Abstract
Background
The C-reactive protein to albumin ratio (CAR) is associated with poor prognosis in various cancers. However, its value in thymic epithelial tumors remains to be elucidated, we aimed to evaluate the prognostic significance of preoperative CAR in patients with surgically resected thymic epithelial tumors (TETs).
Methods
We retrospectively collected data from 125 patients with TETs who underwent thymoma resection at our center. The best cutoff values for the continuous variable, CAR, were obtained using X-tile software. Univariate and multivariate Cox regression analyses were used to evaluate CAR as an independent predictor of overall survival (OS) and recurrence-free survival (RFS). Kaplan–Meier analysis and log-rank tests were used to present risk stratification of patients based on CAR and the Glasgow-prognostic-score (GPS). The prognostic effect of CAR was assessed using a receiver operating characteristic curve.
Results
Patients were categorized into high (≥ 0.17) and low (< 0.17) CAR groups according to the optimal cutoff value of 0.17. Univariate and multivariate analyses showed that CAR was an independent predictor of prognosis. World health organization stage, CAR level, GPS score, and drinking history were important independent prognostic factors for OS (p < 0.05). T stage, CAR level, and drinking history were important independent prognostic factors for RFS (p < 0.05). The area under the curve value of CAR to predict prognosis was 0.734 for OS and 0.680 for RFS.
Conclusions
Elevated preoperative CAR was independently associated with poor OS and RFS after thymectomy. Therefore, CAR may be a valuable biomarker for the postoperative prognosis of TETs.
Similar content being viewed by others
Introduction
Thymic epithelial tumors (TETs) are mediastinal tumors originating from epithelial cells of the thymus [1, 2]. They can be grouped into thymoma, thymic carcinoma, and thymic neuroendocrine tumors based on their histology. Although TETs represent the most common of the anterior mediastinum tumors in adults, their incidence is only 0.13 per 100,000 person-years, according to the American Cancer Registry [3]. In the world health organization (WHO) classification system, TETs are categorized into A, AB, B1, B2, B3, and rarer subtypes [4]. Surgical treatment is still the standard treatment for thymic epithelial tumors [5,6,7,8], and prognostic factors for postoperative survival include tumor size [9, 10], age [11], T stage [12], WHO histological classification [13], tumor vascular invasion [14], completeness of resection [15], and postoperative adjuvant therapy [16]. However, biomarkers with prognostic value have not yet been discovered and confirmed for this type of malignancy.
A growing number of reports indicate that both a systemic inflammatory response and nutritional status are important factors associated with long-term survival outcomes in various malignant tumors. Although the C-reactive protein to albumin ratio (CAR) has been repeatedly reported to be related to poor prognosis in various cancers, including pancreatic [17, 18], gastric [19], lung [20], and esophageal cancer [21], its role in TETs remains to be elucidated.
Given the rarity of TETs, few studies have investigated the preoperative CAR in patients with this tumor. Although thymoma is usually indolent, it is malignant. Therefore, we aimed to evaluate the prognostic value of CAR in patients with TETs. We assessed the relationship of CAR levels with overall survival (OS) and recurrence-free survival (RFS), as well as the predictive effects of CAR and the Glasgow-Prognostic-score (GPS).
Materials and methods
This study was approved by the Medical Ethics Committee of Sun Yat-Sen University Cancer Center (B2020-353–01) and complied with the Declaration of Helsinki. Data were recorded at the Sun Yat-sen University Cancer Center, under the record number: RDDA2021002090.
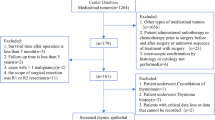
We retrospectively collected the medical records of patients with TETs, who underwent thymoma resection at Sun Yat-sen University Cancer Center between May 2004 and August 2015. The patient inclusion criteria were as follows: (1) patients older than 18 years; (2) presence of histopathologically confirmed TET, including thymoma and thymic carcinoma (TC); (3) complete surgical resection (R0, no residual disease); and (4) complete relevant laboratory tests (routine blood and routine biochemical) within 7 days before surgery. Patients were excluded if: (1) radiotherapy or chemotherapy administered prior to surgery, before and after surgery, or an unknown sequence of treatment with surgery. (2) follow-up time of less than five years. (3) patients with more than one malignancy or history of other malignancies. (4) postoperative survival time of less than 3 months. (5) the patient only underwent thymoma biopsy. (6) the surgical method was cryoablation. (7) incomplete follow-up information.
