOX/kg body weight). We injected the drugs every 3 days to monitor tumor volumes until we euthanized the mouse. We measured tumor weight and volume at the end of the experiment. The tumor volume was computed by 1/2 (length × width × width).
For the tumor metastasis analysis, our team suspended luminescence-labeled MCF-7 cells (2 × 105) in sterile PBS and then injected into individual nude mice tail vein. After 4 weeks, we evaluated lung metastasis by applying an in vivo bioluminescence imaging system. The metastatic foci number in lung tissues was obtained following hematoxylin and eosin (HE) staining.
Immunohistochemical and immunofluorescence analyses
We fixed tumor tissues in 4% paraformaldehyde solution, which we embedded in paraffin sliced them into 5 μm thick sections. Technician stained them using HE or antibodies against Ki67 and examined them under Zeiss Axioplan 2 microscope (Carl Zeiss AG, Oberkochen, Germany) equipped with digital instrument to confirm cell growth.
For the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay in vivo, we first fixed tumor tissues by applying 4% paraformaldehyde and permeabilized them with 1% Triton X-100. We conducted TUNEL process utilizing an in situ cell apoptosis detection kit (Roche, Shanghai, China). We mounted cells in SlowFade Antifade by DAPI (Solarbio, Beijing, China), which we visualized under the Zeiss Axioplan 2 microscope.
For the autophagy assay in vivo, we first fixed tumor tissues with 4% paraformaldehyde, embedded them in paraffin, sliced them into 5 μm thick sections, stained them with LC3 and ULK2, and viewed them under the Axioplan 2 microscope. ImageJ software was used for fluorescence density analysis.
Statistical analyses
Data are represented by means ± standard deviation (SD). Our team performed statistics analyses using GraphPad Prism (La Jolla, CA, USA) to identify significant differences between groups. We considered P-values ≤ 0.05 to be statistical significance. Our team used two-tailed Student’s t-tests to obtain significant differences between 2 groups. One-way analysis of variance with post hoc Bonferroni tests were utilized to compute significant differences among > = 3 groups.
Results
CHI treatment inhibited BC cell proliferation and tumor growth
The CCK8 detection kit showed that the CHI treatment suppressed BC cell growth depending on concentration in T47D and MCF-7 cells. The half-maximal inhibitory concentrations (IC50s) for MCF-7 and T47D were 20 and 25 nM, respectively (Fig. 1A–B), and the colony formation assay showed that CHI treatment significantly suppressed proliferation of T47D and MCF-7 cells (Fig. 1C–D). The in vivo xenograft mouse assay demonstrated that tumor weight and volume in nude mice injected with MCF-7 cells decreased after CHI treatment (Fig. 1E–G). Additionally, immunohistochemical analysis showed that CHI treatment decreased Ki67 expression in tumor tissues (Fig. 1H–I). These results suggested that CHI treatment suppressed BC proliferation and tumor growth.
CHI treatment inhibited BC cell proliferation and tumor growth. A, B CCK8 detection showed the proliferation ability of T47D (A) and MCF-7 (B) cells after treatment with different doses of CHI for 24 h. C, D Colony formation assay results showed T47D and MCF-7 cell proliferation after treatment with different doses of CHI. Data are expressed as means ± SD. ***P < 0.001 vs. 0 nM CHI. E Images of nude mouse xenografts regarding MCF-7 cells. F, G Tumor volumes in mice were measured every 5 days. Data are expressed as means ± SD. ***P < 0.001 vs. CHI. H, I Immunohistochemical detection showed the expression of Ki67 in the tumor tissues. Data are expressed as means ± SD. ***P < 0.001 vs. 0 nM CHI. Scal bar, 100 μm
CHI treatment inhibited BC cell invasion
Results of the transwell migration assay showed that CHI treatment suppressed migration of MCF-7 and T47D cells (Fig. 2A–B). Live imaging detection revealed pulmonary metastasis of MCF-7 cells, and HE staining showed that CHI treatment reduced this metastasis capability by reducing the metastatic foci number in lung tissues (Fig. 2C–E). These findings indicated that CHI treatment inhibited BC cell invasion in vivo and in vitro.
