Abstract
Background
Diet may impact important risk factors for endometrial cancer such as obesity and inflammation. However, evidence on the role of specific dietary factors is limited. We investigated associations between dietary fatty acids and endometrial cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC).
Methods
This analysis includes 1,886 incident endometrial cancer cases and 297,432 non-cases. All participants were followed up for a mean of 8.8 years. Multivariable Cox proportional hazard models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) of endometrial cancer across quintiles of individual fatty acids estimated from various food sources quantified through food frequency questionnaires in the entire EPIC cohort. The false discovery rate (q-values) was computed to control for multiple comparisons.
Results
Consumption of n-6 γ-linolenic acid was inversely associated with endometrial cancer risk (HR comparing 5th with 1st quintileQ5−Q1=0.77, 95% CI = 0.64; 0.92, ptrend=0.01, q-value = 0.15). This association was mainly driven by γ-linolenic acid derived from plant sources (HRper unit increment=0.94, 95%CI= (0.90;0.98), p = 0.01) but not from animal sources (HRper unit increment= 1.00, 95%CI = (0.92; 1.07), p = 0.92). In addition, an inverse association was found between consumption of n-3 α-linolenic acid from vegetable sources and endometrial cancer risk (HRper unit increment= 0.93, 95%CI = (0.87; 0.99), p = 0.04). No significant association was found between any other fatty acids (individual or grouped) and endometrial cancer risk.
Conclusion
Our results suggest that higher consumption of γ-linolenic acid and α-linoleic acid from plant sources may be associated with lower risk of endometrial cancer.
Similar content being viewed by others
Introduction
In 2020, 417,367 new endometrial cancer cases were diagnosed and 97,370 deaths were recorded from endometrial cancer worldwide [1]. In Europe, endometrial cancer is the fourth most common cancer and the sixth most common cause of cancer death in women [1]. Overweight and obesity, poor diet and physical inactivity have been reported to increase the risk of developing endometrial cancer [2, 3]. However, evidence on the role of specific dietary factors in endometrial cancer risk is still limited [4] and prevention strategies are needed.
Experimental studies suggest two major biologically plausible mechanisms that underlie the association between endometrial cancer risk and dietary exposure particularly with regard to saturated fatty acids (SFA), unsaturated fatty acids and cholesterol. Firstly, these dietary components can modulate the production, metabolism, and excretion of endogenous hormones, which influence the proliferation of endometrial cancer cells [5,6,7,8]. Secondly, they can influence inflammatory processes, which are important in the development of many cancer types [9] including endometrial cancer where they play a central role in the regulation of endometrial mucosa growth and shedding during the menstrual cycle [10] and endometrial repair following menstruation [11].
A nutrient-wide association study from the EPIC, the Nurses’ Health Study (NHS) and the NHSII reported a higher risk of endometrial cancer in relation to a higher intake of total fat and monounsaturated fat (MUFA), but the association was primarily driven by findings from EPIC [12]. A meta-analysis by the World Cancer Research Fund (WCRF) Continuous Update Project concluded that there was “limited” evidence for a link between endometrial cancer risk and each for intake of total fat and of saturated/animal fat [4].
Data from a dose-response meta-analysis based on epidemiological studies published up to June 2015 suggested a lack of association between total dietary fat intake and endometrial cancer risk [13]. Conversely, results from another dose-response meta-analysis, including seven cohorts and fourteen case-control studies [14], suggested that higher MUFA intake was associated with lower endometrial cancer risk, while total fat and SFA intake were associated with a higher risk of endometrial cancer in the case–control studies only. The same meta-analysis [14] found no significant association between polyunsaturated fatty acids (PUFA) or linoleic acid and endometrial cancer risk. Another meta-analysis focusing on fish intake and n-3 PUFA suggested that intake of n-3 PUFA may be inversely associated with endometrial cancer risk [15].
The heterogeneity of the results from epidemiological studies in this field and the lack of information on endometrial cancer subtypes and on the types of foods (from animal or plant sources) in these studies call for larger and more in-depth investigations in the field. We therefore analyzed the association between fatty acid intake and endometrial cancer risk, overall and by different of cancer subtype stratifications, and investigated the association of dietary sources of fatty acids (animal or plant)- on endometrial cancer risk.
