Abstract
Combination chemoimmunotherapy (CIT) consisting of anti-CD20 has improved the progression-free survival (PFS) and overall survival (OS) of patients with chronic lymphocytic leukaemia (CLL). We performed a comprehensive synthesis of prognostic factors in patients with CLL on combined CIT with anti-CD20 antibodies compared with standard chemotherapy alone or targeted therapy.
We searched the MEDLINE and academic search complete electronic databases as well as clinicaltrials.gov (from inception up to 01 August 2022) for randomised controlled trials examining chemoimmunotherapy and targeted therapy in patients with CLL. The risk of bias and the quality of evidence was assessed using the quality in prognostic studies tool (QUIPS).
A total of 10 prognostic factors were identified and evaluated in patients with CLL on anti-CD20 antibody-containing CIT. The predictive value of the following prognostic factors was confirmed and associated with poor patient outcomes; deletion 17p (HR = 3.39), Immunoglobulin heavy chain variable region gene mutation status (HR = 0.96) and β2-microglobulin (HR = 1.41).
Conventional predictive factors may have retained prognostic value and could be useful in the stratification of patients who may be non-responsive to CIT.
Trial registration: International Prospective Register of Systematic Reviews (PROSPERO) registry (CRD42021218997).
Similar content being viewed by others
Introduction
The prevalence of chronic lymphocytic leukaemia (CLL) in adults over the age of 65 has gradually increased in high income countries [1, 2]. CLL disproportionately affects males, and an inferior survival rate in males has been reported in several studies [3,4,5].
Over the last two decades, novel clinical and genetic-based prognostic factors have been identified in patients with CLL [6]. These include age, gender, immunoglobulin heavy chain variable region gene (IGHV) mutation status and cytogenetic abnormalities [7, 8], the aberrant expression of CD38 and ZAP70 [9], TP53 mutation [10], β2-microglobulin [11], and the Eastern Cooperative Oncology Group (ECOG) performance status [6, 7]. The development and implementation of prediction models have allowed for the risk-stratification of patients with CLL based on genetic traits [12].
In patients with CLL, therapy consisting of ibrutinib [13, 14], chlorambucil [15], fludarabine and cyclophosphamide [16, 17] yielded low overall response rates (ORR), with treated patients having an estimated 5-year overall survival (OS) of < 40% [18, 19]. These clinical outcomes in patients with CLL led to a shift towards novel antibody-based therapies in the last decade. These include rituximab, an anti-CD20 monoclonal antibody which when administered in combination with standard chemotherapy, improves the patient response rates and is associated with complete remission (CR) in patients with CLL [20,21,22]. However, despite the benefit of chemoimmunotherapy (CIT) with rituximab, patient outcomes are highly variable [23]. The efficacy of rituximab-based CIT has been demonstrated in cohorts of patients without the associated genetic aberrations such as Del(17p) and TP53 mutations [24].
The advances and refinement of prognostic risk scores has led to improved risk stratification of patients with CLL. The cornerstone of these risk scores, are the revised Rai [25] and Binet [26] staging systems, and novel prognostic indices such as CLL International Prognostic Index (CLL-IPI) [27] which allow for a precise risk stratification. Pertinent challenges in the risk stratification of patients with CLL on CIT include the lack of cumulative evidence on the predictive value of integrated cell and genetic based prognostic models [28]. Moreover, the lack of diverse multi-ethnic cohorts and prevalent risk factors also contribute to the imprecision of these predictive models [29, 30]. Therefore, the current systematic review and meta-analysis sought to identify and evaluate studies reporting on prognostic factors in patients with CLL on CIT or targeted therapy. Moreover, we aimed at providing a comprehensive synthesis and confirmation of prognostic factors associated with poor clinical outcomes in patients with CLL on CIT.