Data collection
Data were collected on the following clinical variables: hematological parameters (obtained within 1 week before surgery), albumin level (ALB), C-reactive protein level (CRP), patient's age, sex, drinking history (drinking alcohol every day, although the specific amount of drinking was not limited or described), smoking history, family history of tumor, histological subtype, tumor size, T stage, myasthenia gravis symptoms, and other clinical information.
Follow-up
The follow-up strategy was every 6–12 months for the first two years, every 12 months for the third to fifth years, and then once annually. The follow-up included chest CT plain scan, hematological examination (routine blood, routine biochemical, tumor markers, etc.). The last follow-up was in August 2020. The primary endpoints were the OS and RFS.
Variable definitions
All hematological parameters were collected within 7 days before surgery. Nutritional indicators were calculated as follows: CAR (C-reactive protein/albumin ratio); Glasgow-Prognostic-score (GPS) including CRP and albumin serum levels. CRP > 1 mg/dL was attributed 1 point; otherwise, 0 points were given. Albumin < 35 g/L was attributed 1 point; otherwise, 0 points were given.
Data analysis
In this study, X-tile software was used to obtain the optimal CAR cutoff value (http://www.tissuearray.org/rimmlab). Statistical analyses were performed using SPSS 25.0 (IBM,Chicago, Illinois, USA) and R software (version 4.0.3; https://www.r-project.org/). Univariate and multivariate analyses were performed using Cox proportional hazards regression models, using hazard ratios (HRs) and 95% confidence intervals (CIs) to assess relative risk. Survival analysis was performed using the Kaplan–Meier method, and differences in survival were compared using the log-rank test. Receiver operating characteristic (ROC) curve analysis was performed to analyze the area under the ROC curve (AUC); all tests were two-way, and the significance level was set at p < 0.05.
Result
Patient characteristics
A total of 125 patients with TETs, including 64 men and 61 women, were included in this study. They exhibited an average age of 50.63 ± 12.63 years and an average tumor size of 6.77 ± 3.27 cm. In addition, Table 1 also includes patients with WHO staging, T staging, smoking history, drinking history, myasthenia gravis, and other relevant clinical information.
Optimal cut-off for preoperative CAR
Taking OS as the endpoint, the optimal cut-off value of preoperative CAR was determined to be 0.17 (p < 0.01) using X-Tile software. For further analysis, patients were assigned into high or low CAR groups (> 0.17 or ≤ 0.17, respectively).
Association of CAR and GPS with survival outcomes
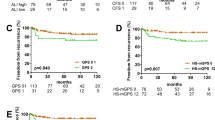
Taking OS and RFS as endpoints, we compared the OS and RFS results of patients assigned to the low-level and high-level CAR and GPS groups, and displayed them by Kaplan–Meier survival curves (Figs. 1 and 2). Finally, the receiver operating characteristic (ROC) curve was used to compare the AUC values of the two groups (Fig. 3).
Univariate and multivariate survival analysis based on overall survival
According to the results of the univariate Cox regression analysis, five variables were significantly associated with OS: WHO stage, T stage, drinking history, CAR, and GPS (Table 2). In multivariate Cox regression analysis, four parameters were defined as independent prognostic factors for OS: WHO stage (A-AB vs. B1-B3, HR = 0.489, at a 95% CI [range: 0.109–2.185] and A-AB vs. C, HR = 4.052, at a 95% CI [range: 0.918–17.886]), drinking history (HR = 6.362, at a 95% CI [range: 1.763–22.955]), CAR (HR = 27.091, at a 95% CI [range: 5.306–138.317]) and GPS (HR = 0.115, at a 95% CI [range: 0.017-0.770]) (Table 2).
Univariate and multivariate survival analysis based on relapse-free survival
According to the results of the univariate Cox regression analysis, four variables were significantly associated with RFS: WHO stage, T stage, drinking history, and CAR (Table 3). In multivariate Cox regression analysis, three parameters were defined as independent prognostic factors for RFS: T stage (T1 vs. T2-3, hazard ratio, HR = 6.992, at a 95% CI [range: 2.585–18.908]), drinking history (HR = 5.549, at a 95% CI [range: 1.833–16.797]), and CAR (HR = 5.930, at a 95% CI [range: 2.019–17.418]) (Table 3).
