Abstract
Background
Treatment options for pretreated triple-negative breast cancer (TNBC) are limited. This study aimed to evaluate the efficacy and safety of apatinib, an antiangiogenic agent, in combination of etoposide for pretreated patients with advanced TNBC.
Methods
In this single-arm phase II trial, patients with advanced TNBC who failed to at least one line of chemotherapy were enrolled. Eligible patients received oral apatinib 500 mg on day 1 to 21, plus oral etoposide 50 mg on day 1 to 14 of a 3-week cycle until disease progression or intolerable toxicities. Etoposide was administered up to six cycles. The primary endpoint was progression-free survival (PFS).
Results
From September 2018 to September 2021, 40 patients with advanced TNBC were enrolled. All patients received previous chemotherapy in the advanced setting, with the median previous lines of 2 (1–5). At the cut-off date on January 10, 2022, the median follow-up was 26.8 (1.6–52.0) months. The median PFS was 6.0 (95% confidence interval [CI]: 3.8–8.2) months, and the median overall survival was 24.5 (95%CI: 10.2–38.8) months. The objective response rate and disease control rate was 10.0% and 62.5%, respectively. The most common adverse events (AEs) were hypertension (65.0%), nausea (47.5%) and vomiting (42.5%). Four patients developed grade 3 AE, including two with hypertension and two with proteinuria.
Conclusions
Apatinib combined with oral etoposide was feasible in pretreated advanced TNBC, and was easy to administer.
Clinical trial registration
Chictr.org.cn, (registration number: ChiCTR1800018497, registration date: 20/09/2018)
Similar content being viewed by others
Introduction
Triple-negative breast cancer (TNBC), characterized by the absence of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) expression, accounting for about 15-20% of breast cancers [1]. Compared to other types of breast cancer, TNBC is associated with rapid disease progression, metastasis, and recurrence, which contribute to a poor prognosis [2]. The treatment landscape of TNBC has undergone significant changes with the emergence of new therapeutic options such as PARP inhibitors for patients with BRCA mutations, immunotherapy targeting PD-L1 positive tumors, and antibody-drug conjugates [3]. Despite this, chemotherapy still plays a critical role in the management of TNBC. However, the median overall survival (OS) of chemotherapy was only 2 to 3 years, indicating a greater need for more treatment options [4].
Vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR) signal pathway plays an important role in tumor angiogenesis, which is essential for tumor growth and spread [5]. Previous studies have demonstrated patients with TNBC showed higher VEGF levels than patients with non-TNBC [6]. As a monoclonal antibody targeting VEGF, bevacizumab alone or in combination with chemotherapy showed promising efficacy for patients with advanced TNBC [7,8,9]. Apatinib is an oral tyrosine kinase inhibitor (TKI) that inhibits tumor angiogenesis by selectively targeting VEGFR-2 [10]. In 2014, a multicenter phase II trial conducted by Hu et al. [11] found that apatinib alone showed potential benefit for patients with heavily pretreated TNBC, with the median progression-free survival (PFS) of 3.3 months. In another phase II trial, camrelizumab (an immune checkpoint inhibitor) was combined with apatinib in advanced TNBC patients receiving fewer than three lines of systemic therapy [12]. The combination of camrelizumab and apatinib showed favorable efficacy and safety profiles in patients with advanced TNBC, and the median PFS was 3.7 months. Moreover, a phase II trial enrolled patients with locally advanced and metastatic TNBC who failed to at least one line of chemotherapy (including anthracycline or taxane), and administrated camrelizumab plus apatinib and eribulin [13]. The results showed the median PFS was 8.1 months, and the toxicities were manageable.
Etoposide, a cell cycle-specific antitumor drug, inhibits tumor cell proliferation by forming complexes with topoisomerases that interfere with DNA repair [14]. It can be easily administered, and is a viable option for patients with advanced metastatic breast cancer. A previous study has shown that etoposide alone is effective and safe for heavily pretreated patients with metastatic breast cancer [15]. Besides, the combination of etoposide and apatinib also showed potential efficacy for pretreated HER-2 negative metastatic breast cancer, with a median PFS of 6.9 months and a median OS of 20.4 months [16].