Methods
Eligibility criteria
The eligibility criteria was based on the Population, Index prognostic factor, Comparator prognostic factors, Outcome, Timing and Setting (PICOTS) guidelines [31]. We included randomised controlled trials (RCTs) reporting on prognostic factors in patients with CLL on CIT containing anti-CD20 monoclonal antibodies (rituximab, obinutuzumab, ofatumumab) or targeted therapy such as Bruton’s tyrosine kinase (BTK) inhibitors. We also included studies that aimed at developing or validating predictive models for mortality in CIT-treated patients with CLL. In addition, we included studies reporting on predictive measures at any time point and setting. Reviews, letters, and case-studies were excluded. In this systematic review, predictive models were considered as multivariable models used to predict survival in patients with CLL using selected predictors. We considered index prognostic factors derived from the CLL International Prognostic Index (CLL-IPI) [27], the German CLL Study Group (GCLLSG) [32], and the MD Anderson Cancer Centre (MDACC) nomogram predictive models [33].
Search strategy and selection process
A systematic literature search was performed by two independent reviewers (ZAM and BBN) on the MEDLINE, MasterFILE premier, Health source: Nursing/Academic edition, and clinical trials.gov. We made use of Medical Subject Headings (MeSH) and related synonyms which included, chronic lymphocytic leukaemia, rituximab, ofatumumab, Obinutuzumab, anti-CD20 monoclonal antibody, ibrutinib, venetoclax, acalabrutinib, idelalisib and prognosis. All electronic databases were searched from inception to the 1st of August 2022. A detailed search strategy is presented in Supplementary Table 1. To augment the database search, we screened the bibliographies of relevant reviews and included studies.
Data extraction
Two reviewers (ZAM and BBN) independently extracted data items from the included studies defined in the critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies for Prognostic factors CHARMS-PF checklist [34]. The extracted study characteristics included, source of data, participant description, sample size, outcomes to be predicted, candidate predictors, type of model.
Risk of bias and quality assessment
The certainty and strength of the evidence was assessed by two independent reviewers (ZAM, SAM) using the Quality In Prognostic Studies (QUIPS) tool [31]. The tool consists of six domains used to appraise studies of prognostic factors (Supplementary Table 2). A third reviewer (BBN) was consulted for arbitration.
Statistical analysis
The Cohen’s kappa was used to assess the inter-rater reliability for the study selection and the study quality and risk of bias assessments [35]. The hazard ratios (HR) or odds ratios (OR) and 95% confidence interval (CI) were pooled to estimate the pooled OS and PFS. The effect estimates of studies were pooled using a random-effects model [36]. The I2 and Chi squared statistical tests were used to assess the levels of statistical heterogeneity [37, 38]. An I2 value of >50% was considered as substantial [36]. All data analysis was performed using STATA 16.0 (StataCorp LP, TX, USA).
Subgroup and sensitivity analyses
To explore the sources of heterogeneity amongst the included studies, we performed a sensitivity analysis based on the study design and quality.
Confirmation of predictive factors
The reported prognostic factors were confirmed based on the robustness of the overall direction of the effect across all eligible studies. Moreover, adjusted effect estimates that remained statistically significant (p < 0.05) after adjusting for covariates in the multivariate analysis were considered as confirmed.
Results
Included studies
We retrieved a total of 4123 citations through the database search, and after excluding 602 duplicated studies only 3521 studies were eligible for screening. Amongst these, 3320 studies were ineligible and excluded during the abstract screening phase. A total of 201 citations were retrieved and 118 articles with available full-texts were assessed for eligibility. A total of 171 studies were excluded for the following reasons: single arm studies (n = 61), ineligible study design (n = 38), clinical endpoint not reported (n = 26); no suitable comparator group (n = 33); only contained post-trial follow-up data (n = 13). In all, 17 studies [14,15,16,17, 39,40,41,42,43,44,45,46,47,48,49,50,51] met the inclusion criteria and were included in the qualitative and quantitative analysis (Fig. 1). The overall reviewer agreement for study selection, was 89% (kappa = 0.82).
Characteristics of included studies
The 17 included studies were published between 2010 and 2021 comprising of a total of 7 349 patients with CLL (Table 1). Most of the included trials were multicentre studies and the study sample size varied from 66 to 817 patients (Median: 389, IQR: 296—532). The age of enrolled participants ranged from 22 – 92 years.