Materials & methods
Study design
The EPIC study includes 521,330 participants recruited between 1992 and 2000 from 23 centers across 10 European countries [16]. The study design, recruitment procedures and data collection have been described previously [17]. Written informed consent was provided by all study participants. Ethical approval for this study was provided by the International Agency for Research on Cancer and the institutional review boards of the local participating EPIC centers. Briefly, dietary information, as well as socio-demographic, and lifestyle data were collected at enrolment from all participants by administration of country-specific questionnaires. Self-administered questionnaires were used in all centers, except in Spain and Ragusa (Italy), where data were collected during personal interviews. In Malmo (Sweden), a combined semi-quantitative food frequency questionnaire and 7-day dietary diary and diet interview was used.
Baseline anthropometric measurements and peripheral blood samples were also collected. Procedures for sample collection, processing and storage are described in detail elsewhere [18].
A total of 308,285 women remained in the study population after exclusion of 35,700 women who had undergone hysterectomy, 25,184 prevalent cancer cases and 4,148 subjects with incomplete follow-up data. Among the included women, 2,023 cases of endometrial cancer were identified by the end of each center’s follow-up period. Further exclusions of cases were based on their tumor morphology (n = 73), lack of completion of lifestyle or dietary questionnaire (26 cases and 2,854 non cases) or classification of the women in the top or bottom 1% of energy intake to energy requirement (38 cases and 5,968 non cases). This left a total of 1,886 cases included in the analysis. Cases were morphologically classified as type I (including adenocarcinoma (NOS), adenocarcinoma in adenomatous polyp, endometrioid adenocarcinoma (NOS), mucinous adenocarcinoma, mucin-producing adenocarcinoma, adenosquamous carcinoma or adenocarcinoma with squamous metaplasia) or Type II (squamous cell carcinoma (NOS), clear cell adenocarcinoma (NOS), mixed cell adenocarcinoma, serous cystadenocarcinoma (NOS) or papillary serous cystadenocarcinoma).
Cancer end point data was based on the latest round of follow-up received from the EPIC centers and centralized at IARC between 2014 and 2016. For each EPIC study center, closure dates of the study period were defined as the latest dates of complete and verified follow-up for both cancer incidence and vital status (dates varied between centers, between June 2008 and December 2013).
Assessment of dietary fatty acids intake
To compile the EPIC Nutrient Database (ENDB) for the EPIC study, a highly standardized procedure was used, adopting nutrient values from ten national food composition databases of the respective EPIC countries. The in-depth process for compiling this ENDB database was described in detail elsewhere [19, 20]. To date, most of the national food composition databases from the ten respective EPIC countries do not contain nutritional values for specific fatty acids isomers. Therefore, the EPIC data was matched with fatty acids isomers using the National Nutrient Database for Standard Reference of the United States (NNDSR; further referred to as USDA table) [21]. Specific foods and recipes that were not included in the USDA were decomposed in ingredients which were available in the USDA table and amounts of fatty acids were obtained through this extra USDA matching. Groupings of FA were defined as: saturated fatty acids (SFA) (4:0, 6:0, 8:0, 10:0, 12:0, 14:0, 15:0, 16:0, 17:0, 18:0, 20:0, 22:0, 24:0), cis-monounsaturated fatty acids (MUFA) (16:1n-7, 16:1n-9, 17:1, 18:1n-5, 18:1n-7, 18:1n-9, 20:1, 22:1, 24:1), n-6 polyunsaturated fatty acids (PUFA) (18:2, 18:3, 20:2, 20:3, 20:4) and n-3 PUFA (18:3, 20:3, 20:5, 22:5, 22:6), long-chain n-6 PUFA (20:2, 20:3, 20:4), long-chain n-3 PUFA (20:3, 20:5, 22:5, 22:6), ruminant trans fatty acids (rTFA) (18:1n-7, CLA), and industrial trans fatty acids (iTFA) (16:1n-9, 18:1n-9, 18:2n-6, 18:3n-3).
