Abstract
Background
Controversy exists regarding the relationship of the outcome of patients with colorectal cancer (CRC) with the time from symptom onset to diagnosis. The aim of this study is to investigate this association, with the assumption that this relationship was nonlinear and with adjustment for multiple confounders, such as tumor grade, symptoms, or admission to an emergency department.
Methods
This multicenter study with prospective follow-up was performed in five regions of Spain from 2010 to 2012. Symptomatic cases of incident CRC from a previous study were examined. At the time of diagnosis, each patient was interviewed, and the associated hospital and clinical records were reviewed. During follow-up, the clinical records were reviewed again to assess survival. Cox survival analysis with a restricted cubic spline was used to model overall and CRC-specific survival, with adjustment for variables related to the patient, health service, and tumor.
Results
A total of 795 patients had symptomatic CRC and 769 of them had complete data on diagnostic delay and survival. Univariate analysis indicated a lower HR for death in patients who had diagnostic intervals less than 4.2 months. However, after adjustment for variables related to the patient, tumor, and utilized health service, there was no relationship of the diagnostic delay with survival of patients with colon and rectal cancer, colon cancer alone, or rectal cancer alone. Cubic spline analysis indicated an inverse association of the diagnostic delay with 5-year survival. However, this association was not statistically significant.
Conclusions
Our results indicated that the duration of diagnostic delay had no significant effect on the outcome of patients with CRC. We suggest that the most important determinant of the duration of diagnostic delay is the biological profile of the tumor. However, it remains the responsibility of community health centers and authorities to minimize diagnostic delays in patients with CRC and to implement initiatives that improve early diagnosis and provide better outcomes.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is one of the most common cancers and a leading cause of cancer deaths in Europe [1]. Although the 5-year overall survival rate in Europe has improved during recent decades, these survival rates are still only about 60%, indicating a need for improvement [2]. The prognosis of a patient with CRC depends on the stage at diagnosis, and many health organizations have therefore recently focused on early diagnosis patients with symptomatic CRC. Population awareness campaigns and urgent referrals are the main initiatives used to increase the early detection of symptomatic CRC [3, 4]. Despite efforts to reduce the time from the onset of first symptoms to diagnosis and after more than four decades of research on this subject, controversy remains about the relationship of the time from symptom onset to diagnosis with survival of patients with CRC [5,6,7].
Some indirect evidence suggested that a long diagnostic interval was associated with poor survival of these patients [7]. However, two systematic reviews that analyzed the relationship of the time from symptom onset to diagnosis with tumor stage and with patient survival found no significant associations [8, 9]. A more recent systematic review that included new studies published up to November 2013 [10] also did not resolve this issue. In particular, this more recent publication [10] identified some studies which reported that a long time from symptom onset to diagnosis was associated with poor survival; some studies which reported that a short diagnostic delay was associated with poor survival, and other studies that found no relationship between time from symptom onset to diagnosis and survival.
In 1994, Macguire et al. [11] proposed that the relationship between time from symptom onset to diagnosis and patient prognosis was nonlinear. In the last decade, some studies incorporated this new paradigm, and examined the nonlinear relationship of the time from symptom onset to diagnosis with cancer outcomes. These studies reported poor survival both in patients with short intervals from symptom onset to diagnosis, as well as in those with very long times from symptom onset to diagnosis [12,13,14,15]. However, some studies that used similar methodologies failed to find this association [16], often because they did not adequately control for potential confounders [17].
The aim of this study was to investigate the association of the time from symptom onset to diagnosis with survival in a cohort of 950 patients with CRC. This study assumed there was a nonlinear relationship between the time from symptom onset to diagnosis and survival, and adjusted for multiple confounders, such as tumor grade, symptoms, and presentation at an emergency department.
Methods
This multicenter study with prospective follow-up examined patients in five regions of Spain (Aragón, Balearic Islands, Catalonia, Galicia, Valencia). The cohort of 950 consecutive patients with incident CRC was examined from September 2006 to September 2008 (DECCIRE Study) [18, 19]. Patients were identified from the pathology services of 9 public hospitals. Patients were excluded if they were younger than 18 years, had prevalent or recurrent CRC, had multiple tumors, were not registered with a general practitioner (GP), or were diagnosed in a private hospital. asymptomatic patients were also excluded (screening detected cases, incidental finding). The cohort of patients was followed up from 2010 to 2012 to assess survival status (DECCIRE II study). The detailed protocol of this study was previously published [20]. This study adheres to STROBE guidelines.