Recently, due to the nature of COVID-19 pandemic, many cancer patients are unable to receive intravenous drug therapy on time. Thus, there is an urgent need to explore the accessible and easy-to-use treatment options for advanced TNBC patients. This study aimed to investigate the efficacy and safety of oral apatinib plus etoposide for pretreated patients with advanced TNBC.
Methods
Study design and patients
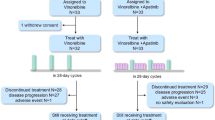
In this phase II trial, pretreated patients with advanced (recurrent or metastatic) TNBC in Harbin Medical University Cancer Hospital were enrolled. ER and PR negative was defined as < 1% in the immunohistochemistry (IHC) nuclear staining. HER-2 negative was defined as IHC staining 0 or 1+, or IHC staining 2 + with fluorescence in situ hybridization or chromogenic in situ hybridization negative. Eligible patients must have received at least one chemotherapy regimen in the advanced setting; had at least one measurable extracranial lesion according to the Response Evaluation Criteria in Advanced Solid Tumors (RECIST) version 1.1; and had an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 or 1. Patients with active brain metastases, leptomeningeal disease or uncontrolled hypertension were excluded. Those who had previously taken antiangiogenic agents (except bevacizumab) or etoposide were also excluded.
The study was approved by the Ethics Committee of the Harbin Medical University Cancer Hospital and was performed in accordance with the Declaration of Helsinki. All patients have signed the informed consent form before any procedure. The study was registered at chictr.org.cn (Number: ChiCTR1800018497) on 20/09/2018.
Procedures
Eligible patients received oral apatinib 500 mg on day 1 to 21, plus oral etoposide 50 mg on day 1 to 14 of a 3-week cycle until disease progression or intolerable toxicities. Etoposide was administered up to six cycles, and patients received apatinib alone after six cycles [17]. In case of grade ≥ 3 hematologic toxicity or grade ≥ 2 non-hematologic toxicity, a dose reduction of apatinib to 250 mg was allowed. Non-hematologic toxicities, including manageable nausea, vomiting, fever with an established cause (e.g., infection or tumor), and grade 3/4 elevated alkaline phosphatase should be treated with symptomatic management without dose suspension or dose reduction. Re-increase of the apatinib dose was not permitted in this study. For the management of treatment toxicity, no more than two dose suspensions per cycle was allowed, and the cumulative duration of dose suspensions should not exceed two weeks per cycle.
Efficacy evaluation was performed using computerized tomography or magnetic resonance imaging every two cycles according to RECIST 1.1 until disease progression or intolerable toxicities. Blood pressure was monitored three times a week. Routine blood, urine, stool, liver and kidney function, electrolytes test and electrocardiogram were performed every cycle. Adverse events (AEs) during the trial were recorded and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 5.0.
Endpoints
The primary endpoint was PFS, which defined as the time from patient enrolment to disease progression or death from any cause, whichever came first. The secondary endpoints included OS (defined as the time from patient enrolment to death from any cause), objective response rate (ORR, complete response and partial response), disease control rate (DCR, complete response, partial response and stable disease) and AEs.
Statistical analysis
Given an accrual period of 12 months, a maximum follow-up time of 18 months, and a two-sided significance level of 0.05, 34 patients with 28 events were allowed detecting an increase of PFS from 2.8 months [18] to 4.3 months with 80% power using a log-rank test. Considering a 20% dropout rate, a total of 40 patients were required. Sample size calculation was determined with PASS 15.0 software (NCSS, LLA, USA).
Descriptive statistical analyses were mainly used. For PFS and OS, patients without document event were censored at last follow-up. The Kaplan-Meier method was utilized to estimate the median PFS and OS, as well as the 95% confidence interval (CI). Subgroup analysis was conducted by liver metastasis, lung metastasis, bone metastasis, prior taxane therapy, prior anthracycline therapy, and prior platinum therapy. Statistical analysis was performed using SPSS version 25.