Statistical analysis
Cox proportional hazards regression using age as the underlying time metric with the subjects’ age at recruitment as the entry time and their age at cancer diagnosis (except for non-melanoma skin cancer), death, emigration or last complete follow-up, whichever occurred first, as the exit time was used to estimate the hazard ratio (HR) and 95% confidence interval (CI) for the association between dietary fatty acids and endometrial cancer risk. Intakes of fatty acids were log-transformed (in order to normalise the distribution) and divided into quintiles based on their distribution in all cohort women participants at baseline, setting women in the lowest category of fatty acids intake as the reference group. All models were stratified by the study center and age at enrolment (in one-year categories). The final multivariable model retained was adjusted for body mass index (BMI) (continuous), number of full term pregnancies (number of live born and/or still born children; 0, 1–2, 3–4; >4; missing), smoking status (never, former, current smokers), oral contraceptive or HRT use (never or ever), menopausal status at enrolment (premenopausal (women are considered premenopausal when they reported having had regular menses over the past 12 months or were younger than 46 years at recruitment); postmenopausal (women were considered postmenopausal when they reported not having had any menses over the past 12 months, reported having had a bilateral ovariectomy, or were older than 55 years); perimenopausal/unknown menopause (women were considered as perimenopausal when they were between age 46 and 55 years and had missing or incomplete questionnaire data), age at menarche (continuous) and total energy intake (continuous). Additional potential confounders (including history of breastfeeding (yes or no), physical activity (active or inactive), usual intake of alcohol (yes or no)) were not included in the final models as they did not alter the relative risk estimates by ˃10% (data not shown). In addition, mutual adjustment of fatty acids for each other did not modify the risk estimates (data not shown). Tests for trend were computed using the quintile specific median of each fatty acid. Stratified analysis by BMI (< 25 vs. ≥ 25 kg/m2), parity (nulliparous vs. parous), or menopausal status (pre vs. postmenopausal), and sensitivity analyses excluding the first 2 years of follow-up. All p for heterogeneity were > 0.05.
Due to the number of tests performed, q-values were calculated using the false discovery rate of the Benjamini-Hochberg procedure [22].
Additionally, associations between individual fatty acids intake (as continuous log-transformed variables) and endometrial cancer risk were investigated by their dietary sources grouping plant sources versus animal sources. The percentage of contribution to individual fatty acids intake was calculated for each food source based on the mean daily intake of dietary sources reported in the questionnaire.
All statistical analyses were carried out using STATA 14.0 (StataCorp, College Station, TX, USA). P-values below 0.05 were considered statistically significant.
Results
Compared to the non-cases, endometrial cancer cases had higher BMI, were more likely to be nulliparous, post-menopausal, to have ever used HRT and to have a lower education status. They also used less oral contraceptives (Table 1).
Intake of n-6 γ-linolenic acid was inversely associated with endometrial cancer risk (HR comparing 5th with 1st quintileQ5−Q1=0.77, 95% CI = 0.64: 0.92, ptrend=0.01, q-value = 0.15). This association was mainly driven by γ-linolenic acid derived from plant sources (contribution of vegetable sources intake to γ-linolenic = 65%, HRper unit increment=0.94, 95%CI= (0.90;0.98), ptrend=0.01)) (Fig. 1). An inverse association was also found between intake of n-3 α-linolenic acid from vegetable sources and endometrial cancer risk (contribution of vegetable sources intake to α-linolenic = 87.1%, HRper unit increment= 0.93, 95%CI = (0.87;0.99), ptrend=0.04) (Fig. 1).
