Abstract
Background
This analysis presents the outcomes of the operations of the National Cancer Network (NCN) pilot project in Lower Silesian Voivodeship, Poland, for lung cancer for the period of 2019–2021. The results concerning measures of the quality of medical processes were analysed.
Methods
Twenty-one measures used to gauge the quality of oncological care for lung cancer were assessed. Data collection and processing for the purpose of calculating the measures were carried out as part of the NCN pilot project based on the Regulation of the Ministry of Health enacted on 13 December 2018. The measures were calculated at the Voivodeship Coordination Center, and the data were derived from the centres included in the network in the area of the analysed voivodeship.
Results
A total of 3,638 patients diagnosed with lung cancer were enrolled in the NCN pilot program during the analysed period. For 3 measures, out of 21, target values were obtained. For 2 measures, the values differed significantly from the assumed target value.
Conclusion
In our opinion, the NCN pilot study, as a test of the network’s functioning, meets the assumed goal. The NCN assessment is based on, inter alia, analysis of the outcomes of oncological quality of care measures for lung cancer, and facilitates monitoring of the quality of medical services provided and the identification of areas for improvement. In addition, the pilot program, which will last until the end of 2022, will allow for further in-depth analysis regarding the network’s limitations before implementing the system on a national scale in Poland. This will be the subject of further investigation.
Similar content being viewed by others
Introduction
Cancer has become one of the greatest challenges faced by medicine in the twenty-first century. Increased average life expectancy and exposure to carcinogens are the main factors related to the observed rise in the number of malignancies. Among them, lung cancer constitutes a significant problem for the Polish health care system. Between 2015 and 2019, lung cancer accounted for 17% and 9% of all cases of malignancy recorded in Poland in men and women, respectively. Currently – it is first cause of cancer deaths, both among men and women in Poland and one of the most frequent cancers [1]. To quote data concerning the projected epidemiology of lung cancer in Poland, by 2030, the number of new cases is expected to increase by 17% in men and 10% in women [2].
To improve treatment outcomes, in addition to high-quality medical services in the field of oncology, efficient coordination of diagnostic and therapeutic processes is necessary.
In early 2019, a pilot project for the National Cancer Network (NCN) was launched in Poland, with the goal of (among others) improving the coordination of treatment and introducing a system to monitor the quality of oncological procedures for the most common types of cancer [3].
The pilot project covers four provinces: Lower Silesia Province, Holy Cross Province, Pomerania Province, and Podlasie Province, with a total population of 7.6 million [4]. The aim of this paper is to present the initial results of the NCN’s functioning in the scope of lung cancer diagnosis and treatemnt for Lower Silesia Province between 2019 and 2021.
Materials and methods
The pilot project for the Polish NCN is based on the Regulation of the Ministry of Health enacted on 13 December 2018; its goal is to assess the organisation and quality of oncological care within an oncology network operating in the provinces covered by the pilot project [3]. Patients diagnosed with lung cancer, breast cancer, colorectal cancer, prostate cancer, and ovarian cancer were included. In Lower Silesia Province, whose population is 2.9 million, data concerning lung cancer have been reported since 1 February 2019. The pilot project was originally set to end on 31 December 2021 [5]. However, under the Regulation of the Ministry of Health enacted on 23 December 2021, the pilot project has been extended until 31 December 2022 [6].
The network comprises a Regional Coordination Centre as well as first- and second-level support centres, which cooperate in the scope of oncological care provided to patients covered by the pilot project.
In Lower Silesia Province (the subject of this analysis), the Regional Coordination Centre’s role was entrusted to the Wroclaw Comprehensive Cancer Centre in Wroclaw as the centre with the highest capacity in terms of medical personnel and the ability to provide comprehensive oncological treatment (i.e.surgery, radiation therapy, systemic treatment, palliative care, rehabilitation). Coordination between the 15 centres included in the pilot project and the coordination centre in Wroclaw within the oncology network created in Lower Silesia Province became an additional aspect.
The centres that form the network in the given province were selected on the basis of quality and organisational criteria, such as the lowest number of procedures performed per year or resources in terms of staff and equipment.
One of the NCN’s primary goals is to introduce a system to monitor the quality of oncological care. For this purpose, registers specific for particular types of cancer were created, and a list of 46 indicators for monitoring the analysed processes was compiled. Twenty-one of these indicators were used to examine the quality of oncological care for lung cancer. Table 1 describes all indicators employed in the Polish National Oncology Network.
