Abstract
Background
The presence of mesorectal fascia (MRF) invasion, grade 4 extramural venous invasion (EMVI), tumour deposits (TD) or extensive or bilateral extramesorectal (lateral) lymph nodes (LLN) on MRI has been suggested to identify patients with indisputable, extensive locally advanced rectal cancer (LARC), at high risk of treatment failure. The aim of this study is to evaluate whether or not intensified chemotherapy prior to neoadjuvant chemoradiotherapy improves the complete response (CR) rate in these patients.
Methods
This multicentre, single-arm, open-label, phase II trial will include 128 patients with non-metastatic high-risk LARC (hr-LARC), fit for triplet chemotherapy. To ensure a study population with indisputable, unfavourable prognostic characteristics, hr-LARC is defined as LARC with on baseline MRI at least one of the following characteristics; MRF invasion, EMVI grade 4, enlarged bilateral or extensive LLN at high risk of an incomplete resection, or TD. Exclusion criteria are the presence of a homozygous DPD deficiency, distant metastases, any chemotherapy within the past 6 months, previous radiotherapy within the pelvic area precluding standard chemoradiotherapy, and any contraindication for the planned treatment. All patients will be planned for six two-weekly cycles of FOLFOXIRI (5-fluorouracil, leucovorin, oxaliplatin and irinotecan) prior to chemoradiotherapy (25 × 2 Gy or 28 × 1.8 Gy with concomitant capecitabine). A resection will be performed following radiological confirmation of resectable disease after the completion of chemoradiotherapy. A watch and wait strategy is allowed in case of a clinical complete response. The primary endpoint is the CR rate, described as a pathological CR or a sustained clinical CR one year after chemoradiotherapy. The main secondary objectives are long-term oncological outcomes, radiological and pathological response, the number of resections with clear margins, treatment-related toxicity, perioperative complications, health-related costs, and quality of life.
Discussion
This trial protocol describes the MEND-IT study. The MEND-IT study aims to evaluate the CR rate after intensified chemotherapy prior to concomitant chemoradiotherapy in a homogeneous group of patients with locally advanced rectal cancer and indisputably unfavourable characteristics, defined as hr-LARC, in order to improve their prognosis.
Trial registration
Clinicaltrials.gov: NCT04838496, registered on 02–04-2021 Netherlands Trial Register: NL9790.
Protocol version
Version 3 dd 11–4-2022.
Similar content being viewed by others
Background
Locally advanced rectal cancer (LARC) is often defined as a clinically staged (c) T3 tumour within 1 mm from the mesorectal fascia (MRF), a cT4 tumour, cN2 disease, or rectal cancer in the presence of extramesorectal lymph nodes [1,2,3]. Standard treatment consists of neoadjuvant chemoradiotherapy prior to surgery, i.e. a total mesorectal excision [1, 4, 5]. However, despite optimal treatment, LARC is associated with local recurrence and distant metastasis rates ranging between 5–10% and 25–40%, respectively [5,6,7,8,9,10,11,12]. This has resulted in an ongoing search for better treatment regimens in patients at high risk of treatment failure.
The main aim of neoadjuvant treatment is to obtain downstaging to facilitate a resection with clear resection margins (R0) [5, 6, 13]. An R0 resection is an important prognostic factor for disease-free survival, particularly due to an improved local recurrence-free survival [5, 8, 14, 15]. In addition, the introduction of neoadjuvant treatment regimens has translated into new treatment strategies, focusing on organ preservation [16,17,18,19]. Previous studies reported a pathological complete response (pCR) rate, i.e. the absence of malignant cells on pathological examination after surgery, in over 15% of the patients treated with neoadjuvant chemoradiotherapy [16, 20,21,22]. Improved long-term outcomes, probably related to a favourable tumour biology, have been suggested in patients with a pCR [22, 23]. Subsequently, an organ preservation strategy with active surveillance, referred to as a watch and wait (W&W) strategy, has been introduced to prevent unnecessary surgical morbidity and mortality without comprising (disease-free) survival [16,17,18,19].