CHI treatment inhibited BC cell invasion. A, B Transwell data showed the migration ability of both T47D and MCF-7 cells after treatment with CHI. Data are expressed as means ± SD. ***P < 0.001 vs. 0 nM CHI. Scal bar, 50 μm. C Live imaging detection showed pulmonary metastasis of the MCF-7 cells. D, E The numbers of metastatic foci in lung tissues were computed following HE staining. Data are expressed as means ± SD. ***P < 0.001 vs. NC. Scal bar, 2.5 mm
CHI treatment promoted BC cell apoptosis by promoting autophagy
Flow cytometry revealed that CHI treatment promoted cell apoptosis in MCF-7 and T47D cells, but 3-Ma (autophagy inhibitor) treatment reversed CHI-induced cell apoptosis (Fig. 3A–B). These results suggested that autophagy played a role in CHI-mediated BC cell apoptosis. Immunofluorescence detection showed that CHI treatment promoted LC3 expression, but 3-Ma treatment inhibited CHI-induced LC3 expression (Fig. 3C–D). Thus, CHI treatment appeared to promote BC cell apoptosis by promoting autophagy.
CHI treatment promoted BC apoptosis by promoting autophagy. A, B Flow cytometry detection showed the apoptosis of both MCF-7 and T47D cells after treatment with CHI and 3-Ma (autophagy inhibitor). Results are expressed as means ± SD. **P < 0.01, ***P < 0.001 vs. NC. C, D Immunofluorescence detection showed the LC3 expression in T47D and MCF-7 cells. The results are expressed as means ± SD. ***P < 0.001 vs. NC. Scal bar, 20 μm
CHI treatment increased MCF-7 and T47D cell sensitivity to DOX
The CCK8 detection results showed that DOX treatment suppressed cell proliferation in a dose-dependent manner in T47D and MCF-7 cells (Fig. 4A). In CHI + DOX treatment group, flow cytometry revealed that DOX treatment increased CHI-induced apoptosis in T47D and MCF-7 cells (Fig. 4B–C). This result suggested that CHI treatment improved T47D and MCF-7 cell sensitivity to DOX.
CHI treatment increased the sensitivity of both MCF-7 and T47D cells to DOX. A CCK8 detection showed the proliferation ability of T47D and MCF-7 cells after treatment with different doses of DOX for 24 h. B, C Flow cytometry detection illustrated the MCF-7 and T47D cell apoptosis after treatment with CHI and DOX. The results are expressed as means ± SD. *P < 0.05, ***P < 0.001 vs. NC
CHI treatment increased MCF-7 cell sensitivity to DOX via increased ULK2-mediated autophagy and decreased tumor growth
The in vivo xenograft mouse assay showcased that tumor volume and weight in nude mice injected with MCF-7 cells decreased after CHI treatment (Fig. 5A–C) but that treatment with 15 mg/kg of DOX did not inhibit MCF-7 tumor growth. However, treatment with the combination of CHI + DOX significantly decreased MCF-7 tumor growth. Immunohistochemical staining for Ki67 expression also showed that CHI treatment increased the MCF-7 cell sensitivity to DOX through decreased tumor growth (Fig. 5D–E). TUNEL staining showed that the combination of CHI + DOX resulted in significantly higher apoptosis in tumor tissues compared to CHI alone (Fig. 5F–G). Immunofluorescence detection showed that CHI treatment promoted LC3 expression, and autophagy-related protein LC3 expression was also increased with CHI + DOX treatment (Fig. 5H–I). Thus, CHI treatment appeared to have improved MCF-7 cell sensitivity to DOX by promoting autophagy.
CHI treatment increased the sensitivity of MCF-7 cells to DOX, as indicated by decreased tumor growth. A Illustrative images of nude mouse xenografts regarding MCF-7 cells. B, C Tumor volumes in mice were detected every 5 days. Results are expressed as means ± SD. ***P < 0.001 vs. CHI. D, E Immunohistochemical detection showed the expression of Ki67 in the tumor tissues. Results are expressed as means ± SD. ***P < 0.001 vs. NC. Scal bar, 100 μm. F, G TUNEL staining showed the apoptosis in tumor tissues. Data are expressed as means ± SD. ***P < 0.001 vs. NC. Scal bar, 100 μm. H, I Immunofluorescence detection showed the expression of autophagy related protein LC3 in the tumor tissues. Results are expressed as means ± SD. ***P < 0.001 vs. NC. Scal bar, 20 μm
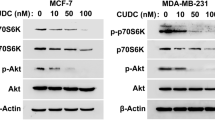
High-throughput sequencing revealed that CHI treatment regarding MCF-7 cells affected gene expression at mRNA level (Fig. 6A and B, Supplementary materials 1). The KEGG pathway enrichment analyses inferred that autophagy pathways participated in CHI-induced autophagy apoptosis via increased ULK2 expression [11] (Fig. 6C, Supplementary materials 2). Immunofluorescence detection showed that CHI treatment promoted ULK2 expression and that autophagy-related ULK2 protein expression was also increased with CHI + DOX treatment (Fig. 6D and E).