Associations between plant and animal sources of gamma- and alpha-linolenic acids with endometrial cancer risk
gamma-linolenic acid (18:3n-6): The percentage of contribution next to the food sources was calculated for each food (sub-) group based on the mean daily intake reported in the dietary questionnaire. It represents the contribution of the corresponding source to the gamma-linolenic acid intake. Contribution of the plant sources (potatoes and other tubes (0.5%), vegetables (2%), fruit, nuts and seeds (0.2%), cereal and cereal products (14%), fat (19.7%), condiments and sauces (27.6%), soups and bouillons (0.3%) and miscellaneous (0.7%)) to γ-linolenic acid = 65.0% vs. animal sources (dairy products (6.6%), meat and meat products (13.7%), fish and shellfish (2.8%) and egg and egg products (7.8%)) = 30.9%
alpha-linolenic acid (18:3n-3): The percentage of contribution next to the food sources was calculated for each food (sub-) group based on the mean daily intake reported in the dietary questionnaire. It represents the contribution of the corresponding source to the alpha-linolenic acid intake. Contribution of the plant sources (potatoes and other tubes (0.3%), vegetables (0.3%),legumes (1.7%), cereal and cereal products (27.9%), fat (33.4%), sugar and confectionery (0.7%) non-alcoholic beverages (0.1%), condiments and sauces (22.4%) and soups and bouillons (0.3%)) to α-linolenic acid = 87.1% vs. animal sources (dairy products (3.8%), meat and meat products (3.5%), fish and shellfish (0.7%), egg and egg products (0.4%) and butter (2.3%)) = 10.7%
HR = Hazard Ratio; CI = confidence interval. The multivariable model was adjusted for BMI (continuous), number of full-term pregnancies (number of live born and/or still born children; 0, 1–2, 3–4; >4; missing), smoking status (never, former, current), oral contraceptive or HRT use (never or ever), menopausal status at enrolment (premenopausal; postmenopausal; perimenopausal/unknown menopause), age at menarche (continuous) and total energy intake (continuous)
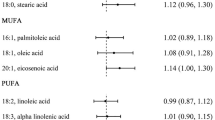
No other statistically significant associations were identified between the other fatty acids (palmitic, stearic, oleic and linoleic acids) including the trans FA (iTFA or rTF) and endometrial cancer risk (Table 2).
Finally, the association between fatty acids and endometrial cancer did not vary according to histological subtypes of endometrial cancer (type I vs. type II). No substantial difference in the risk estimate was shown in the stratified analysis by BMI (< 25 vs. ≥ 25 kg/m2), parity (nulliparous vs. parous), or menopausal status (pre vs. postmenopausal), and in the sensitivity analyses excluding the first 2 years of follow-up. In all stratified analyses, no significant association was reported between fatty acids (grouped or individual) and endometrial cancer risk (data not shown). All p for heterogeneity were > 0.05.
Discussion
In this large-scale prospective analysis, an inverse association between the consumption of n-6 γ-linolenic acid and n-3 α-linolenic acid and endometrial cancer risk was found and this association was mainly driven by the vegetable sources of these two fatty acids. These associations did not vary according to histological subtypes of endometrial cancer.
Besides γ-linolenic acid and α-linolenic acid from vegetable sources, no significant association between any other dietary fatty acids and endometrial cancer was reported in this study. Our results align with results from the NHS and NHSII studies [12] but not with findings from a previous analysis within the EPIC study, which reported an inverse association between total fat intake, total MUFA and endometrial cancer [12]. This is probably due to the fact that in the current EPIC analysis, in addition to having a longer follow-up and more endometrial cancer cases, a better separation between cis and trans MUFA isomers was available after the USDA matching (the mean of total MUFA intake in the previous EPIC analysis was 29 g/day vs. 24 g/day of cis-MUFA in this current analysis) [18].
The overall inverse association between γ-linolenic acid and endometrial cancer risk was mainly driven by its vegetable sources, mainly from cereal and cereal products (14%), fat (19.7%) and condiments and sauces (27.6%). In addition, the inverse association between endometrial cancer and n-3 α-linolenic acid from vegetable sources (mainly cereal and cereal products (27.9%), fat (33.4%) and condiments and sauces (22.4%)) was not observed for n-3 α-linolenic acid from animal sources.
Our study is the first to investigate the associations between animal and plant sources of fatty acids and endometrial cancer risk. MUFA from plant sources also have an aded value in decreasing overall mortality including cardiovascular and cancer mortality, as recently reported in the NHS and Health Professionals Follow-Up Study (HPFS) [23]. Our data suggest that vegetable sources of γ-linolenic acid and α-linolenic acid may exert a protective effect on endometrial cancer risk. The mechanisms underlying these inverse associations might be explained by the fact that γ-linolenic acid and α-linolenic were both reported to induce apoptosis in an experimental study on cancer cell lines. However, this in vitro analysis has also reported additional differential antitumor effects of γ-linolenic acid and α-linolenic acid. α-linolenic was reported to affect some cellular pathways, particularly the mitochondrial protein import pathway and the cycle of citric acid whereas γ-linolenic acid has no specific actions on these pathways [24]. Besides their direct effects on cancer development, anti-carcinogenic components of the vegetable sources of these fatty acids (mainly nuts and seeds) which are rich in vitamins, minerals and a range of active metabolites such as phenolic acids, phytosterols, carotenoids, and polyphenolic compounds, might also contribute to this inverse association [25]. Moreover, the mixture of all these components or the called “matrix effect” might explain this association and not necessarily each component by itself [26], the modulation of steroid hormone concentrations and metabolism, the activation of antioxidant mechanisms, the regulation of detoxification enzymes, and/or the stimulation of the immune system [27].