Measurements
Data collection at cohort inception
Data were from patient interviews that were conducted by trained GPs and nurses immediately after diagnosis. These data included initial CRC symptoms, date of first presentation, perception of the seriousness of symptoms, help-seeking behavior, socio-demographic factors (age, sex, marital status, and level of education), and history of cancer in family members or acquaintances. Each patient was asked how long he/she felt unwell. If the patient remembered the exact date, that date was recorded; if the patient could not remember the exact date, then an approximate date was recorded. The first symptoms were the symptoms spontaneously reported by the patient without prompting by a GP or nurse. After recording the first symptoms and the date of onset, the interviewer asked each patient if he/she presented with any other symptoms on a checklist of 22 frequent CRC symptoms. For patients who were not interviewed, the date of first symptoms recorded in the primary health care record or the hospital record was used. Data from the hospital records included date of first symptoms (recorded at the first visit), tumor characteristics (grade, TNM stage, and location), and date of diagnosis (based on the date of the first histology report), and the presence of an intestinal occlusion. The first hospital service that evaluated the patient was classified as an emergency department or an outpatient service. The Charlson Comorbidity Index (CCI) at diagnosis was recorded based on comorbidities registered in clinical records. In addition, we collected the following treatment-related variables: resection (curative or palliative) and oncological treatment (chemotherapy and radiotherapy: before or after resection).
Data collection during follow up
During follow-up, survival time and mortality (including date and cause of death) were recorded using data from the Regional Mortality Registries.
Statistical analysis
Survival time (all-cause mortality) was the date from diagnosis to the final outcome. The main predictor of survival was the time from symptom onset to diagnosis.
Time intervals are presented as medians and inter-quartile ranges (IQRs) and categorical variables as proportions. Patients were allocated into quartiles according to their times from symptom onset to diagnosis: 0–1.9 months (0–57 days), 1.91–4.20 months (57–127 days), 4.21–8.4 months (127–257 days), and more than 8.4 months (257–639 days). The relationship of the time from symptom onset to diagnosis with co-variables was assessed using the chi square test (categorical variables) and ANOVA (continuous variables). A relationship was considered significant if the associated P value was 0.05 or less. Bivariate and multivariate Cox regression analyses were used to analyze the relationship of the duration of diagnostic delay with mortality after adjusting for co-variables. Hazard ratios (HRs) for mortality and 95% confidence intervals (CIs) were calculated. Patients were censored if they were not in the mortality registry or did not have contact with a health service point during the prior 6 months. Predictors considered as prior potential confounders and those associated with the exposure (time from symptom onset to diagnosis) and the outcome (mortality) were included in the analysis. Multivariate regression models were fitted with adjustment for the colon and rectum jointly, and with separate adjustment for the colon and rectum. Final models were subjected to manual backward fitting using the likelihood-ratio test. Determination of Schoenfeld residuals and a log–log graph indicated there were no violations of the proportional hazards assumption. Tumor stage at diagnosis was considered an intermediate variable and was not included in the multivariate analysis.
Although the proportional hazard assumption was not violated, a nonlinear relationship between time from symptom onset to diagnosis and mortality was assumed. This approach allowed identification of a more flexible association between the time from symptom onset to diagnosis and mortality. Cubic spline regression analysis was performed using restricted cubic splines with four knots and 4.19 days (colon and rectum), 4.11 days (colon), and 4.26 days (rectum) as the reference points. A sensitivity analysis was also conducted measuring CRC -specific survival (please refer to Supplementary Tables S1, S2 and supplementary figures S1, S2 and S3. Also, we did an additional stage specific cubic spline regression analysis for overall mortality presented in Fig. 4 and for CRC-specific survival (Supplementary figure S4). Data were analyzed using STATA version 13 (Stata Corp, TX, USA).