Results
Baseline characteristics of patients
From September 2018 to September 2021, 40 patients with advanced TNBC were included in this study. The median age was 56, with an age range of 27 to 76. Three patients (7.5%) had an ECOG PS score of 0, while 37 (92.5%) had an ECOG PS score of 1. The median number of metastatic sites of all patients was 2 (0–5). Nine (22.5%), nine (22.5%) and thirteen (32.5%) developed lung metastasis, liver metastasis and bone metastasis, respectively. There was no patient had bone-only disease. All patients received previous chemotherapy in the advanced setting, with the median previous lines of 2 (1–5). A total of 24, 32 and 20 patients received prior taxane therapy, anthracycline therapy and platinum therapy, respectively (Table 1).
Efficacy endpoints
At the cut-off date on January 10, 2022, the median follow-up was 26.8 (1.6–52.0) months. A total of 30 patients experienced disease progression or death, and 2 patients were still on the treatment. The median PFS was 6.0 (95%CI: 3.8–8.2) months (Fig. 1). Seventeen patients died, and the median OS was 24.5 (95%CI: 10.2–38.8) months. Four patients (10.0%) and 21 patients (52.5%) achieved partial response and stable disease, respectively, with an ORR of 10.0% and a DCR of 62.5%. The subgroup analysis is shown in Table 2. The median PFS was generally consistent among all subgroups. Of the patients included in the study, 42.5% had visceral metastases. In the 22.5% patients who had liver metastases, the median PFS was 7.1 months, in the 22.5% who had lung metastatic disease, the median PFS was 5.3 months, and in the 32.5% who had bone metastatic disease, the median PFS was 7.4 months. In terms of prior treatment with chemotherapeutic agents, 60% of patients had received prior taxane therapy, 80% of patients used anthracyclines, and 50% of patients received platinum. The median PFS for them was 7.1 months, 7.5 months and 8.1 months, respectively.
Safety profiles
A total of 38 patients (95.0%) experienced AE of any grade, and most of them were grade 1 or 2. The most common AEs in this study was hypertension (65%), followed by nausea (47.5%), vomiting (42.5%), and hand-foot syndrome (32.5%), which were all well controlled with symptomatic treatment (Table 3). Four cases of grade 3 AE were observed, two of which were hypertension and the other two were proteinuria, all possibly related to the use of apatinib. One patient permanently discontinued apatinib due to grade 3 hypertension, while the other three patients temporarily suspended their treatment with both apatinib and etoposide after experiencing grade 3 AEs. After the AEs were resolved to grade ≤ 1, these patients continued apatinib at a reduced dose of 250 mg while etoposide was resumed at the same dose. Besides, five patients reduced etoposide dose due to AE (mainly nausea, vomiting and hand-foot syndrome). No death related to the treatment was reported.
Discussion
TNBC is associated with poor prognosis, especially for heavily pretreated patients [19]. Novel treatment strategies are urgently needed. In this study, TNBC patients who failed to at least one line of chemotherapy in the advanced setting were enrolled. The results showed that apatinib combined with etoposide was well-tolerated in most patients, and yielded an ORR of 10.0%, and a DCR of 62.5%. The median PFS and OS were 6.0 months and 24.5 months, respectively.
Chemotherapy still plays a critical role in the treatment of TNBC. However, numerous clinical studies and practices show that chemotherapy has limited benefits, with the median OS ranged from 12.5 months to 13.4 months and the median PFS ranged from 2.8 months to 5.3 months for pretreated TNBC [20,21,22,23]. Angiogenesis is closely related to tumor growth and metastasis, and antiangiogenic treatment is one of the important strategies in the treatment of metastatic breast cancer [24]. Several studies have demonstrated the promising response rate of the bevacizumab alone or addition to chemotherapy for advanced TNBC [7,8,9], but there was no significant improvement in OS [25]. These results showed the antiangiogenic agents may benefit TNBC patients.
Apatinib selectively binds to and inhibits VEGFR-2 activity, preventing tumor angiogenesis and inhibiting tumor growth [10]. Several studies have evaluated the role of apatinib in the treatment of advanced TNBC. In a two-stage phase II study, 25 patients with heavily pretreated TNBC received oral apatinib 750 mg daily, and an additional of 59 patients received apatinib 500 mg [11]. The results showed that both doses were effective, and 500 mg is safer than 750 mg. Thus, we chose the 500 mg as the initial dose of apatinib in this study. Besides, recent studies have investigated the role of the combination of camrelizumab and apatinib in pretreated TNBC. The results showed that camrelizumab plus apatinib with and without chemotherapy was effective, with acceptable safety profiles [12, 13]. All these results demonstrated the benefit of apatinib in patients with TNBC.