CHI treatment increased ULK2-mediated autophagy. A Representative images of the scatter plot. B Clustered heat map for MCF-7 cells after CHI treatment compared with the normal control cells (NC). C Results of high-throughput sequencing showing differential expression of genes and results of KEGG pathway enrichment analyses after CHI treatment [11]. D, E Immunofluorescence detection showed ULK2 expression in the tumor tissues. Results are expressed as means ± SD. ***P < 0.001 vs. NC. Scal bar, 20 μm
Discussion
DOX is an indispensable chemotherapy for BC treatment. However, tumor cells can become resistant to chemotherapy over time, which is the main reason for chemotherapy failure along with tumor recurrence [12,13,14]. Drug resistance is a complicated process that involves large-scale mechanisms [15]. Current study found that CHI treatment significantly decreased BC cell proliferation in dose-dependent manner. Additionally, IC50 values of CHI for T47D and MCF-7 cells were 25 and 20 nM, respectively, which were similar to those previously reported [7]. DOX based combinations with TOR, CQ, or CNC were found to affect positively DOX effectiveness and reduce DOX doses applied to BC cells via autophagy modulation [16,17,18]. Thus, combined treatment might be a therapeutic agent candidate targeting various targets such like autophagy, apoptosis, and notch signaling pathways [19]. We also found that CHI treatment inhibited MCF-7-mediated tumor growth and BC cell invasion in in vivo and in vitro experiments, likely by promoting BC cell apoptosis and autophagy. Treatment with the autophagy inhibitor 3-Ma reversed the CHI-induced BC cell apoptosis, suggesting that CHI triggered autophagy and promoted cell autophagy and apoptosis.
To determine whether CHI could reverse the resistance of BC to DOX, we first determined the IC50 of DOX using CCK8 analysis and found that DOX treatment suppressed cell proliferation. The dose depends on T47D and MCF-7 cells. Subsequent flow cytometry analysis showed that CHI treatment increased T47D and MCF-7 cell sensitivity to DOX. Our in vivo experiment showed that CHI treatment improved MCF-7 cell sensitivity to DOX via decreasing cancer cell growth and increasing autophagy and apoptosis. Previous studies also reported that CHI treatment increased cleaved caspase-7 and LC3 II/I expression [20]. The autophagy assay and Annexin V data showcased that autophagosome microtubule associated protein LC3-II abnormally incremented. Previously, Zhou et al. [21] reported that CHI induced cells to enter the late phase of apoptosis. However, the regulatory mechanisms involved in these processes are still largely unclear.
Therefore, in this study we also used high-throughput sequencing to analyze the abnormal expression of genes at the mRNA level in MCF-7 cells with or without CHI treatment. The result showed that CHI treatment promoted ULK2 expression. ULK2 is homologous to mammalian autophagy-associated proteins that are essential for autophagy initiation [18, 19]. However, validating the underlying intercellular molecular mechanisms and the involvement of ULK2 in autophagy induced by CHI is still needed.
Conclusion
In conclusion, CHI combined with DOX inhibited cell growth synergistically, increased autophagy activation, and induced apoptosis. This could be an essential mechanism by which CHI combined with DOX can prevent BC drug resistance. More studies are needed to verify the CHI + DOX treatment efficiency and safety in vivo and in vitro to suppress drug resistance in BC.
Availability of data and materials
The data used and/or analyzed during present investigation are available from corresponding author upon requests.
Abbreviations
- BC:
-
Breast cancer
- CHI:
-
Chidamide
- DOX:
-
Doxorubicin
- HDACi:
-
Histone deacetylase inhibitor
- PBS:
-
Phosphate buffered saline
- HE:
-
Hematoxylin and eosin
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Polyak K. Breast cancer: origins and evolution. J Clin Invest. 2007;117(11):3155–63.
Maishman T, Cutress RI, Hernandez A, Gerty S, Copson ER, Durcan L, et al. Local recurrence and breast oncological surgery in young women with breast cancer: The POSH observational cohort study. Ann Surg. 2017;266(1):165–72.
Przanowski P, Lou S, Tihagam RD, Mondal T, Conlan C, Shivange G, et al. Oncogenic TRIM37 links Chemoresistance and metastatic fate in triple-negative breast cancer. Cancer Res. 2020;80(21):4791–804.