Discussion
In this study, we show that a high preoperative CAR is associated with poor patient outcomes after thymectomy, including overall survival and recurrence-free survival. Additionally, we confirm that GPS is an independent prognostic factor for overall survival in patients with TETs.
Consistent with previous findings, we show that T stage and WHO stage are independently associated with the postoperative prognosis of TETs [12, 22, 23]. Interestingly, our multivariate Cox analysis found that a history of drinking was independently associated with poor OS and RFS. Indeed, in a multicenter case–control study, Sabroe et al. found that alcohol consumption is a risk factor for thymoma [24]. However, its relationship with postoperative prognosis of patients is unclear, and further research is therefore needed.
CRP is an important acute-phase reactive protein that is predominantly produced by hepatocytes, and its level increases with the inflammatory response [25]. In 2017, Moser et al. found that serum CRP levels help to indicate highly aggressive TETs and may be an indicator of tumor recurrence during patient follow-up [26]. In 2019, Moser et al. found that the CRP-fibrinogen score (CFS) was an independent predictor of recurrence in patients with TETs [27].
Albumin is also generated by the liver and maintain intravascular osmotic pressure and promote substance transport. At the same time, albumin is an important nutrient, reflecting the nutritional status of the human body. Additionally, hypoalbuminemia is associated with a persistent systemic inflammatory response [28]. Multiple studies have shown that the development of hypoalbuminemia is secondary to elevated serum CRP levels [29, 30]. Malnutrition is related to a poor prognosis in patients with thymic epithelial tumors [31]. Furthermore, Ma et al. reported that preoperative albumin levels were associated with postoperative RFS in TET patients [32].
The impact of systemic inflammatory responses on the short- and long-term outcomes of various tumors has been widely reported [33,34,35]. Among them, the prognostic value of a neutrophil count, a neutrophil-to-lymphocyte ratio (NLR), and a platelet-lymphocyte ratio (PLR) in TETs has been reported and confirmed [36, 37]. The association between albumin and nutritional status reflects the systemic inflammatory response and nutritional status of patients and has been confirmed [38]. In addition, Okamoto et al. indicated that CAR may be the most useful prognostic indicator in postoperative immuno-nutritional parameters of non-small cell lung cancer [39]. Due to the low incidence of thymoma, the current research on these potentially effective prognostic markers is relatively limited. In our study, CAR showed the greatest discriminative power, which was shown to be independently associated with OS and RFS. By comparing the area under the ROC curve (AUC), it can be seen that the prognostic prediction ability of CAR is superior to that of the GPS.
This study includes some limitations: First, it was performed at a single institution using a retrospective design and relatively few patients. It is therefore necessary to conduct a prospective multicenter study to test our findings in a larger patient cohort. When the model is applied to an external database, its validity needs to be verified. Additionally, only patients with primary TET surgery were included in this study; therefore, our findings may not be suitable for prognosis prediction in patients with recurrent TETs. and then, the threshold of the indicator CAR in this study was calculated based on the patients in this study, and a more precise threshold may be obtained with a larger sample size in the future. Finally, we were unable to investigate the impact of postoperative CAR dynamics in patients with thymoma.
Conclusions
Through retrospective analysis of more than 10 years of patient data, Our findings suggest that preoperative serum CAR level is an independent prognostic factor for OS and RFS. Therefore, CAR appears to be a powerful biomarker for the postoperative prognosis of TETs, but further prospective and multicenter studies are needed to confirm our results.
Availability of data and materials
If the investigator is interested in the clinic data, all clinicopathological information about our patient can be obtained by contacting the corresponding author, We have uploaded the data of this study in the Research Data Deposit of SYSUCC, and its number was RDDA2021002090 (http://www.resea rchdata.org.cn/).
Abbreviations
- CAR:
-
C-reactive protein/albumin ratio
- GPS:
-
Glasgow-Prognostic-Score
- pT stage:
-
Pathological T stage NLR:neutrophil-to-lymphocyte ratio
- ALB:
-
Albumin
- PLR:
-
Platelet-lymphocyte ratio
- pT stage:
-
Pathological T stage
- OS:
-
Overall survival
- RFS:
-
Recurrence-free survival
- TC:
-
Thymic carcinoma
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- ROC:
-
Receiver operating characteristic curve
- AUC:
-
Area under the curve
- TET:
-
Thymic epithelial tumor
References
Thomas CR Jr, Cameron D. et al. Thymoma: State of the Art. J Clin Oncol. 1999. 17(7):2280–2289
Marx A, Ströbel P, Badve SS, et al. ITMIG Consensus Statement on the Use of the WHO Histological Classification of Thymoma and Thymic Carcinoma: Refined Definitions, Histological Criteria, and Reporting. J Thorac Oncol. 2014;9:596–611.
Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol. 2010;5(10 Suppl 4):S260–5.
Marx A, Chan JK, Coindre JM, et al. The 2015 World Health Organization Classification of Tumors of the Thymus: Continuity and Changes. J Thorac Oncol. 2015;10(10):1383–95.
Kondo K. Monden, Yasumasa, Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg. 2003;76(3):878–84.
Weksler B, Dhupar R, Parikh V, et al. Thymic carcinoma: a multivariate analysis of factors predictive of survival in 290 patients. Ann Thorac Surg. 2013;95(1):299–303.
Ahmad U, Yao X, Detterbeck F, et al. Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg. 2015;149(1):95–100 (101 e1-2).
Lee CY, Bae MK, Park IK, et al. Early Masaoka stage and complete resection is important for prognosis of thymic carcinoma: a 20-year experience at a single institution. Eur J Cardiothorac Surg. 2009;36(1):159–62 (discussion 163).
Fukui T, Fukumoto K, Okasaka T, et al. Prognostic impact of tumour size in completely resected thymic epithelial tumours. Eur J Cardiothorac Surg. 2016;50(6):1068–74.
Yun JK, Kim HR, Kim DK, et al. Tumor size as a prognostic factor in limited-stage thymic epithelial tumors: A multicenter analysis. J Thorac Cardiovasc Surg. 2021;162(1):309-317 e9.
Okereke IC, Kesler KA, Morad MH, et al. Prognostic indicators after surgery for thymoma. Ann Thorac Surg. 2010;89(4):1071–7 (discussion 1077-9).
Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol. 2014;9(9 Suppl 2):S65-72.
Okumura M, Ohta M, Tateyama H, et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer. 2002;94(3):624–32.
Tseng Y-L, Wang ST, Wu MH, et al. Thymic carcinoma: involvement of great vessels indicates poor prognosis. Ann Thoracic Surg. 2003;76(4):1041–5.
Detterbeck F, Youssef S, Ruffini E, Okumura M. A review of prognostic factors in thymic malignancies. J Thorac Oncol. 2011;6(7 Suppl 3):S1698–704.
Ruffini E, Detterbeck F, Van Raemdonck D, et al. Tumours of the thymus: a cohort study of prognostic factors from the European Society of Thoracic Surgeons database. Eur J Cardiothorac Surg. 2014;46(3):361–8.
Arakawa Y, Miyazaki K, Yoshikawa M. et al. Value of the CRP-albumin ratio in patients with resectable pancreatic cancer. J Med Invest. 2021;68(34):244-255.
Ikuta S, Aihara T, Yamanaka N. Preoperative C-reactive protein to albumin ratio is a predictor of survival after pancreatic resection for pancreatic ductal adenocarcinoma. Asia Pac J Clin Oncol. 2019;15(5):e109–14.
Kudou K, Saeki H, Nakashima Y, et al. C-reactive protein/albumin ratio is a poor prognostic factor of esophagogastric junction and upper gastric cancer. J Gastroenterol Hepatol. 2019;34(2):355–63.
Yamauchi Y, Safi S, Muley T, et al. C-reactive protein-albumin ratio is an independent prognostic predictor of tumor recurrence in stage IIIA-N2 lung adenocarcinoma patients. Lung Cancer. 2017;114:62–7.
Zhang Y, Xiao G, Wang R. Clinical significance of systemic immune-inflammation index (SII) and C-reactive protein-to-albumin ratio (CAR) in patients with esophageal cancer: a meta-analysis. Cancer Manag Res. 2019;11:4185–200.
Kondo K, Yoshizawa K, Tsuyuguchi M, et al. WHO histologic classification is a prognostic indicator in thymoma. Ann Thorac Surg. 2004;77(4):1183–8.
Rea F, Marulli G, Girardi R, et al. Long-term survival and prognostic factors in thymic epithelial tumours. Eur J Cardiothorac Surg. 2004;26(2):412–8.
Eriksson M, Kaerlev L, Johansen P, et al. Tobacco smoking and alcohol consumption as risk factors for thymoma - A European case-control study. Cancer Epidemiol. 2019;61:133–8.