In this study, no significant association was found between n-6 and n-3 PUFA overall, or long-chain PUFA, and endometrial cancer risk. However, these two families are known to playing a significant role in cancer by generating modulatory molecules for inflammatory responses, including eicosanoids (prostaglandins and leukotrienes), and cytokines (interleukins) and by affecting the gene expression of several bioactive molecules. Linoleic acid is an essential FA, derived only from diet and mainly from seeds, nuts vegetable oils (safflower oil, maize oil, sunflower oil and soybean oil), meat and eggs. γ-linolenic acid is derived from linoleic acid, (by Δ6-desaturase) and can be prolongated by the enzyme elongase 5 to dihomo-γ-linolenic acid (20:3n-6; DGLA) [28]. After this, DGLA go through oxidative metabolism by cyclooxygenases and lipoxygenases to generate anti-inflammatory eicosanoids (prostaglandins of series 1 and leukotrienes of series 3) [29, 30]. With these same series of enzymes, n-3 α-linolenic acid (the essential n-3 PUFA derived mainly from seeds (flaxseeds and flaxseed oils) and nuts), competing with linoleic and γ-linolenic acid, is converted into long-chain fatty acids (LC-PUFA): eicosapentaenoic acid (20:5n-3; EPA) and docosahexaenoic acid (22:6n-3;DHA). Found in oily fish and fish supplements, these fatty acids can increase anti-inflammatory and inflammation resolving mediators called resolvins, protectins and maresins. They can also inhibit many inflammation facets including leucocyte chemotaxis, adhesion molecule expression and leucocyte–endothelial adhesive interactions, production of eicosanoids like prostaglandins and leukotrienes from the n-6 arachidonic acid, and production of pro-inflammatory cytokines [28].
Inflammation has been linked to endometrial cancer in several cohort and case-control studies [31,32,33]. LC-PUFA (EPA and DHA), which are suggested to be anti-inflammatory (as described above), could potentially reduce endometrial cancer risk [34]. However, epidemiological results in this field are inconclusive. One Japanese case-control study reported a lower risk of endometrial cancer in association with higher fish consumption (significant inverse association with 16.02 g/1000Kcal of the mean intake of fish in the Japanese study vs. 13.80 g/1000Kcal of fish intake in this current study) [35], whereas several other case-control and cohort studies reported no statistically significant associations [15, 36,37,38]. Similarly, our data showed no significant association between n-3 LC-PUFA and endometrial cancer risk. This is probably due to the fact that the mean intake of EPA (57 mg/day) and DHA (97 mg/day) in our study was lower than the one recommended in the US (> 500 mg/day EPA + DHA) and in Europe (250 mg/day EPA + DHA) [39]. In addition, we didn’t have data regarding PUFA supplementation to consider. Further studies are needed to clarify the potential association between n-3 LC-PUFA, fish intake and endometrial cancer risk.
iTFA consumption is associated with an increased risk of all-cause mortality [40], and the WHO supports actions to eliminate these fatty acids from the diet [41]. Epidemiological data on the association between iTFA and cancer risk are few [42]. However, in agreement with other studies [14, 15], we did not find any significant association between iTFA and endometrial cancer risk. Contrary to the positive association that we reported with breast and ovarian cancer development in the EPIC cohort [43, 44], this present study suggests that iTFA from industrial processes are not associated with endometrial cancer development.
The strengths of our study are several including its prospective design, the very large number of incident endometrial cancer cases and our ability to separate n-6 and n-3 cis PUFA isomers. The main limitation of this study is the single collection at baseline of questionnaire dietary data potentially causing random measurement errors and failing to reflect long-term habits. These biases may underestimate true associations. In addition, no information was provided regarding PUFA supplementation, so our analyses were limited to dietary intake only. Another limitation is that the biomarkers of fatty acids were not available in this study; their availability would have allowed a complementary assessment for the associations between fatty acids and endometrial cancer risk. In addition, results regarding analysis by histological subtypes were not conclusive and probably underpowered due to the small sample size of type II tumors.