The geographic distribution of the included studies consisted of Europe, Americas, Asia, Australia (Table 1). The included studies comprised of 64% (n = 4 700) patients who were treatment-naïve, 11% (n = 815) of patients who were previously treated and 22.3% (n = 1 642) who were relapsed/refractory. In addition, 47% (n = 8) of the included studies reported on the Rai staging whereas 41% (n = 7) reported on Binet staging system. One study (6%) reported both Rai and Binet staging systems and another study (6%) did not specify the staging system used.
Prognostic factors in patients with CLL
In the included studies, prognostic factors were analysed before the start of treatment (Table 2). Overall, the studies comprised of 25.5% (n = 1 823) of patients who were 70 years or older, 55.7% (n = 3 984) of patients with an unmutated IGHV status, 17.4% (n = 1 245) with del11q, 6.8% (n = 489) with a del17p, 26.8% of the patients (n = 1 915) had del13q, and 3.9% (n = 264) had TP53 mutation. Notably, 6% (n = 429) patients were reported to have Trisomy 12. In the reported cell-based prognostic factors the included studies reported on ZAP-70 expression in 12.2% (n = 872) of the patients, and CD38 expression was reported in 12% (n = 863) of the included patients, 21.3 (n = 1 526) patients had elevated B2M levels (≥ 3.5 mg/L). In all, 36.7% (n = 2 625) of the included patients with CLL were in the advanced stage of the disease.
Risk of bias and quality assessment
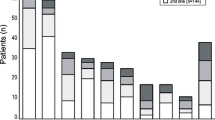
We assessed the quality of all included studies using the QUIPS tool for assessing risk of bias in prognostic factor studies [31]. The study-level risk of bias assessment is presented in Supplementary Table 2. Briefly, two studies were scored as high-risk [16, 41], five as moderate risk [39, 40, 47, 48, 50], whilst the rest were deemed to be at low risk of bias [14, 15, 17, 42,43,44,45,46, 49, 51]. Overall, the included studies were scored as low risk for study participation (k = 0.76, minimal agreement), and outcome measurement (k = 0.88, strong agreement), moderate risk for study attrition (k = 0.88, moderate agreement) and confounding measurement (k = 0.65, minimal agreement) and high risk for prognostic factor measurement (k = 0.90, strong agreement) and statistical analysis and reporting (k = 0.76, minimal agreement) (Fig. 2).
Primary outcomes
Survival outcomes of patients with CLL receiving CIT containing anti-CD20
A total of 5 studies [15,16,17, 42, 47] reported on an improved PFS in patients with CLL, when an anti-CD20 mAbs were concurrently used with standard chemotherapy. CIT in combination with anti-CD20 monoclonal antibodies, was associated with improved PFS (HR = 0.50 Cl [0.35–0.65], p < 0.01). There were high levels of heterogeneity (I2 = 90.78%) in the included studies. Overall, the pooled effect estimate showed no statistically significant difference in OS in patients with CLL treated with CIT and chemotherapy alone (p = 0.22) (Fig. 3).
Survival outcomes of patients with CLL on maintenance therapy with anti-CD20
A total of 4 studies [43,44,45, 51] reported on an improved PFS following maintenance therapy with anti-CD20 therapy as compared to patients who did not receive any treatment (observation group). The pooled effect estimate showed improved albeit non-significant PFS (HR = 0.51 [0.42–0.60], p = 0.93). There were no differences in OS between patients receiving maintenance therapy compared to those who were not on treatment. There were no significant differences in the pooled effect estimates (p = 0.96) and there were low levels of statistical heterogeneity amongst included studies, I2 = 0%.
Survival outcomes of patients with CLL receiving targeted therapy
In the meta-analysis, a total of eight studies [14, 39, 40, 46, 48,49,50] reported an improved PFS with novel targeted agents as compared to chemoimmunotherapy. Target therapy containing BTK and BLC2 inhibitors was associated with significantly improved PFS as compared to CIT (HR = 0.25 Cl [0.19–0.30], p = 0.07). OS data was available for seven studies [39,40,41, 46, 48,49,50]. Overall, targeted therapy was associated with improved OS (HR = 0.56 [0.33–0.80], p = 0.05). There were substantial levels of heterogeneity in the included studies (I2 = 51.67%).