Results
There were 795 CRC patients who were symptomatic, 779 (97.9%) of them had data on the time from symptom onset to diagnosis, and 769 (96.7%) of them had complete follow-up data (In 10 cases we cannot confirm their situation as alive or dead). We initially analyzed the sociodemographic and clinical characteristics of patients diagnosed with CRC after presentation with symptoms (Table 1). The median age at diagnosis was 72 years (interquartile range [IQR]:62–78), most patients only completed elementary education, and there were more men (62.2%) than women (37.8%). The median time from symptom onset to diagnosis was 4.2 months (IQR: 1.9–8.4). Most tumors were in the colon (61.1%) and were grade I or II (89.3%). More than half of the patients had tumor stage II or III at diagnoses, and 19.4% had stage IV metastatic tumors. Most surgeries had a curative intention (92%), 30% did not receive oncological treatment, 48% received chemotherapy,and 21% both radiotherapy and chemotherapy. The median follow-up time was 57.9 months (IQR: 23.6–67.2). The total overall survival rate was 68.3% at 3 years and 57.3% at 5 years, and the mean survival time was 45.0 months (95%CI: 43.6–46.3). There was no information about CRC-specific deaths in 196 cases. CRC-specific mortality was 67.2% at 3 years and 65.5% at 5 years.
We also performed a bivariate Cox regression analysis to determine the relationship of different factors, including time from symptom onset to diagnosis, with 5-year overall survival. In the unadjusted analysis, times from symptom onset to diagnosis of 4.2 to 8.4 months and more than 8.4 months were associated with a decreased risk of mortality. Patients who were older, had an advanced stage tumor, had a grade III or IV tumor, presented at an emergency department, had an intestinal obstruction, had a higher CCI, had anorexia and constipation as first symptoms and those treated with chemotherapy or radiotherapy-chemotherapy had an increased risk of death. Rectal bleeding as a first symptom was associated with a decreased risk of mortality. There were no significant associations of death with sex, level of education, tumor localization, perception of symptom severity, help-seeking behavior, and other symptoms and type of resection.
We then examined the relationship of different patient characteristics with the duration of the diagnostic delay (Table 2). The results indicated the diagnostic delay was longer for patients who were older, female, perceived their symptoms as not serious, or had weight loss as a first symptom. The time from symptom onset to diagnosis was shorter for those who had an intestinal obstruction, presented at an emergency department, exhibited help-seeking behavior, had abdominal pain, and had constipation. There were no significant associations between time from symptom onset to diagnosis and other factors, including level of education, history of cancer in the family and acquaintances, other comorbidities, other first symptoms, tumor grade, tumor stage, and tumor location.
We performed multivariate Cox regression analysis to identify factors significantly associated with cancer of the colon or rectum (Model-1), cancer of the colon alone (Model-2), and cancer of the rectum alone (Model-3; Table 3). The results indicated that age (all models), tumor location (Model-1), tumor grade (all models), intestinal obstruction (Model-1 and -2), emergency presentation (Model-1 and -2), and Charlson index (all models) were associated with an increased risk of 5-year mortality. Rectal bleeding (Model-1) was inversely associated with 5-year mortality. Notably, the time from symptom onset to diagnosis was not significantly associated with 5-year mortality.
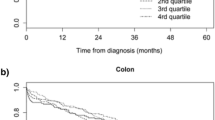
We also performed cubic spline regression analysis of the relationship of the duration of diagnostic delay with 5-year overall survival in patients with cancer of the colon or rectum (Fig. 1), cancer of the colon alone (Fig. 2) and cancer of the rectum alone (Fig. 3).
These models adjusted for age, sex, tumor location, tumor grade, emergency presentation, intestinal obstruction, comorbidities, and symptoms. In each case, there was an inverse association between time from symptom onset to diagnosis and 5-year survival, but all the confidence intervals were very wide and none of these associations were statistically significant. We also found an overall inverse association in the analysis of stage specific spline curves for the adjusted model (Fig. 4).
We performed a sensitivity analysis of the association of time to diagnosis and 5-year CRC-specific mortality and obtained similar results (Supplementary material Tables S1 and S2; Figures S1, S2, S3 and S4).
Discussion
The results of our multivariate adjusted Cox proportional hazard models indicated that a longer time from symptom onset to diagnosis was not associated with increased hazard of mortality in patients with symptomatic CRC. Our cubic spline analysis, which considered nonlinearities, indicated an inverse association between the duration of diagnostic delay and survival; however, this association was not statistically significant. Other CRC studies that examined the time from symptom onset to diagnosis and also used restricted cubic spline analyses reported similar results [16, 17].