To ensure a uniform standard of calculations for all indicators, indicator assessment sheets were proposed; these sheets contain data concerning the process to which a given indicator applies, instructions for calculating the indicator, and sources of data and target value (PROQUAL Management Institute). Table 2 provides an example of an indicator assessment sheet.
The Regional Coordination Centre is the unit responsible for calculating the indicators on the basis of clinical (qualitative) and settlement (quantitative) data. These data come from the centres participating in the pilot project in the given province and from the National Health Fund and are stored in a central data warehouse. A uniform electronic format for inputting the data makes it possible to quickly filter the data to calculate specific indicators.
The results are published quarterly. Graphical presentations of the calculated indicators are cumulative quarter-to-quarter and can be accessed through a web application (Oncoindiv3.0).
In the presented results, we decided to focus on three indicators that achieved the expected values and two indicators with results significantly below standards.
Results
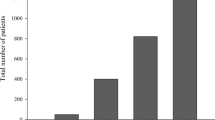
Over the course of the pilot project, in Lower Silesia Province, 3,638 patients—2,161 (59%) men and 1,477 (41%) women—with a diagnosis of lung cancer were included between 2019 and 2021. Patients over 60 were the most numerous group among both men and women, with 1,891 (87%) and 1,267 (85%), respectively included. Of the total number of patients, 420 (12%) were reported by the Regional Coordination Centre (the Wroclaw Comprehensive Cancer Centre), 2,649 (73%) by the Lower Silesia Centre for Pulmonary Diseases in Wroclaw, and 569 (15%) by other centres in Lower Silesia Province.
Expected values were obtained for three indicators. For ‘Percentage of patients who required hospitalisation due to complications following radiation therapy for cancer’, a result of 0% was obtained. For ‘Percentage of diagnostic procedures repeated within a 6-week period (computed tomography, endoscopy, biopsy, pathological analysis, molecular analysis)’, the desired value of 0% was also obtained for the assessed diagnostic procedures, except for pathological analysis, for which a value of 0.05% was derived. The last indicator for which the outcome was consistent with the expected value specified in the indicator assessment sheet was ‘Percentage of diagnostic procedures thatrequired reimpression or reassessment of material within a 6-week period’, with a value of 0% obtained for computed tomography, pathological analysis, and molecular analysis.
For the two indicators, the obtained values were significantly different from the expected values of 100%. For ‘Percentage of patients with suspected lung cancer and pleural effusion in whom aetiology of the effusion was identified’, this aetiology was identified in only 8% of patients. For ‘Percentage of patients with stage 3 non-small-cell lung cancer who received concurrent chemoradiotherapy’, the final outcome was 6%.
A list of all indicators discussed in this analysis, together with the obtained results, is shown in Table 3.
Discussion
One of the network’s main goals is to introduce indicators specific to particular disease entities for use in monitoring the quality of services provided within oncological care. The proposed system is rooted in a mechanism that enables constant improvement under a plan-do-check-act (PDCA) cycle.
The data suggest a correlation between the quality of the medical process, its organisation, and the therapeutic effect [7,8,9,10]. Moreover, an idea of quality indicators in oncology is introduced in different areas of cancer care [11,12,13]. Until now, Poland has had no unified system for managing a large database containing data on cancer patients, which would make it possible to use such data to calculate indicators related to outcomes and internal processes, i.e. the quality of individual hospitals that provide oncological care. The pilot project for the network is meant to test the functioning of specially designed data warehouses, the quality of data stored in these warehouses, the process of collecting the data, and the flow of data between individual components in the network.
When the NCN was being designed, emphasis was placed on creating a unified data structure for all centres participating in the pilot project and on automating processes (e.g.the calculation of individual indicators) to the greatest extent possible. This was done to facilitate and accelerate data processing. In addition, these assumptions meant that the obtained results could be expected to be reliable and transparent and to enable easy comparison between centres or provinces.