Overall, the meta-analysis shows that chemoimmunotherapy and maintenance therapy with anti-CD20 antibodies is superior to chemotherapy, and targeted therapy is superior to CIT in terms of PFS with HR = 0.39 [0.31–0.47], p < 0.01 and OS (HR = 0.66 [0.53–0.78], p < 0.02 (Fig. 4). There were high levels of heterogeneity on studies assessed for PFS (I = 88.16%).
Prognostic factors associated with poor patient outcomes in CLL patients
Prognostic markers ranged from host factors, such as age and cytogenetics, whereby 10 (58.8%) studies reported Del(17p) as a prognostic factor for PFS [14, 15, 17, 40,41,42,43,44, 47,48,49,50]. Two studies excluded patients with Del(17p) [45, 46] and in another study, del(17p) and del (11q) did not impact PFS [44]. Whereas 10 studies reported unmutated IGHV as a prognostic factor [17, 39,40,41,42, 45, 46, 48,49,50]. Trisomy 12 was identified as a prognostic factor in three studies [39, 42, 46] and TP aberrations was reported in four studies [40, 41, 48, 49].
The reported prognostic factors associated with early disease progression included elevated B2M levels (levels of ≥ 3.5 mg/L) [17, 43], thymidine kinase (concentration of 10 µ/L), white cell count (10 × 109 per L) and ECOG PS of 2 [17] and advanced disease stage III/IV [17]. After adjusting for covariates, Del(17p), unmutated IGVH status and elevated B2M (Table 4).
Discussion
We conducted a systematic review and meta-analysis of prognostic factors associated with poor survival in patients with chronic lymphocytic leukemia on CIT and novel targeted agents. The available data on the use of ICIs and targeted therapy in the management of CLL is limited to predominantly European and American populations (Table 1). The current study also highlights the lack of multi-ethnic RCTs with diverse population with CLL. The included studies reported on various candidate predictors of survival in patients with CLL on CIT and targeted therapy (Table 3).
Amongst the reported prognostic factors only one protein factor (β2-microglobulin) retained predictive value in patients with CLL on anti-CD20-containing CIT, after multivariable analysis. Only two other prognostic factors met our criteria for confirmed prognostic factors and these included, cytogenetic factors (deletion 17p, IGHV status). Notably, in our meta-analysis we pooled studies that reported on adjusted estimates and the levels of statistical heterogeneity were high (I2 > 70%) for the confirmed cytogenetic factors and for β2-microglobulin (Table 4). Interestingly, the value of β2-microglobulin as an independent prognostic marker has not been extensively assessed in patients with CLL on CIT and targeted therapy, although in a previous study its predictive value for treatment-free survival was retained after adjusting for factors such as CD38 expression and IGHV mutation status [52].
The cut-off levels of B2M associated with poor prognosis remain unclear and in untreated CLL patients a value of 2 mg/L [54] while in our analysis B2M levels ≥ 3.5 mg/L [17, 43] were associated with disease progression in treated patients with CLL. Notably in the current analysis, we report on the retained predictive value of B2M in CLL patients on rituximab-containing CIT and maintenance therapy with rituximab. Future studies comprised of diverse patient populations are needed especially in minority ethnic groups to allow for validation of this prognostic marker in the era of CIT and novel targeted therapy. In the era of CIT, and chemotherapy-free CLL management, future studies evaluating the correlations between B2M levels and expression of CD20 and other immune checkpoints in patients with CLL, may assist in the stratification of patients who are most responsive to immunotherapy.
To the best of our knowledge this systematic review and meta-analysis provides the first analysis of prognostic factors in anti-CD20-containing CIT and targeted therapy. The current study has several limitations, firstly these findings are mainly derived from American and European populations. This limits the extrapolation of these findings into other low-to-middle income countries. Lastly, due to the low number of studies reporting on these prognostic factors in patients with CLL on CIT and targeted therapy, we could not explore the sources of heterogeneity in a subgroup analysis based on the potential differences in disease stage and duration of follow-up.