In contrast, Tørring et al. [14] found a U-shaped relationship between the diagnostic interval and mortality in five common cancers. In particular, for patients attended by a GP due to alarming symptoms, the risk of dying decreased as the diagnostic interval increased up to 5 weeks, and the risk then increased. These results were contrary to those for patients with vague or non-specific symptoms, although that relationship was not significant [12]. Importantly, Tørring et al. [14] found similar U-shaped relationships for patients with melanoma, breast cancer, prostate cancer, and lung cancer, but only for those with alarming symptoms [14]. However, neither of these studies adjusted for potential confounders.
The ‘waiting time paradox’, first described in a clinical setting by Crawford et al. [21], refers to the inverse association of survival with time until onset of treatment or diagnosis. A likely explanation of this paradox is that the nature of the disease affects the delay, and delay is therefore a confounder. In other words, when patients first present with symptoms, clinicians can reliably identify patients with more severe disease and then administer further diagnostic testing or treatment to these patients. Although we were able to adjust for some potential confounders, such as tumor grade, emergency presentation, symptoms, and comorbidities, it is possible that residual and unmeasured confounders contributed to the waiting time paradox. Furthermore, the relationship between cancer symptoms and stage is not straightforward and rarely linear; symptoms are often totally absent in patients with late-stage cancers. There is also high heterogeneity in the stage of CRC when the initial symptoms appear. We previously proposed that the CRC stage when symptoms first appear is an important and unknown confounder, reasonably the time to diagnosis and survival time would be shorter if symptoms of CRC appeared at stage III or IV [22] and this made it difficult for us to ascertain whether the time duration between symptom onset and diagnosis influenced survival. Majumbar et al. suggested that the symptomatic phase in some patients with CRC may be a very late event during the course of this disease, and that a difference of several months in the time from symptom onset to diagnosis may not have a significant effect on outcome [23]. This interpretation is consistent with our findings.
In the last decade, researchers examining the relationship of the time from symptom onset to diagnosis with survival have used more rigorous study designs in an effort to better define diagnosis-treatment intervals, as recommended by Arhi et al. [24]. Thus, recent studies of this topic have used larger sample sizes, imposed fewer restrictions on patient inclusion, and considered potential confounders. Nevertheless, comparisons of studies are still difficult because there are significant differences in the methods, patient characteristics, and inclusion/exclusion criteria, i.e., focusing on patients admitted to emergency departments or older patients [21, 24, 25]. Many studies have measured the time from symptom onset to diagnosis [16, 21, 26], as in the present study, but others measured the time from initial presentation to diagnosis [12,13,14, 17, 23, 25, 27]. Studies that measured the total diagnostic interval reported no association between long diagnostic interval and risk of death [16, 26, 28] but Pita et al. found that a shorter lag time was associated with lower survival in patients with rectal cancer [16]. However, using the interval from onset of symptoms to diagnosis, let have a broad idea of the diagnostic interval as it includes patient delay that is, from symptoms onset to presentation to a health professional. This time is relevant as it could account for 20% to 40% of the total diagnostic interval [19, 27]. Interventions centered on health professionals and organizations are important, but it is also necessary to increase interventions that increase population awareness of cancer symptoms and address help-seeking behaviors.
Limitations and strengths
The present study had some limitations. First, as in all studies based on interview data, there was a risk of recall bias. Because we interviewed most patients after surgery (an average of 47 days after diagnosis) they may have had difficulties recalling the exact time of symptom onset, particularly if the symptoms were mild or non-specific, and this could have led to information misclassification. A patient with poor prognosis or with symptoms that appeared more recently might also provide more reliable information regarding the date of presentation and symptoms. However, any source of information is susceptible to misclassification. A previous study compared how the date of symptom onset from three sources affected the total diagnostic interval, and demonstrated that data were affected by information bias and non-random classification [22]. We measured all-cause mortality from the date of diagnosis to death or censoring. Some authors suggested that this might cause lead-time bias, but sensitivity analysis that measured the time from diagnosis at presentation led to similar results [17]. We found similar results when analyzing all-cause mortality and CRC-specific mortality. Pruitt et al. [29] found divergences between all-cause and CRC-specific death when measuring the relationship between time to diagnosis or treatment and survival. Despite high variation of time to treatment between CRC patients [18, 29], we did not measure the relationship of treatment delay and survival both as an independent interval or added to the diagnosis interval. Again, there are controversial results in studies that measured treatment delay and mortality. Some authors found that shorter treatment delays had the higher odds of all-cause [29] but not for CRC-specific death. Others observed that the risk of death significantly increased in those patients with treatment delay > = 30 days [30]. Nevertheless, analogous results have been found with those of Murchie et al. [17] where after adjusting for multiple confounders no relationship between time of presentation to treatment was observed.