Antiangiogenic agents combined with chemotherapy may have a synergistic effect, thus improving the prognosis of cancer patients [26]. The combination of apatinib and chemotherapy for patients with cancer has also been evaluated. AEROC trial found apatinib plus oral etoposide is effective and safe for patients with platinum-resistant or platinum-refractory ovarian cancer [17]. Besides, both apatinib and etoposide are oral agents, and thus the treatment can be easily administrated by patients at home without the need for infusion devices [27]. Moreover, a single-arm, phase II trial conducted by Hu et al. [16] evaluated the efficacy and safety of apatinib combined with etoposide in the later-line treatment of HER2-negative metastatic breast cancer. The initial dose of apatinib was 500 mg in patients with ECOG scores of 0–1, and 425 mg in patients with ECOG scores of 2, respectively. According to the study results, ORR was 35.5%, DCR was 87.1%, median PFS was 6.9 months, and median OS was 20.4 months. Compared to our results, the median PFS was prolonged, probably because the hormone receptor-positive patients included in that study had a better prognosis. The study also found that hormone receptor-positive patients (n = 19) had a better PFS (median: 7.4 vs. 3.1 months) than hormone receptor-negative patients (n = 12) [16].
Various other TKIs, either alone or in combination with chemotherapy, have been investigated for treating metastatic TNBC. A randomized clinical trial has compared sorafenib plus capecitabine with capecitabine alone in patients with HER2-negative metastatic breast cancer, and the median PFS was 4.3 months for sorafenib plus capecitabine and 3.0 months for capecitabine in patients with HR-negative disease [28]. A randomized phase II trial compared the efficacy of sunitinib alone and single-agent chemotherapy in patients with pretreated TNBC. The median PFS was 2.0 months in patients receiving sunitinib, and 2.7 months in patients receiving chemotherapy [29]. In addition, a phase III trial also failed to demonstrated the superiority of sunitinib plus docetaxel over docetaxel alone in terms of PFS and OS for the first-line treatment of patients with HER2-negative metastatic breast cancer [30]. The regimens in our study yielded a median PFS of 6.0 months, which was numerically higher than that of these studies. In this study, the median OS was 24.5 months, which was longer than previous studies [20,21,22,23]. This may be due to the smaller proportion of patients with visceral metastases, and the possible synergistic effect of apatinib plus etoposide in this study. In order for these findings to be confirmed, further studies are necessary.
Overall, the treatment combination appeared to be well-tolerated in most patients. A total of 65% patients developed hypertension, and most of them were well controlled after anti-hypertension treatment. Nausea and vomiting occurred in 47.5% of patients. Four patients developed grade 3 AEs, including two with hypertension and two with proteinuria. No treatment-related death was reported. Similarly, the most common grade 3 or 4 treatment-related AEs of apatinib plus etoposide were hypertension, fatigue, and thrombocytopenia in previous studies [16]. Our preliminary results suggest that the treatment may be well-tolerated, but further studies with larger sample sizes are needed to confirm these findings.
This study has some limitations. This is a single-arm trial with only 40 patients, so the results may be biased due to the small sample size and lack of a control. Therefore, further phase III randomized controlled trial with increased sample size should be conducted to further investigate the role of apatinib plus etoposide for patients with TNBC.
In conclusion, apatinib in combination with oral etoposide was feasible in pretreated advanced TNBC, with the advantage of ease of administration.
Data Availability
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Abbreviations
- TNBC:
-
Triple-negative breast cancer
- ER:
-
estrogen receptor
- PR:
-
Progesterone receptor
- HER2:
-
Human epodermal growth factor receptor 2
- VEGF:
-
vascular endothelial growth factor
- TKI:
-
tyrosine kinase inhibitor
- IHC:
-
immunohistochemistry
- RECIST:
-
Response Evaluation Criteria in Advanced Solid Tumors
- ECOG PS:
-
Eastern Cooperative Oncology Group performance status
- AEs:
-
Adverse Events
- ORR:
-
Objective response rate
- DCR:
-
Disease control rate
References
Garrido-Castro AC, Lin NU, Polyak K. Insights into Molecular Classifications of Triple-Negative breast Cancer: improving patient selection for treatment. Cancer Discov. 2019;9(2):176–98.