The purpose of monitoring oncological care processes is to identify areas that require an increase in quality level or that should be maintained at a specified satisfactory level. Our analysis showed that the results obtained through the NCN indeed identified such areas. Of the 21 indicators, two were at an unsatisfactory level compared to values specified as target values. For ‘Percentage of patients with stage 3 non-small-cell lung cancer who received concurrent chemoradiotherapy’, the obtained result was only 6%. Similar analyses conducted in other European countries revealed a higher percentage of administration of this treatment regimen [14, 15]. Such low results in our province may be related to the different structures of patients with stage 3 cancer included in our analysis. It is possible that the vast majority of patients were stage 3B and 3C patients, who are more commonly qualified for sequential chemoradiotherapy. However, the definition of the indicator failed to differentiate between stages 3A, 3B, and 3C. Another reason for the unsatisfactory outcome may have been organisational or logistical obstacles. Even in Wroclaw, the capital of the province, hospitals that provide systemic treatment for lung cancer patients and the facilities that provide radiation therapy are located in different parts of the city, which could have affected the choice of treatment regimen and led to a preference for sequential therapy. It is also possible that there was a methodological error that affected the reliability of the findings. Further studies are needed to either confirm the above reasoning or to identify other factors that influence the result obtained for the indicator in question. Such internal analysis is currently being conducted by the Regional Coordination Centre.
Increasing the number of patients qualified for concurrent treatment seems vital given the current indications for immunotherapy, which in this instance led to an increase in the overall survival rate in patients who received concurrent chemoradiotherapy [16, 17].
The second outcome, which fell significantly below the set standard, was the very low percentage of lung cancer patients with a diagnosis of pleural effusion at only 8%. Of the 25 patients included in the assessment through this indicator (‘Percentage of patients with suspected lung cancer and pleural effusion in whom aetiology of the effusion was identified’), only 2 received such a diagnosis. However, this indicator received negative opinions from experts who approved it in Lower Silesia Province and will likely not be included in the NCN after the pilot project ends.
One argument supporting the exclusion of this indicator is its doubtful function in the clinical realm. Pleural effusion is typically seen in palliative care patients, for whom delaying symptomatic treatment due to commencement of a pleural effusion diagnosis seems unacceptable.
Furthermore, the number of included patients for this indicator was very low at only 25. The same was true for ‘Percentage of patients with suspected lung cancer seen by a pulmonary specialist within 14 working days of registering a referral with the health care provider’ at only 62 patients. This may have been caused by incorrect design of the indicators and the lack of information concerning the described event (such as being seen by a pulmonary specialist) in the patient’s medical history, which makes it impossible to correctly assign the patient within the indicator in question. This indicator may also be rejected following the conclusion of the pilot project according to the reasons stated above.
Analysis of the results obtained through the NCN for our province also identified indicators that met their target values or were very close. Two of these described the patients’ condition. The first measured the share of patients who required hospitalisation due to complications following the conclusion of radiation therapy for cancer and had a value of 0% at the end of the assessed period. The second evaluated the number of patients who required hospitalisation due to complications following systemic treatment. In this case, the data concerned the periods of 30, 60, and 90 days after the assessed therapy ended. A result of 2% was obtained for each period. When compared to similar analyses, the obtained outcomes may be regarded as indicating that the frequency of complications requiring hospitalisation following non-surgical treatment in our region is optimal [18, 19].
We believe, however, that the introduction of an additional indicator—which would make it possible to assess the number of hospitalisations required during radiation therapy administered primarily on an outpatient basis—should be considered. Other new indicators could also be included in the NCN, e.g. indicators evaluating aspects related to immunotherapy or stereotactic radiation therapy for lung cancer.
Another group of indicators that met the levels expected under the assumptions of the NCN involves diagnostic procedures. These indicators describe internal processes and the quality of specific procedures. Therefore, the percentage of imaging, endoscopic, and molecular procedures that required reimpression or reassessment or were repeated within a 6 week-period was 0%. This outcome reflects the high quality of initial and follow-up diagnostic procedures for lung cancer in centres that form a part of the NCN in the analysed region of Poland.
It is worth referring to an analysis of tests performed for genetic and molecular predictors in patients at clinical stages 3 and 4 and the epidemiological structure of this group of patients. Compared to the findings presented in that analysis, which cover the NCN’s period of functioning between 2019 and 2020 in Lower Silesia Province, the current study showed no significant differences in the scope of the analysed aspects [20]. Only the percentage of stage 3 patients who underwent an assessment of predictors decreased, from 40% at the end of 2020 to 35% at the end of 2021.
A significant disproportion between the number of lung cancer patients reported by individual centres is noticeable. It stems from the specific nature of the organisation of health care related to pulmonary diseases in Lower Silesia Province. As many as 73% of patients included in the pilot project for the NCN were reported by a single centre, the Lower Silesia Centre for Pulmonary Diseases in Wroclaw. This unit has been the primary centre for the treatment of pulmonary diseases in our region for many years, which explains the high number of patients reported by it among those included in the pilot project.