One of the strengths of our study is that we included incident cases and calculated the interval from the onset of symptoms until diagnosis, similar to the studies of Pita et al. [16] and Singh et al. [25]. As these authors mentioned, studies that measure the diagnosis time independently of the location of diagnosis (out-patient clinic, emergency department, etc.) may have more external validity because they include all types of patients. In comparison, studies that only examine patients who visited primary care centers probably have more homogenous patient populations and greater internal validity, but they may have excluded 20 to 30% of the patients who were diagnosed with CRC at emergency departments or other outpatient referral services [19, 31, 32]; the different characteristics of these groups could be related to differences outcome. Several studies showed that CRC patients who bypassed their GPs were diagnosed earlier [33] and had poorer outcomes [34]. The 2011 study of Tørring et al. [12] found that 56% of the patients in which the GP was not involved in the diagnosis were admitted to an emergency room. Moreover, the present study examined CRC incident cases in different public tertiary hospitals and general hospitals, and thus considered a wide variety of patients whose diagnoses were by a wide variety of clinicians. We had complete data for most patients; these data were about 90% complete for tumor stage and grade and more than 96% complete for follow up and diagnostic delay. Furthermore, the diverse variables that we examined enabled adjustment for potential confounding, as in other studies of this topic. We included variables that were significantly associated with risk of death in the bivariate analysis. Variables such as grade, emergency department presentation, and intestinal occlusion may be markers of fast-growing tumors and could potentially contribute to the account for a ‘waiting time paradox’ (poorer prognosis in patients with shorter times from symptom onset to diagnosis). Moreover, adjustment for age and comorbidities is essential because they are related to patient mortality and time from symptom onset to diagnosis [35]. We found treatment as factors associated with survival. However, the treatment the patient received is closely related to the stage at diagnosis. We did not adjust by tumor stage because the relationship between diagnostic delay, tumor stage, and survival is complex. This relationship is often considered an intermediate factor between time from symptom onset to diagnosis and survival because it is on the causal pathway that connects time from symptom onset to diagnosis and survival. The stage specific analysis did not show significant association between length of diagnosis and survival.
Conclusions
Our results provided no evidence that the time from symptom onset to diagnosis affected the prognosis of patients with symptomatic CRC. These results are similar to those of several previous studies, although some other studies reported contrary results. We suggest that the most important determinant affecting the diagnostic duration is the biological profile of the tumor and its consequent clinical effects.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CRC:
-
Colorectal cancer
- GP:
-
General practitioner
- CCI:
-
The Charlson Comorbidity Index
- IQRs:
-
Inter-quartile ranges
- CIs:
-
Confidence intervals
- HRs:
-
Hazard ratios
References
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018 GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–53. https://doi.org/10.1002/ijc.31937.
Brenner H, Bouvier AM, Foschi R, Hackl M, Larsen IK, Lemmens V, Mangone L, Francisci S, EUROCARE Working Group. Progress in colorectal cancer survival in Europe from the late 1980s to the early 21st century: the EUROCARE study. Int J Cancer. 2012;131(7):1649–58. https://doi.org/10.1002/ijc.26192.
Prades J, Espinàs J, Font R, Argimon JM, Borras JM. Implementing a Cancer Fast-track Programme between primary and specialized care in Catalonia (Spain): a mixed methods study. Br J Cancer. 2011;105(6):753–9. https://doi.org/10.1038/bjc.2011.308.
Probst HB, Hussain ZB, Andersen O. Cancer patient pathways in Denmark as a joint effort between bureaucrats, health professionals and politicians–a national Danish project. Health Policy. 2012;105(1):65–70. https://doi.org/10.1016/j.healthpol.2011.11.001.