Conclusion
A plethora of novel prognostic factors have been described in untreated patients with CLL. However, in the era of CIT there is a lack of adequate studies exploring the predictive value of the conventional and novel prognostic factors in a multi-ethnic cohort of patients with CLL. In this systematic review and meta-analysis of prognostic factors, classical cytogenetic factors such as deletion 17p retained predictive value in patients with CLL on CIT. Lastly, the white cell count and conventional prognostic markers such as B2M and LDH levels were also regarded as confirmed prognostic factors in patients with CLL on rituximab containing CIT. These factors should be included in future prognostic factors in the era of CIT and chemotherapy-free era of CLL patient management.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
References
Gribben JG. Chronic lymphocytic leukemia: planning for an aging population. Expert Rev Anticancer Ther. 2010;10:1389–94.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30.
Catovsky D, Wade R, Else M. The clinical significance of patients’ sex in chronic lymphocytic leukemia. Haematologica. 2014;99:1088–94.
Molica S, Mauro FR, Callea V, Gentile M, Giannarelli D, Lopez M, et al. A gender-based score system predicts the clinical outcome of patients with early B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2005;46:553–60.
Kristinsson SY, Dickman PW, Wilson WH, Caporaso N, Björkholm M, Landgren O. Improved survival in chronic lymphocytic leukemia in the past decade: a population-based study including 11,179 patients diagnosed between 1973–2003 in Sweden. Haematologica. 2009;94:1259–65.
Cohen JA, Bomben R, Pozzo F, Tissino E, Härzschel A, Hartmann TN, et al. An updated perspective on current prognostic and predictive biomarkers in chronic lymphocytic leukemia in the context of chemoimmunotherapy and novel targeted therapy. Cancers. 2020;12:1–17.
Rosenquist R, Cortese D, Bhoi S, Mansouri L, Gunnarsson R. Prognostic markers and their clinical applicability in chronic lymphocytic leukemia: Where do we stand? Leuk Lymphoma. 2013;54:2351–64.
Pflug N, Bahlo J, Shanafelt TD, Eichhorst BF, Bergmann MA, Elter T, et al. Development of a comprehensive prognostic index for patients with chronic lymphocytic leukemia. Blood. 2014;124:49–62.
Rassenti LZ, Jain S, Keating MJ, Wierda WG, Grever MR, Byrd JC, et al. Relative value of ZAP-70, CD38, and immunoglobulin mutation status in predicting aggressive disease in chronic lymphocytic leukemia. Blood. 2008;112:1923–30.
Landau DA, Tausch E, Taylor-Weiner AN, Stewart C, Reiter JG, Lawrence M, et al. Mutations driving CLL and their evolution in progression and relapse HHS public access cologne cluster of excellence in cellular stress responses in aging-associated diseases. Ivana Bozic Nature. 2015;6814149:525–30.
Hallek M, Wanders L, Ostwald M, Busch R, Senekowitsch R, Stern S, et al. Serum β2-microglobulin and serum thymidine kinase are independent predictors of progression-free survival in chronic lymphocytic leukemia and immunocytoma. Leuk Lymphoma. 1996;22:439–47.
Gaidano G, Rossi D. The mutational landscape of chronic lymphocytic leukemia and its impact on prognosis and treatment. Hematology. 2017;329–37.
Burger JA, Sivina M, Jain N, Kim E, Kadia T, Estrov Z, et al. Randomized trial of ibrutinib vs ibrutinib plus rituximab in patients with chronic lymphocytic leukemia. Blood. 2019;133:1011–9.
Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379:2517–28.
Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–10.
Robak T, Dmoszynska A, Solal-Céligny P, Warzocha K, Loscertales J, Catalano J, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1756–65.
Hallek M, Fischer K, Fink AM, Busch R, Mayer J, Hensel M, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. 2010;376:1164–74.
Jaglowski S, Jones JA. Choosing first-line therapy for chronic lymphocytic leukemia. Expert Rev Anticancer Ther. 2011;11:1379–90.