Marnell L, Mold C, Du Clos TW. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005;117(2):104–11.
Janik S, Bekos C, Hacker P, et al. Elevated CRP levels predict poor outcome and tumor recurrence in patients with thymic epithelial tumors: A pro- and retrospective analysis. Oncotarget. 2017;8(29):47090–102.
Veraar C, Janik S, Thanner J, et al. Clinical prognostic scores for patients with thymic epithelial tumors. Sci Rep. 2019;9(1):18581.
Esper DH, Harb WA. The cancer cachexia syndrome: a review of metabolic and clinical manifestations. Nutr Clin Pract. 2005;20(4):369–76.
Al-Shaiba R, McMillan DC, Angerson WJ, et al. The relationship between hypoalbuminaemia, tumour volume and the systemic inflammatory response in patients with colorectal liver metastases. Br J Cancer. 2004;91(2):205–7.
Argiles JM, Busquets S, Lopez-Soriano FJ. Cytokines in the pathogenesis of cancer cachexia. Curr Opin Clin Nutr Metab Care. 2003;6(4):401–6.
Xin Y, Cai H, Wu L, Cui Y. The Effect of Immunonutrition on the Postoperative Complications in Thymoma with Myasthenia Gravis. Mediators Inflamm. 2016;2016:8781740.
Huang YY, Wu LL, Liu X, Liang SH, Ma GW. Nomogram predict relapse-free survival of patients with thymic epithelial tumors after surgery. BMC Cancer. 2021;21(1):847.
Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):493–503.
McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534–40.
Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–30.
Wang L, Ruan M, Yan H, et al. Pretreatment serum neutrophil-to-lymphocyte and monocyte-to-lymphocyte ratios: Two tumor-related systemic inflammatory markers in patients with thymic epithelial tumors. Cytokine. 2020;133:155149.
Okada S, Shimomura M, Tsunezuka H, et al. High Neutrophil Count as a Negative Prognostic Factor for Relapse in Patients with Thymic Epithelial Tumor. Ann Surg Oncol. 2020;27(7):2438–47.
Almasaudi AS, Dolan RD, Edwards CA, McMillan DC. Hypoalbuminemia Reflects Nutritional Risk, Body Composition and Systemic Inflammation and Is Independently Associated with Survival in Patients with Colorectal Cancer. Cancers (Basel). 2020;12(7):1986.
Matsubara T, et al. Identification of the Best Prognostic Marker Among Immunonutritional Parameters Using Serum C-Reactive Protein and Albumin in Non-Small Cell Lung Cancer. Ann Surg Oncol. 2021;28(6):3046–54.
Acknowledgements
We would like to thank the Sun Yat-sen University Cancer Center for providing valuable clinical data, Thanks to our respected pioneer, Professor Tie-Hua Rong, who oriented us to surgical oncology. We thank our patients who silently provided data, Finally, we are grateful to Editage (https://app.editage.com/) for its excellent polishing work.
Funding
This work was supported by grants from the Wu Jie-ping Medical Foundation. (Nos. 320.6750.2020–15-7).
Author information
Authors and Affiliations
Contributions
Conception and design of the work: MGW, HYY. Provision of study materials or patients: MGW. Acquisition of data: HYY and HY. Analysis of data: LSH, HYY and HY. Interpretation of data: MGW, HYY. HYY drafted the manuscript; MGW, HY and HYY substantially revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of Sun Yat-Sen University Cancer Center (B2020-353–01) and complied with the Declaration of Helsinki. Data were recorded at the Sun Yat-sen University Cancer Center, under the record number: RDDA2021002090.At the same time, this study has obtained the exemption of informed consent application from the Ethics Committee of Sun Yat-sen University Cancer Center.
Consent for publication
Not applicable.
Competing interests
The authors disclose no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure 1.
KM analysis of T stage (A)、WHO(B) and Drinking history (C) based on overall survival.
Additional file 2: Figure 2.
KM analysis of T stage (A)、WHO(B) and Drinking history (C) based on relapse-free survival.
Additional file 3: Figure 3.
Nomogram predicting 3- ,5- and 10- overall survival after thymectomy for thymic epithelial tumors patients.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huang, YY., Liu, X., Liang, SH. et al. Prognostic value of preoperative C-reactive protein to albumin ratio in patients with thymic epithelial tumors: a retrospective study. BMC Cancer 22, 1183 (2022). https://doi.org/10.1186/s12885-022-10234-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-10234-x