Rai S, Kelly MJ. Priorization of colorectal referrals: a review of the 2-week wait referral system. Colorectal Dis. 2007;9(3):195–202. https://doi.org/10.1111/j.1463-1318.2006.01107.x.
Moller H, Gildea C, Meechan D, Rubin G, Round T, Vesdsted P. Use of the English urgent referral pathway for suspected cancer and mortality in patients with cancer: cohort study. BMJ. 2015;351:h5102.
L Thornton H Reader S Stojkovic V Allgar N Woodcock 2016 Has the 'Fast-Track' referral system affected the route of presentation and/or clinical outcomes in patients with colorectal cancer? World J Surg Oncolhttps://doi.org/10.1186/s12957-016-0911-8
Ramos M, Esteva M, Cabeza E, Llobera J, Ruiz A. Lack of association between diagnostic and therapeutic delay and stage of colorectal cancer. Eur J Cancer. 2008;44(4):510–21. https://doi.org/10.1016/j.ejca.2008.01.011.
Ramos M, Esteva M, Cabeza E, Campillo C, Llobera J, Aguiló A. Relationship of diagnostic and therapeutic delay with survival in colorectal cancer: A review. Eur J Cancer. 2007;43(17):2467–78. https://doi.org/10.1016/j.ejca.2007.08.023.
Neal RD, Tharmanathan P, France B, Din NU, Cotton S, Fallon-Ferguson J, Hamilton W, Hendry A, Hendry M, Lewis R, Macleod U, Mitchell ED, Pickett M, Rai T, Shaw K, Stuart N, Tørring ML, Wilkinson C, Williams B, Williams N, Emery J. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review Br J Cancer. 2015;112(Suppl 1):S92-107. https://doi.org/10.1038/bjc.2015.48.
Maguire A, Porta M, Malats N, Gallén M, Piñol JL, Fernandez E. Cancer survival and the duration of symptoms. An analysis of possible forms of the risk function. ISDS II Project Investigators. Eur J Cancer. 1994;30A(6):785–92. https://doi.org/10.1016/0959-8049(94)90293-3.
Tørring ML, Frydenberg M, Hansen RP, Olesen F, Hamilton W, Vedsted P. Time to diagnosis and mortality in colorectal cancer: a cohort study in primary care. Br J Cancer. 2011;104:934–40. https://doi.org/10.1038/bjc.2011.60.Erratum.In:BrJCancer.2011;104:1930.
Tørring ML, Frydenberg M, Hamilton W, Hansen RP, Lautrup MD, Vedsted P. Diagnostic interval and mortality in colorectal cancer: U-shaped association demonstrated for three different datasets. J Clin Epidemiol. 2012. https://doi.org/10.1016/j.jclinepi.2011.12.006.
Tørring ML, Frydenberg M, Hansen RP, Olesen F, Vedsted P. Evidence of increasing mortality with longer diagnostic intervals for five common cancers: a cohort study in primary care. Eur J Cancer. 2013. https://doi.org/10.1016/j.ejca.2013.01.025.
Tørring ML, Murchie P, Hamilton W, Vedsted P, Esteva M, Lautrup M, Winget M, Rubin G. Evidence of advanced stage colorectal cancer with longer diagnostic intervals: a pooled analysis of seven primary care cohorts comprising 11 720 patients in five countries. Br J Cancer. 2017. https://doi.org/10.1038/bjc.2017.236.
Pita-Fernández S, González-Sáez L, López-Calviño B, Seoane-Pillado T, Rodríguez-Camacho E, Pazos-Sierra A, González-Santamaría P, Pértega-Díaz S. Effect of diagnostic delay on survival in patients with colorectal cancer: a retrospective cohort study. BMC Cancer. 2016. https://doi.org/10.1186/s12885-016-2717-z.
Murchie P, Raja EA, Brewster DH, Campbell NC, Ritchie LD, Robertson R, Samuel L, Gray N, Lee AJ. Time from first presentation in primary care to treatment of symptomatic colorectal cancer: effect on disease stage and survival. Br J Cancer. 2014. https://doi.org/10.1038/bjc.2014.352.