Gökbuget N, Dombret H, Ribera JM, Fielding AK, Advani A, Bassan R, et al. International reference analysis of outcomes in adults with B-precursor Ph-negative relapsed/refractor y acute lymphoblastic leukemia. Haematologica. 2016;101:1524–33.
Lee LJ, Toze CL, Huang SJT, Gillan TL, Connors JM, Sehn LH, et al. Improved survival outcomes with the addition of rituximab to initial therapy for chronic lymphocytic leukemia : a comparative effectiveness analysis in the province of British Columbia. Canada. 2018;59:1356–63.
Keating MJ, O’Brien S, Albitar M, Lerner S, Plunkett W, Giles F, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–88.
Wierda W, O’Brien S, Faderl S, Ferrajoli A, Wang X, Do KA, et al. A retrospective comparison of three sequential groups of patients with recurrent/refractory chronic lymphocytic leukemia treated with fludarabine-based regimens. Cancer. 2006;106:337–45.
Brown JR, Hallek MJ, Pagel JM. Chemoimmunotherapy versus targeted treatment in chronic lymphocytic leukemia: When, How Long, How Much, and in Which Combination? American Society of Clinical Oncology Educational Book. 2016;36:e387–98.
Brown JR, Cymbalista F, Sharman J, Jacobs I, Nava-Parada P, Mato A. The role of rituximab in chronic lymphocytic leukemia treatment and the potential utility of biosimilars. Oncologist. 2018;23:288–96.
Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–34.
Binet JL, Auquier A, Dighiero G, Chastang C, Piguet H, Goasguen J, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48:198–206.
International T. An international prognostic index for patients with chronic lymphocytic leukaemia (CLL-IPI): a meta-analysis of individual patient data. Lancet Oncol. 2016;17:779–90.
Kreuzberger N, Jaag D, Trivella M, Lj E, Aldin A, Umlau L, et al. Kreuzberger N, Damen JAAG, Trivella M, Estcourt LJ, Aldin A, Umlau L, Vazquez-Montes MDLA, Wol R, Moons KGM, Monsef I, Foroutan F, Kreuzer KA, Skoetz N. 2020. https://doi.org/10.1002/14651858.CD012022.pub2.www.cochranelibrary.com.
Parikh SA. Chronic lymphocytic leukemia treatment algorithm 2018. Blood Cancer J. 2018;8:1–10.
Yun X, Zhang Y, Wang X. Recent progress of prognostic biomarkers and risk scoring systems in chronic lymphocytic leukemia. Biomarker Res. 2020;8:1–11.
Hayden JA, Cô P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. 2006.
Tam CS, Seymour JF. A new prognostic score for CLL. Blood. 2014;124:1–2.
Munk Pedersen I, Reed J. Microenvironmental interactions and survival of CLL B-cells. Leuk Lymphoma. 2004;45:2365–72.
Moons KGM, de Groot JAH, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS Checklist. PLoS Med. 2014;11:1–12.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74.
Schroll JB, Moustgaard R, Gøtzsche PC. Dealing with substantial heterogeneity in Cochrane reviews. Cross-sectional study. BMC Med Res Methodol. 2011;11:1–8.
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:1–9.
Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–29.
Shanafelt TD, Wang XV, Kay NE, Hanson CA, O’Brien S, Barrientos J, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381:432–43.
Seymour JF, Kipps TJ, Eichhorst B, Hillmen P, D’Rozario J, Assouline S, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378:1107–20.
Ghia P, Pluta A, Wach M, Lysak D, Kozak T, Simkovic M, et al. Ascend: Phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38:2849–61.
Robak T, Warzocha K, GovindBabu K, Kulyaba Y, Kuliczkowski K, Abdulkadyrov K, et al. Ofatumumab plus fludarabine and cyclophosphamide in relapsed chronic lymphocytic leukemia: results from the COMPLEMENT 2 trial. Leuk Lymphoma. 2017;58:1084–93.