Zagami P, Carey LA. Triple negative breast cancer: pitfalls and progress. NPJ Breast Cancer. 2022;8(1):95.
Kumar H, Vishal Gupta N, Jain R, Madhunapantula SV, Saravana Babu C, Kesharwani SS, Dey S, Jain V. A Review of Biological Targets and Therapeutic Approaches in the Management of Triple-Negative Breast Cancer.J Adv Res2023.
Sharma P. Biology and Management of Patients with Triple-Negative breast Cancer. Oncologist. 2016;21(9):1050–62.
Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res. 2006;12(17):5018–22.
Linderholm BK, Hellborg H, Johansson U, Elmberger G, Skoog L, Lehtio J, Lewensohn R. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer. Ann Oncol. 2009;20(10):1639–46.
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–76.
Miles DW, Chan A, Dirix LY, Cortes J, Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28(20):3239–47.
Ferrero JM, Hardy-Bessard AC, Capitain O, Lortholary A, Salles B, Follana P, Herve R, Deblock M, Dauba J, Atlassi M, et al. Weekly paclitaxel, capecitabine, and bevacizumab with maintenance capecitabine and bevacizumab as first-line therapy for triple-negative, metastatic, or locally advanced breast cancer: results from the GINECO A-TaXel phase 2 study. Cancer. 2016;122(20):3119–26.
Tian S, Quan H, Xie C, Guo H, Lu F, Xu Y, Li J, Lou L. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102(7):1374–80.
Hu X, Zhang J, Xu B, Jiang Z, Ragaz J, Tong Z, Zhang Q, Wang X, Feng J, Pang D, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer. 2014;135(8):1961–9.
Liu J, Liu Q, Li Y, Li Q, Su F, Yao H, Su S, Wang Q, Jin L, Wang Y et al. Efficacy and safety of camrelizumab combined with apatinib in advanced triple-negative breast cancer: an open-label phase II trial.J Immunother Cancer2020, 8(1).
Liu J, Wang Y, Tian Z, Lin Y, Li H, Zhu Z, Liu Q, Su S, Zeng Y, Jia W, et al. Multicenter phase II trial of Camrelizumab combined with apatinib and eribulin in heavily pretreated patients with advanced triple-negative breast cancer. Nat Commun. 2022;13(1):3011.
Hande KR. Etoposide: four decades of development of a topoisomerase II inhibitor. Eur J Cancer. 1998;34(10):1514–21.
Yuan P, Xu BH, Wang JY, Ma F, Fan Y, Li Q, Zhang P. Oral etoposide monotherapy is effective for metastatic breast cancer with heavy prior therapy. Chin Med J (Engl). 2012;125(5):775–9.
Hu N, Zhu A, Si Y, Yue J, Wang X, Wang J, Ma F, Xu B, Yuan P. A phase II, single-arm study of apatinib and oral etoposide in heavily pre-treated metastatic breast Cancer. Front Oncol. 2020;10:565384.
Lan CY, Wang Y, Xiong Y, Li JD, Shen JX, Li YF, Zheng M, Zhang YN, Feng YL, Liu Q, et al. Apatinib combined with oral etoposide in patients with platinum-resistant or platinum-refractory ovarian cancer (AEROC): a phase 2, single-arm, prospective study. Lancet Oncol. 2018;19(9):1239–46.
Twelves C, Cortes J, Vahdat L, Olivo M, He Y, Kaufman PA, Awada A. Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res Treat. 2014;148(3):553–61.
Li Y, Zhang H, Merkher Y, Chen L, Liu N, Leonov S, Chen Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J Hematol Oncol. 2022;15(1):121.
Yuan P, Hu X, Sun T, Li W, Zhang Q, Cui S, Cheng Y, Ouyang Q, Wang X, Chen Z, et al. Eribulin mesilate versus vinorelbine in women with locally recurrent or metastatic breast cancer: a randomised clinical trial. Eur J Cancer. 2019;112:57–65.