Esteva M, Ramos M, Cabeza E, Llobera J, Ruiz A, Pita S, Segura JM, Cortes JM, Gonzalez-Lujan L; DECCIRE research group. Factors influencing delay in the diagnosis of colorectal cancer: a study protocol. BMC Cancer. 2007 https://doi.org/10.1186/1471-2407-7-86.
Esteva M, Leiva A, Ramos M, Pita-Fernández S, González-Luján L, Casamitjana M, Sánchez MA, et al; DECCIRE GROUP. Factors related with symptom duration until diagnosis and treatment of symptomatic colorectal cancer.BMC Cancer. 2013 https://doi.org/10.1186/1471-2407-13-87.
Pita Fernández S, Pértega Díaz S, López Calviño B, González Santamaría P, Seoane Pillado T, Arnal Monreal F, et al. Diagnosis delay and follow-up strategies in colorectal cancer. Prognosis implications: a study protocol. BMC Cancer. 2010 https://doi.org/10.1186/1471-2407-10-528.
Terhaar sive Droste JS, Oort FA, van der Hulst RWN, Coupé VMH, Craanen ME, Meijer GA, Morsink GM, Visser O, van Wanrooij RLJ, Mulder CJJ. Does delay in diagnosing colorectal cancer in symptomatic patients affect tumor stage and survival? A population-based observational study. BMC Cancer. 2010 https://doi.org/10.1186/1471-2407-10-332.
Leiva A, Esteva M, Llobera J, Macià F, Pita-Fernández S, González-Luján L, Sánchez-Calavera MA, Ramos M. Time to diagnosis and stage of symptomatic colorectal cancer determined by three different sources of information: A population based retrospective study. Cancer Epidemiol. 2017. https://doi.org/10.1016/j.canep.2016.10.021.
Majundar SR, Flechter RH, Evans At. How does colorectal cancer present? Symptoms, duration and clues to location. Am J Gastroenterol 1999 https://doi.org/10.1111/j.1572-0241.1999.01454.x
Arhi CS, Burns EM, Bottle A, Bouras G,Aylin P, Ziprin P, Darzi A. Delays in referral from primary care worsen survival for patients with colorectal cancer: a retrospective cohort study. Br J General Pract 2020; 2020 https://doi.org/10.3399/bjgp20X710441.
Singh H, Shu E, Demers A, Bernstein CN, Griffith J, Fradette K. Trends in time to diagnosis of colon cancer and impact on clinical outcomes.Can J Gastroenterol. 2012; 26:877–80. https://doi.org/10.1155/2012/363242.
Thomson CS, Forman D. Cancer survival in England and the influence of early diagnosis: what can we learn from recent EUROCARE results? Br J Cancer. 2009;101(Suppl 2):S102–9. https://doi.org/10.1038/sj.bjc.6605399.
Weller D, Vedsted P, Rubin G, Walter FM, Emery J, Scott S, et al. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer. 2012. https://doi.org/10.1038/bjc.2012.68.
Crawford SC, Davis JA, Siddiqui NA, de Caestecker L, Gillis CR, Hole D, Penney G. The waiting time paradox: population based retrospective study of treatment delay and survival of women with endometrial cancer in Scotland. BMJ. 2002. https://doi.org/10.1136/bmj.325.7357.196.
Pruitt SL, Harzke AJ, Davidson NO, Schootman M. Do diagnostic and treatment delays for colorectal cancer increase risk of death? Cancer Causes Control. 2013. https://doi.org/10.1007/s10552-013-0172-6.
Young-Heeng L, Pei-Tseng K, Yueh-Hsin W, Wei-Yin K, Su-ling K, Wen-Chen T. Effect of length of time from diagnosis to treatment on colorectal cancer survival: A population-based study. Plos One 2019 https://doi.org/10.1371/journal.pone.0210465. eCollection 2019.
Elliss-Brokers L, McPhail S, Ives A, Greenslade M, Shelton J, Hiom S, Richards M. Routes to diagnosis for cancer – determining the patient journey using multiple routine data sets. Br J Cancer. 2012. https://doi.org/10.1038/bjc.2012.408.