Robak T, Błoński J, Skotnicki AB, Piotrowska M, Wróbel T, Rybka J, et al. Rituximab, cladribine, and cyclophosphamide (RCC) induction with rituximab maintenance in chronic lymphocytic leukemia: PALG - CLL4 (ML21283) trial. Eur J Haematol. 2018;100:465–74.
Greil R, Obrtlíková P, Smolej L, Kozák T, Steurer M, Andel J, et al. Rituximab maintenance versus observation alone in patients with chronic lymphocytic leukaemia who respond to fi rst-line or second-line rituximab-containing chemoimmunotherapy: final results of the AGMT CLL-8a Mabtenance randomised trial. 2016. https://doi.org/10.1016/S2352-3026(16)30045-X.
Dartigeas C, Neste E Van Den, Léger J, Maisonneuve H, Berthou C, Dilhuydy M, et al. Articles Rituximab maintenance versus observation following abbreviated induction with chemoimmunotherapy in elderly patients with previously untreated chronic lymphocytic leukaemia (CLL 2007 SA): an open-label, randomised phase 3 study. 2017;3026.
Chanan-Khan A, Cramer P, Demirkan F, Fraser G, Silva RS, Grosicki S, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17:200–11.
Hillmen P, Robak T, Janssens A, Babu KG, Kloczko J, Grosicki S, et al. Chlorambucil plus ofatumumab versus chlorambucil alone in previously untreated patients with chronic lymphocytic leukaemia (COMPLEMENT 1): a randomised, multicentre, open-label phase 3 trial. Lancet. 2015;385:1873–83.
Moreno C, Greil R, Demirkan F, Tedeschi A, Anz B, Larratt L, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:43–56.
Fischer K, Al-Sawaf O, Bahlo J, Fink A-M, Tandon M, Dixon M, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380:2225–36.
Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Ian W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE-TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395:1278–91.
van Oers MHJ, Kuliczkowski K, Smolej L, Petrini M, Offner F, Grosicki S, et al. Ofatumumab maintenance versus observation in relapsed chronic lymphocytic leukaemia (PROLONG): An open-label, multicentre, randomised phase 3 study. Lancet Oncol. 2015;16:1370–9.
Delgado J, Pratt G, Phillips N, Briones J, Fegan C, Nomdedeu J, et al. Beta 2-microglobulin is a better predictor of treatment-free survival in patients with chronic lymphocytic leukaemia if adjusted according to glomerular filtration rate. Br J Haematol. 2009;145:801–5.
Dartigeas C, Van Den Neste E, Léger J, Maisonneuve H, Berthou C, Dilhuydy MS, et al. Rituximab maintenance versus observation following abbreviated induction with chemoimmunotherapy in elderly patients with previously untreated chronic lymphocytic leukaemia (CLL 2007 SA): an open-label, randomised phase 3 study. Lancet Haematol. 2018;5:e82–94.
Tsimberidou AM, Wen S, O’Brien S, McLaughlin P, Wierda WG, Ferrajoli A, et al. Assessment of chronic lymphocytic leukemia and small lymphocytic lymphoma by absolute lymphocyte counts in 2,126 patients: 20 years of experience at the University of Texas MD Anderson Cancer Center. J Clin Oncol. 2007;25:4648–56.
Acknowledgements
None.
Funding
There was no funding source for this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
ZAM and BBN conceptualised and designed the study. BBN provided supervision. ZAM was responsible for the writing of the original draft. ZAM, BBN, SAM, TMN reviewed, edited, and approved the final manuscript. BBN is the guarantor of the study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable The current study reports on publicly available data and no participant-level data were obtained.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial or academic interests that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Search strategy.
Additional file 2: Supplementary Table 2.
Risk of bias assessment of individual studies using the QUIPS tool.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mkhwanazi, Z.A., Nyambuya, T.M., Mfusi, S.A. et al. Prognostic markers in patients with chronic lymphocytic leukaemia on targeted therapy, chemoimmunotherapy with anti-CD20 monoclonal antibody: a systematic review and meta-analysis of prognostic factors. BMC Cancer 22, 1218 (2022). https://doi.org/10.1186/s12885-022-10223-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-10223-0