Conclusion
To our knowledge, this is the first large-scale study to scrutinize the effects of dietary sources of fatty acids (animal or plant)- on endometrial cancer risk. Our findings in EPIC showed that plant sources of the essential n-6 and n-3 PUFA were inversely associated with endometrial cancer development, suggesting that the dietary source of fatty acids (animal versus plant) may be important when investigating the association between fatty acids and cancer risk.
Data Availability
For information on how to submit an application for gaining access to EPIC data and/or biospecimens, please follow the instructions at http://epic.iarc.fr/access/index.php.
Abbreviations
- CI:
-
Confidence Interval
- DGLA:
-
Dihomo-γ-Linolenic Acid
- DHA:
-
Docosahexaenoic acid
- ENDB:
-
EPIC Nutrient Database
- EPA:
-
Eicosapentaenoic Acid
- EPIC:
-
European Prospective Investigation into Cancer and Nutrition
- HPFS:
-
Health Professionals Follow-Up Study
- HR:
-
Hazard Ratio
- HRT:
-
Hormonal Replacement Therapy
- IARC:
-
International Agency for Cancer Reasearch
- iTFA:
-
industrial Trans fatty acids
- LCPUFA:
-
Long Chain Polyunsaturated Fatty Acids
- MUFA:
-
Monosaturated Fatty acids
- NHS:
-
Nurses’ Health Study
- NNDSR:
-
National Nutrient Database for Standard Reference of the United States
- PUFA:
-
Polyunsaturated Fatty Acids
- rTFA:
-
ruminant Trans Fatty Acids
- SFA:
-
Saturated Fatty acids
- USDA:
-
United States Department of Agriculture
- WCRF:
-
World Cancer Research Fund
- WHO:
-
World Health Organization
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021 May;71(3):209–249. https://doi.org/10.3322/caac.21660. Epub 2021 Feb 4. PMID: 33538338.
Forouzanfar MH, Afshin A, Alexander LT, Anderson RH, Bhutta ZA, Biryukov S, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the global burden of Disease Study 2015. The Lancet. 2016;388:10053.
Moore K, Brewer MA. Endometrial Cancer: is this a New Disease? Am Soc Clin Oncol Educ Book. 2017;37:435–42.
World Cancer Research Fund / American Institute for Cancer Research. Diet, Nutrition, Physical activity and Endometrial cancer, revised 2018.
Bruning PF, Bonfrer JM. Free fatty acid concentrations correlated with the available fraction of estradiol in human plasma. Cancer Res. 1986;46(5):2606–9.
Nagaoka T, Onodera H, Hayashi Y, Maekawa A. Influence of high-fat diets on the occurrence of spontaneous uterine endometrial adenocarcinomas in rats. Teratog Carcinog Mutagen. 1995;15(4):167–77.
Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34(3):362–6.
Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1531–43.
Calder PC. Functional roles of fatty acids and their Effects on Human Health. JPEN J Parenter Enteral Nutr. 2015;39(1 Suppl):18S–32S.
Kelly RW, King AE, Critchley HO. Cytokine control in human endometrium. Reproduction. 2001;121(1):3–19.
Salamonsen LA. Tissue injury and repair in the female human reproductive tract. Reproduction. 2003;125(3):301–11.
Merritt MA, Tzoulaki I, Tworoger SS, De Vivo I, Hankinson SE, Fernandes J et al. Investigation of dietary factors and endometrial cancer risk using a nutrient-wide association study approach in the EPIC and Nurses’ Health Study (NHS) and NHSII. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24(2):466–71.
Jiang L, Hou R, Gong TT, Wu QJ. Dietary fat intake and endometrial cancer risk: dose-response meta-analysis of epidemiological studies. Sci Rep. 2015;5:16693.
Zhao J, Lyu C, Gao J, Du L, Shan B, Zhang H, et al. Dietary fat intake and endometrial cancer risk: a dose response meta-analysis. Med (Baltim). 2016;95(27):e4121.
Hou R, Yao SS, Liu J, Wang LL, Wu L, Jiang L. Dietary n-3 polyunsaturated fatty acids, fish consumption, and endometrial cancer risk: a meta-analysis of epidemiological studies. Oncotarget. 2017;8(53):91684–93.