From the perspective of the idea of the project, a sufficiently high level of inclusion of patients in the network is essential. Leaving patients out of the network leads to results that may provide an inadequate picture of the current situation as assessed by a particular indicator. Likewise, consistent reporting of patients by the same centre or within the same province is key with respect to the reliability of the outcomes. In our analysis, we noticed differences in the number of reported clinical stage 3 patients for two indicators: the statistical indicator that evaluated the percentage of stage 3 patients encompassed 890 patients, while the indicator that assessed the share of patients in the same stage who received concurrent chemoradiotherapy had only 516 patients. The cause of this difference can be traced to different definitions of indicators and criteria for inclusion. It also seems likely that, simultaneously, there may have been errors in reporting caused by imprecise information contained, e.g. in medical histories of patients included in the pilot project or by patients ‘escaping’ the NCN due to errors in the data management process, which was not automated.
It is clear that working with such a large database concerning cancer patients requires many years of observation. This condition allows us to assume that it will be possible to correlate results obtained through the NCN during its operation for individual types of malignancies with direct treatment effects, expressed through a local control group or the overall survival rate. Furthermore, identifying such correlations will make it possible in the future to modify specific steps of oncological care and thus create conditions to improve the effectiveness of cancer treatment in a given area. Adopting the NCN concept into the Polish oncological care system would create an opportunity.
At the moment, it is difficult to compare the outcomes obtained and presented in this paper with the period before the pilot project was implemented for the NCN, as Poland had no similar system for quality assessment in oncology in place.
This paper presents findings on a selective basis, concentrating on the analysis of indicators related to lung cancer diagnosis and treatment. Other components of the NCN—such as the creation of the position of Coordinator for Oncological Care; measurement of the level of satisfaction with health care, assessed by the patient using a dedicated survey; or standardisation of reports from pathological and radiological exams—require further, separate analyses.
The limitation of the presented work is the pilot nature of the project. Errors in data reporting, incomplete data, and elements of the NCN, such as the aforementioned measures that do not fulfill the intended role, are some of the limitations of the pilot.
We believe that the ongoing pilot project for the NCN, understood as a platform for testing the NCN’s components, will make it possible to identify significant limitations; once these are eliminated, we can take advantage of the potential stemming from coordinated and high-quality oncological care.
Conclusions
The pilot project for the NCN is an ambitious and innovative for Poland as a whole, aimed at assessing the functioning of a network of oncological centres and managing data used to calculate quality indicators for oncological care. The analysis shows the results of the operations of the NCN in Lower Silesia Province for lung cancer. Further examination of the NCN spanning its entire 3-year period—including a focus on the shortcomings and irregularities in its functioning with a view to eliminating them in the future—will prove invaluable.
The NCN in Poland offers opportunities to improve the quality of cancer care, including diagnostics and treatment, and to select centers that meet the established standards. In our opinion, this may translate into an improvement in the results of oncological treatment in the future.
Availability of data and materials
The data underlying this article will be shared on reasonable request to the corresponding author.
Abbreviations
- NCN:
-
National Cancer Network
- KSO:
-
Polish National Oncology Network (Krajowa Sieć Onkologiczna, polish)
- PDCA:
-
Plan-Do-Check-Act
References
Wojciechowska U, Didkowska J, Morbidity and deaths from malignant neoplasms in Poland. National Registry Cancer, The Maria Sklodowska-Curie National Research Institute of Oncology (in Polish). http://onkologia.org.pl/raporty. (Accessed 22 Apr 2022)
Ferlay J, Laversanne M, Ervik M, et al. (2020). Global Cancer Observatory: Cancer Tomorrow. International Agency for Research on Cancer. Lyon, France. https://gco.iarc.fr/tomorrow. (Accessed 28 Feb 2022)
Regulation of the Minister of Health of 13 December 2018 on the pilot program of care for the patients within the oncological network, Dz.U. 2018 poz.2423 (in Polish). http://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20180002423. (Accessed 21 Feb 2022)
Population. Size and structure and vital statistics in Poland by territorial division in 2020. As of 30th June. Statistics Poland, Warsaw 2020. https://stat.gov.pl/obszary-tematyczne/ludnosc/ludnosc/ludnosc-stan-i-struktura-ludnosci-oraz-ruch-naturalny-w-przekroju-terytorialnym-stan-w-dniu-30-06-2020,6,28.html. (Accessed 20 Apr 2022)
Regulation of the Minister of Health of 18 August 2020 amending the regulation on a pilot program of care for the patient within oncological networks, Dz.U.2020 poz.1433 (in Polish). https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20200001433 (Accessed 19 Feb 2022)
Regulation of the Minister of Health of 23 December 2021 on the pilot program of care for the patients within the oncological network, Dz.U.2021 poz.2412 (in Polish). https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20210002412 (Accessed 29 Feb 2022)
Osarogiagbon RU, D’Amico TA. Improving lung cancer outcomes by improving the quality of surgical care. Transl Lung Cancer Res. 2015;4(4):424–31.