Kim C, Gao R, Sei E, Brandt R, Hartman J, Hatschek T, et al. Chemoresistance evolution in triple-negative breast cancer delineated by single-cell sequencing. Cell. 2018;173(4):879-93.e13.
Gao S, Li X, Zang J, Xu W, Zhang Y. Preclinical and Clinical Studies of Chidamide (CS055/HBI-8000), An Orally Available Subtype-selective HDAC Inhibitor for Cancer Therapy. Anticancer Agents Med Chem. 2017;17(6):802–12.
Cao L, Zhao S, Yang Q, Shi Z, Liu J, Pan T, et al. Chidamide Combined With Doxorubicin Induced p53-driven cell cycle arrest and cell apoptosis reverse multidrug resistance of breast cancer. Front Oncol. 2021;11:614458.
Krusche CA, Wülfing P, Kersting C, Vloet A, Böcker W, Kiesel L, et al. Histone deacetylase-1 and -3 protein expression in human breast cancer: a tissue microarray analysis. Breast Cancer Res Treat. 2005;90(1):15–23.
Seo J, Min SK, Park HR, Kim DH, Kwon MJ, Kim LS, et al. Expression of Histone Deacetylases HDAC1, HDAC2, HDAC3, and HDAC6 in Invasive Ductal Carcinomas of the Breast. J Breast Cancer. 2014;17(4):323–31.
Jiang Z, Li W, Hu X, Zhang Q, Sun T, Cui S, et al. Tucidinostat plus exemestane for postmenopausal patients with advanced, hormone receptor-positive breast cancer (ACE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(6):806–15.
Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45(D1):D353–61.
Nedeljković M, Damjanović A. Mechanisms of chemotherapy resistance in triple-negative breast cancer-how we can rise to the challenge. Cells. 2019;8(9):957.
Chun KH, Park JH, Fan S. Predicting and overcoming chemotherapeutic resistance in breast cancer. Adv Exp Med Biol. 2017;1026:59–104.
Tang Y, Wang Y, Kiani MF, Wang B. Classification, treatment strategy, and associated drug resistance in breast cancer. Clin Breast Cancer. 2016;16(5):335–43.
Xu X, Zhang L, He X, Zhang P, Sun C, Xu X, et al. TGF-β plays a vital role in triple-negative breast cancer (TNBC) drug-resistance through regulating stemness, EMT and apoptosis. Biochem Biophys Res Commun. 2018;502(1):160–5.
Abdel-Mohsen MA, Abdel Malak CA, El-Shafey ES. Influence of copper (I) nicotinate complex and autophagy modulation on doxorubicin-induced cytotoxicity in HCC1806 breast cancer cells. Adv Med Sci. 2019;64(1):202–9.
Klionsky DJ, Abdel-Aziz AK, Abdelfatah S, Abdellatif M, Abdoli A, Abel S, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)(1). Autophagy. 2021;17(1):1–382.
Abdel-Mohsen MA, Abdel Malak CA, Abou Yossef MA, El-Shafey ES. Antitumor Activity of Copper (I)-Nicotinate Complex and Autophagy Modulation in HCC1806 Breast Cancer Cells. Anticancer Agents Med Chem. 2017;17(11):1526–36.
El-Shafey ES, Elsherbiny ES. Possible selective cytotoxicity of vanadium complex on breast cancer cells involving pathophysiological pathways. Anticancer Agents Med Chem. 2019;19(17):2130–9.
Lu CT, Leong PY, Hou TY, Huang SJ, Hsiao YP, Ko JL. Ganoderma immunomodulatory protein and chidamide down-regulate integrin-related signaling pathway result in migration inhibition and apoptosis induction. Phytomedicine. 2018;51:39–47.
Zhou W, Han H, Xu J, Sun T, Feng X. Autophagic Vacuole Secretion Triggered by Chidamide Participates in TRAIL Apoptosis Effect in Breast Cancer Cells. Curr Pharm Des. 2021;27(20):2366–80.
Acknowledgements
I sincerely thank KEGG database with the permission.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All works including study conception and design, collecting the data and performing the data analysis, interpretation of the data and the completion of figures and tables and draft of the article and final approval of the submitted version were solely done by the only author.
Corresponding author
Ethics declarations
Ethics approval and participate consent
Ethics Committee at Guangdong Provincial People’s Hospital (KY2020-675–01) approved all procedures, which we conducted following the guidelines. All methods were carried out following ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, J. Chidamide enhances cytotoxicity of doxorubicin by promoting autophagy and apoptosis in breast cancer. BMC Cancer 23, 353 (2023). https://doi.org/10.1186/s12885-023-10774-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-10774-w