Bingham S, Riboli E. Diet and cancer–the european prospective investigation into Cancer and Nutrition. Nat Rev Cancer. 2004;4(3):206–15.
Riboli E, Kaaks R. The EPIC Project: rationale and study design. European prospective investigation into Cancer and Nutrition. Int J Epidemiol. 1997;26(Suppl 1):6–14.
Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, et al. European prospective investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5(6B):1113–24.
Slimani N, Deharveng G, Unwin I, Southgate DA, Vignat J, Skeie G, et al. The EPIC nutrient database project (ENDB): a first attempt to standardize nutrient databases across the 10 european countries participating in the EPIC study. Eur J Clin Nutr. 2007;61(9):1037–56.
Nicolas G, Witthoft CM, Vignat J, Knaze V, Huybrechts I, Roe M, et al. Compilation of a standardised international folate database for EPIC. Food Chem. 2016;193:134–40.
Van Puyvelde H, Perez-Cornago A, Casagrande C, Nicolas G, Versele V, Skeie G et al. Comparing Calculated Nutrient Intakes Using Different Food Composition Databases: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC) Cohort.Nutrients. 2020;12(10).
Benjamini YHY. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B. 1995;57:289–300.
Guasch-Ferre M, Zong G, Willett WC, Zock PL, Wanders AJ, Hu FB, et al. Associations of Monounsaturated fatty acids from plant and animal sources with total and cause-specific mortality in two US prospective cohort studies. Circ Res. 2019;124(8):1266–75.
González-Fernández MJ, Ortea I, Guil-Guerrero JL. α-Linolenic and γ-linolenic acids exercise differential antitumor effects on HT-29 human colorectal cancer cells. Toxicol Res (Camb). 2020 Jul;27(4):474–83.
Balakrishna R, Bjørnerud T, Bemanian M, Aune D, Fadnes LT. Consumption of nuts and seeds and Health Outcomes Including Cardiovascular, Diabetes and Metabolic Disease, Cancer, and mortality: an Umbrella Review. Adv Nutr. 2022 Aug;30:nmac077.
Aguilera JM. The food matrix: implications in processing, nutrition and health. Crit Rev Food Sci Nutr. 2019;59(22):3612–29. https://doi.org/10.1080/10408398.2018.1502743. Epub 2018 Sep 10. PMID: 30040431.
Lampe JW. Health effects of vegetables and fruit: assessing mechanisms of action in human experimental studies. Am J Clin Nutr. 1999;70(3 Suppl):475S–90S.
Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. 2017;45(5):1105–15.
Kapoor R, Huang YS. Gamma linolenic acid: an antiinflammatory omega-6 fatty acid. Curr Pharm Biotechnol. 2006;7(6):531–4.
Schulze MB, Minihane AM, Saleh RNM, Risérus U. Intake and metabolism of omega-3 and omega-6 polyunsaturated fatty acids: nutritional implications for cardiometabolic diseases. Lancet Diabetes Endocrinol. 2020 Nov;8(11):915–30. https://doi.org/10.1016/S2213-8587(20)30148-0. Epub 2020 Sep 16. PMID: 32949497.
Fortner RT, Husing A, Kuhn T, Konar M, Overvad K, Tjonneland A, et al. Endometrial cancer risk prediction including serum-based biomarkers: results from the EPIC cohort. Int J Cancer. 2017;140(6):1317–23.
Dossus L, Rinaldi S, Becker S, Lukanova A, Tjonneland A, Olsen A, et al. Obesity, inflammatory markers, and endometrial cancer risk: a prospective case-control study. Endocr Relat Cancer. 2010;17(4):1007–19.
Wang T, Rohan TE, Gunter MJ, Xue X, Wactawski-Wende J, Rajpathak SN, et al. A prospective study of inflammation markers and endometrial cancer risk in postmenopausal hormone nonusers. Cancer Epidemiol Biomarkers Prev. 2011;20(5):971–7.
Fernandez E, Chatenoud L, La Vecchia C, Negri E, Franceschi S. Fish consumption and cancer risk. Am J Clin Nutr. 1999;70(1):85–90.
Takayama S, Monma Y, Tsubota-Utsugi M, Nagase S, Tsubono Y, Numata T, et al. Food intake and the risk of endometrial endometrioid adenocarcinoma in japanese women. Nutr Cancer. 2013;65(7):954–60.