Bach PB, Cramer LD, Schrag D, et al. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345(3):181–8.
Denton E, Conron M. Improving outcomes in lung cancer: the value of the multidisciplinary health care team. J Multidiscip Healthc. 2016;9:137–44.
Jakobsen E, Green A, Oesterlind K, et al. Nationwide quality improvement in lung cancer care: the role of the Danish Lung Cancer Group and Registry. J Thorac Oncol. 2013;8(10):1238–47.
Wang C, Li X, Su S, et al. Factors analysis on the use of key quality indicators for narrowing the gap of quality of care of breast cancer. BMC Cancer. 2019;19:1099. https://doi.org/10.1186/s12885-019-6334-5.
Mathoulin-Pélissier S, Bécouarn Y, Belleannée G, et al. Quality indicators for colorectal cancer surgery and care according to patient-, tumor-, and hospital-related factors. BMC Cancer. 2012;12:297. https://doi.org/10.1186/1471-2407-12-297.
Guarneri V, Pronzato P, Bertetto O, et al. Use of electronic administrative databases to measure quality indicators of breast cancer care: experience of five regional oncology networks in Italy. JCO Oncol Pract. 2020;16(2):e211–20.
Evison M, Limited AUK. The current treatment landscape in the UK for stage III NSCLC. Br J Cancer. 2020;123(Suppl 1):3–9.
Walraven I, Damhuis RA, Ten Berge MG, et al. Treatment Variation of Sequential versus Concurrent Chemoradiotherapy in Stage III Non-Small Cell Lung Cancer Patients in the Netherlands and Belgium. Clin Oncol (R Coll Radiol). 2017;29(11):e177–85.
Antonia S, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018;379:2342–50.
Faivre-Finn C, Vicente D, Kurata T, et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update From the PACIFIC Trial. J Thorac Oncol. 2021;16(5):860–7.
Hazell SZ, Mai N, Fu W, et al. Hospitalization and definitive radiotherapy in lung cancer: incidence, risk factors and survival impact. BMC Cancer. 2020;20(1):334.
Waddle MR, Chen RC, Arastu NH, et al. Unanticipated hospital admissions during or soon after radiation therapy: incidence and predictive factors. Pract Radiat Oncol. 2015;5(3):e245–53.
Trembecki Ł, Sztuder A, Pawlak I, et al. Analysis of lung cancer measures of the National Cancer Network pilot study in Poland for potential improvement in the quality of advanced-stage lung cancer therapy. BMC Cancer. 2021;21(1):1252.
Acknowledgements
Not applicable.
Funding
This research was financed through a statutory subsidies by the Minister of Science and Higher Education as part of the Wroclaw Medical University Department of Oncology research grant: SUBZ.C280.22.001 (record number in the Simple System).
Author information
Authors and Affiliations
Contributions
Ł.T. conceived and designed the analysis, undertook data abstraction, data analyses and drafted and finalized the paper. A.Sz. co-ordinated the analysis. I.D., A.Sz., R.M., A.M., contributed to interpretation of analysed data. R.M., participated in drafting of the manuscript. A.M., participated in drafting and finalizing the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Wroclaw Medical University.
Approval number KB-289/2022.
All patients included in the National Cancer Network (NCN) pilot program in Poland made a written declaration of informed consent to participate in the NCN pilot program. The Additional file 1: provides Declaration Form (in polish).
Before enrollment, each patient obtained information about the principles of the pilot program and the detailed information of personal data processing as part of the NCN.
All methods were carried out in accordance with the Helsinki declaration and with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
Authors declare that they have no conflict of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Trembecki, Ł., Sztuder, A., Dębicka, I. et al. The pilot project of the National Cancer Network in Poland: Assessment of the functioning of the National Cancer Network and results from quality indicators for lung cancer (2019–2021). BMC Cancer 22, 939 (2022). https://doi.org/10.1186/s12885-022-10020-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-10020-9