Esteva M, Ruiz Díaz M, Sánchez MA, Pértega S, Pita-Fernández S, Macià F, Posso M, González-Luján L, Boscá-Wats MM, Leiva A, Ripoll J; DECCIRE GROUP. Emergency presentation of colorectal patients in Spain. PLoS One. 2018 https://doi.org/10.1371/journal.pone.0203556.
Murchie P, Adam R, McNair E, Swann R, Witt J, Wood R, Weller D. Cancer diagnosis in Scottish primary care: Results from the National Cancer Diagnosis Audit. Eur J Cancer Care. 2020. https://doi.org/10.1111/ecc.13234.
Algar VL, Neal RD. Delays in diagnosis of six cancers: analysis of data from de National Survey of NHS Patients: Cancer. Br J Cancer. 2005. https://doi.org/10.1038/sj.bjc.6602587.
Renzi C, Kaushal A, Emery J, Hamilton W, Neal RD, Rachet B, Rubin G, Singh H, Walter FM, de Wit NJ, Lyratzopoulos G. Comorbid chronic diseases and cancer diagnosis: disease-specific effects and underlying mechanisms. Nat Rev Clin Oncol. 2019. https://doi.org/10.1038/s41571-019-0249-6.
Acknowledgements
In memory of Salvador Pita-Fernandez who passed out in 2018. He has been the study coordinator and a reference for cancer research in our country. We would thank him for his enthusiasm, expertise and teaching qualities. Also, the authors would like to thank all the patients in the cohort for generously contributing their time to the development of this study.
Funding
This study received two grants for each participating group from the Ministry of Science and Innovation, Carlos III Institute, Healthcare Research Fund. Grants PI: 052273, PI050787, PI050700, PI052692, and PI052141 for the DECCIRE study. The second one, for the DECCIRE II study grants: PS09/00663, PI09/01800, PS09/00954, PS09/01614 and PS09/01375. In addition, the study has received the support of the Health Promotion and Preventive Activities—Primary Healthcare Network, which is supported by other grants from the Ministry of Health ISCIII-RETCI G03/170 and RD06/0018. The study was also partially supported by a XUGA grant (08CSA073916PR) and the Galician Network for Colorectal Cancer Research. Also, this project was founded by research grant from the Carlos III Institute of Health (Ministry of Science, Innovation and Universities, Spain; reference PI18/01676) which was co-funded with European Union ERDF funds (European Regional Development Fund, “A way to make Europe”). The study has undergone peer-review by the funding body. In addition, the study has been also partially supported by the Galician Network for Colorectal Cancer Research (REGICC).
Author information
Authors and Affiliations
Contributions
SPD, ME, FM, MASC, MRM and AE participated in the design and methodology. ME, MASC, AE, TSP, FM, were project administrators in their region. All of these individuals (MRM, LGL, CMN, RM, VBB, TSP) have been involved in the management of data collection. VBB and TSP did data curation. SPD, AL and ME did the statistical analysis and interpreted the results. ME and SPD drafted the manuscript on comments from all contributors. All of the authors read, revised and approved the final manuscript. Salvador Pita Fernandez conceptualized the study and together with SPD was the coordinator of the multicenter study. He passed out two in 2018.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study has been carried out according to the good clinical practice guidelines of the Helsinki declaration. Informed consent was obtained from each patient to take part in the study and to review their clinical records. This project was approved by the ethics review board of each one of the sites participating in the study: Clinical Research Ethical Committee of Galicia (decision no. 2009/110), Clinical Research Ethical Committee of Balearic Islands (decision no. 1167/09 PI), Clinical Research Ethical Committee of the Municipal Institute of Health Care (CEIC-IMAS, Barcelona) (decision no. 2009/3556/I), Clinical Research Ethical Committee of Aragón (decision no. 06/2009), and the Clinical Research Ethical Committee of the University Clinic Hospital of Valencia (date of approval June 5, 2009).
Consent for publication
Not applicable. No data from any individual person were included in this manuscript.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary tables
Additional file 2.
Supplementary figures 1-3
Additional file 3.
Supplementary fig 4
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Esteva, M., Leiva, A., Ramos-Monserrat, M. et al. Relationship between time from symptom’s onset to diagnosis and prognosis in patients with symptomatic colorectal cancer. BMC Cancer 22, 910 (2022). https://doi.org/10.1186/s12885-022-09990-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09990-7