Arem H, Neuhouser ML, Irwin ML, Cartmel B, Lu L, Risch H, et al. Omega-3 and omega-6 fatty acid intakes and endometrial cancer risk in a population-based case-control study. Eur J Nutr. 2013;52(3):1251–60.
Brasky TM, Sponholtz TR, Palmer JR, Rosenberg L, Ruiz-Narvaez EA, Wise LA. Associations of Dietary Long-Chain omega-3 polyunsaturated fatty acids and Fish Consumption with Endometrial Cancer Risk in the Black Women’s Health Study. Am J Epidemiol. 2016;183(3):199–209.
Daniel CR, Cross AJ, Graubard BI, Hollenbeck AR, Park Y, Sinha R. Prospective investigation of poultry and fish intake in relation to cancer risk. Cancer Prev Res (Phila). 2011;4(11):1903–11.
https://health.gov/dietaryguidelines/2015/resources/2015-2020_Dietary_Guidelines.pdf.
de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. 2015;351:h3978.
Ghebreyesus TA, Frieden TR. REPLACE: a roadmap to make the world trans fat free by 2023. Lancet. 2018;391(10134):1978–80.
Nathalie Michels IOS, Berit L, Heitmann V, Chajès. Inge Huybrechts. Dietary trans-fatty acid intake in relation to cancer risk: a systematic review and meta-analysis. 2020.
Chajes V, Assi N, Biessy C, Ferrari P, Rinaldi S, Slimani N, et al. A prospective evaluation of plasma phospholipid fatty acids and breast cancer risk in the EPIC study. Ann Oncol. 2017;28(11):2836–42.
Yammine SG, Hybrechts I, Biessy C, Dossus L, Aglago E, Gunter MJ et al. Dietary and circulating fatty acids and ovarian cancer risk in the European Prospective Investigation into Cancer and Nutrition. 2019.
Acknowledgements
We would like to thank ‘the National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands’ for their contribution and ongoing support to the EPIC Study.
Funding
This work was undertaken during the tenure of a doctoral Fellowship supported by INCa (INCa 2016 − 184), by Fondation ARC pour la recherche sur le cancer (ARC 2019-DOC4) and by IARC.
The coordination of EPIC is financially supported by International Agency for Research on Cancer (IARC) and by the Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London which has additional infrastructure support provided by the NIHR Imperial Biomedical Research Centre (BRC). The national cohorts are supported by: Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); German Cancer Aid, German Cancer Research Center (DKFZ), German Institute of Human Nutrition PotsdamRehbruecke (DIfE), Federal Ministry of Education and Research (BMBF) (Germany); Associazione Italiana per la Ricerca sul Cancro-AIRC-Italy, Compagnia di SanPaolo and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (The Netherlands); Health Research Fund (FIS) - Instituto de Salud Carlos III (ISCIII), Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra, and the Catalan Institute of Oncology - ICO (Spain); Swedish Cancer Society, Swedish Research Council and County Councils of Skåne and Västerbotten (Sweden); Cancer Research UK (C864/A 14136 to EPIC-Norfolk (DOI https://doi.org/10.22025/2019.10.105.00004); C8221/A29017 to EPIC-Oxford), Medical Research Council (MR/N003284/1 and MC_UU_00006/1 to EPIC-Norfolk; MR/M012190/1 to EPIC-Oxford). (United Kingdom).
Author information
Authors and Affiliations
Contributions
SY, VC and MJG conceived the study. SY and CB performed the statistical analyses. SY and VC drafted the manuscript. All other authors contributed to the acquisition and interpretation of data and critically revised the manuscript for important intellectual content. All authors have read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participants provided written informed consent to participate in the EPIC study. The EPIC study was approved by the Ethics Committee of the International Agency for Research on Cancer (IARC) and all EPIC centers. The present study has been approved by the IARC Ethics Committee (IEC meeting 2015-05) and the EPIC Steering Committee with representation from IARC, Imperial College, and the EPIC centers. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/ World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer / World Health Organization.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yammine, S.G., Huybrechts, I., Biessy, C. et al. Dietary fatty acids and endometrial cancer risk within the European Prospective Investigation into Cancer and Nutrition. BMC Cancer 23, 159 (2023). https://doi.org/10.1186/s12885-023-10611-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-10611-0