Cortes J, O’Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Dieras V, Delozier T, et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377(9769):914–23.
Zhang J, Wang L, Wang Z, Hu X, Wang B, Cao J, Lv F, Zhen C, Zhang S, Shao Z. A phase II trial of biweekly vinorelbine and oxaliplatin in second- or third-line metastatic triple-negative breast cancer. Cancer Biol Ther. 2015;16(2):225–32.
Laessig D, Stemmler HJ, Vehling-Kaiser U, Fasching PA, Melchert F, Kolbl H, Stauch M, Maubach P, Scharl A, Morack G, et al. Gemcitabine and carboplatin in intensively pretreated patients with metastatic breast cancer. Oncology. 2007;73(5–6):407–14.
Zhang M, Liu J, Liu G, Xing Z, Jia Z, Li J, Wang W, Wang J, Qin L, Wang X, et al. Anti-vascular endothelial growth factor therapy in breast cancer: molecular pathway, potential targets, and current treatment strategies. Cancer Lett. 2021;520:422–33.
Miles DW, Dieras V, Cortes J, Duenne AA, Yi J, O’Shaughnessy J. First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: pooled and subgroup analyses of data from 2447 patients. Ann Oncol. 2013;24(11):2773–80.
Bodzioch M, Bajger P, Forys U. Angiogenesis and chemotherapy resistance: optimizing chemotherapy scheduling using mathematical modeling. J Cancer Res Clin Oncol. 2021;147(8):2281–99.
Gourley C. Apatinib and etoposide: surprising efficacy of an oral combination. Lancet Oncol. 2018;19(9):1146–7.
Baselga J, Segalla JG, Roche H, Del Giglio A, Pinczowski H, Ciruelos EM, Filho SC, Gomez P, Van Eyll B, Bermejo B, et al. Sorafenib in combination with capecitabine: an oral regimen for patients with HER2-negative locally advanced or metastatic breast cancer. J Clin Oncol. 2012;30(13):1484–91.
Curigliano G, Pivot X, Cortes J, Elias A, Cesari R, Khosravan R, Collier M, Huang X, Cataruozolo PE, Kern KA, et al. Randomized phase II study of sunitinib versus standard of care for patients with previously treated advanced triple-negative breast cancer. Breast. 2013;22(5):650–6.
Bergh J, Bondarenko IM, Lichinitser MR, Liljegren A, Greil R, Voytko NL, Makhson AN, Cortes J, Lortholary A, Bischoff J, et al. First-line treatment of advanced breast cancer with sunitinib in combination with docetaxel versus docetaxel alone: results of a prospective, randomized phase III study. J Clin Oncol. 2012;30(9):921–9.
Acknowledgements
We thank Xuwei Fu (Jiangsu Hengrui Pharmaceuticals Co., Ltd.) for the assistance in data interpretation, and Zhongjiang Chen (Jiangsu Hengrui Pharmaceuticals Co., Ltd.) for the writing assistance.
Funding
This study was funded by National Cancer Fund Climbing Fund (NCC201808B018).
Author information
Authors and Affiliations
Contributions
MRC and HLL have contributed equally to this work. MRC, HLL, and SY are responsible for the conception of the trial. HP, LCS, CHL, XSC, WL, JH, JH and YX were involved in the design of the trial. NZZ, YQC, TH, DNZ, YYS, LZ and XML were involved in the acquisition and analysis of data for the trial. MRC and HLL were involved in drafting the manuscript. LC, MRC, and YX were involved in revising the manuscript. All authors have agreed the final version of manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Harbin Medical University Cancer Hospital and was performed in accordance with the Declaration of Helsinki. All patients have signed the informed consent form before any procedure.
Consent for publication
Not applicable.
Competing Interest
The authors have no competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cao, M., Lu, H., Yan, S. et al. Apatinib plus etoposide in pretreated patients with advanced triple-negative breast cancer: a phase II trial. BMC Cancer 23, 463 (2023). https://doi.org/10.1186/s12885-023-10768-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-10768-8