Nonetheless, substantial improvements in distant metastasis rates and overall survival for patients with LARC are lagging behind [3]. The administration of adjuvant chemotherapy to reduce distant metastasis rates and improve overall survival in rectal cancer is controversial [3, 24, 25]. As an alternative, the addition of neoadjuvant chemotherapy to the current treatment regimen has been suggested [26,27,28,29]. A matched case–control study observed an improved complete response (CR) rate of 28% compared to 9% (p = 0.013) with the addition of doublet chemotherapy to neoadjuvant treatment in patients with unfavourable LARC, defined as any cT4 rectal cancer, or cT2/3 rectal cancer with extramural venous invasion (EMVI), and/or tumour deposits, and/or cN2 disease on magnetic resonance imaging (MRI) [30]. These results on CR are in line with those of three recently published, phase III randomised controlled trials in LARC [28, 31, 32]. In the PRODIGE 23 trial patients with cT3 rectal cancer eligible for chemoradiotherapy and cT4 rectal cancer were randomly assigned to either chemoradiotherapy alone or neoadjuvant FOLFIRINOX (fluorouracil, irinotecan, leucovorin, oxaliplatin) followed by chemoradiotherapy prior to surgery [32]. The RAPIDO trial included patients with an involved MRF (the primary tumour or a lymph node on a distance of ≤ 1 mm from the MRF), cT4 disease, extramural vascular invasion, cN2 disease, or enlarged lateral lymph nodes [33]. These patients were treated with either chemoradiotherapy alone or short-course radiotherapy followed by neoadjuvant doublet chemotherapy (a fluoropyrimidine in combination with oxaliplatin; CAPOX/FOLFOX) [33]. Despite the improvement in CR rate and 3-year disease-free survival / disease-related treatment failure in patients treated with neoadjuvant chemotherapy, no substantial improvements in overall survival were reported in either trial [32, 33]. Moreover, in the RAPIDO trial, the locoregional failure rate at 5 years was higher in the experimental group compared to the standard of care group (10% versus 7%, p = 0.038), whereas the significant difference in distant metastasis rate (23% versus 31%, p = 0.011) remained favourable for the experimental group [34]. In the STELLAR trial, patients with cT3-4 and/or node-positive disease were randomised between either neoadjuvant chemoradiotherapy or short-course radiotherapy followed by doublet chemotherapy [28]. A non-inferior 3-year disease-free survival was observed in the experimental arm, and preliminary results suggested a benefit in 3-year overall survival in this group [28]. However, a longer follow-up is awaited to draw definite conclusions, considering the disappearance of an overall survival benefit in the Polish II trial after a 8 year follow-up period [35]. Apart from these survival outcomes, an increase in preoperative ≥ grade 3 adverse events was reported in the experimental arms [28, 32, 33]. Nevertheless, the administration of both doublet and triplet chemotherapy did not result in significantly less patients proceeding to surgery compared to the standard treatment arms [32, 33].
Hence, neoadjuvant chemotherapy in combination with (chemo)radiotherapy may improve short- and long-term outcomes in LARC. However, the absence of a substantial improvement in overall survival, the increased risk of treatment-related toxicity, possibly resulting in treatment delays, the differences in patient selection and treatment regimens among different studies, and the underrepresentation of patients with the prognostic most unfavourable rectal tumours hamper the clinical implementation of neoadjuvant chemotherapy in rectal cancer [29, 31, 36,37,38,39,40]. Moreover, overrepresentation of patients who do not need additional systemic treatment for cure, may also dilute positive oncological results. As a consequence, it has been hypothesised that total neoadjuvant treatment, consisting of both neoadjuvant chemotherapy and chemoradiotherapy, should be preserved for a select group of patients facing the worst prognosis [31].
Since the start of the RAPIDO trial in 2011, imaging-based staging has been further refined [33]. High-risk imaging-based features were more routinely described and studied, which resulted in the recognition of clinically important, unfavourable characteristics on MRI [33, 41,42,43]. An involved MRF, defined as a distance of ≤ 1 mm to the MRF, has already been recognized as an important, unfavourable prognostic feature for several years [44, 45]. In addition, the identification of EMVI, tumour deposits, or enlarged extramesorectal (lateral) lymph nodes (LLN) with a short-axis of at least 7 mm on baseline MRI has been suggested to identify patients with the most ‘ugly’ LARC, at high risk of failure on current treatment regimens [33, 42, 46,47,48,49,50,51]. A significantly decreased disease-free and overall survival rate has been described for patients with EMVI-positive rectal cancer, with 5-year distant metastasis rates up to 45.2%, compared to EMVI-negative rectal cancer (25.7%) [12]. In addition, the presence of tumour deposits on baseline MRI has been identified as an independent prognostic factor, indicating a poor survival [42, 52]. Lastly, a lateral local recurrence rate of up to 19.5% has been described in patients with lateral lymph nodes, located in the iliac or obturator compartment, with a short-axis of at least 7 mm on baseline MRI despite treatment with chemoradiotherapy prior to a total mesorectal excision [48].
Hence, these imaging-based, high-risk features may be used to identify patients with LARC facing the worst prognosis, at high risk of failing on current treatment regimens. Nevertheless, it has been described that the recognition of imaging-based MRF involvement might be overestimated [45, 51, 53, 54]. In addition, the presence of EMVI is evaluated based on a scoring system, classifying both grade 3 and 4, in contrast to grade 0–2, as EMVI-positive [55]. Grade 3 might comprise more subtle, possibly debatable, EMVI, whereas grade 4 describes evident EMVI with obvious vessel abnormalities [55]. Therefore, we hypothesise that the presence of evident MRF invasion, EMVI grade 4, extensive or bilateral enlarged lateral lymph nodes, or tumour deposits, we refer to as MEND criteria, will correctly identify a homogeneous group of patients with high-risk (hr-) LARC, facing the worst prognosis.
This select group of patients fulfilling the MEND criteria might benefit from an intensified treatment regimen. However, these patients were underrepresent in previous trials [28, 32, 33]. The addition of intensified induction chemotherapy (IT), consisting of FOLFOXIRI (5-fluorouracil, irinotecan, leucovorin, oxaliplatin) has been suggested. This triplet chemotherapy regimen has been associated with a significantly improved tumour response of over 10%, progression-free survival and overall survival compared to a doublet chemotherapy regimen in patients diagnosed with metastatic colorectal cancer [56,57,58]. The PRODIGE 23 trial confirmed that the majority of the patients in the experimental arm was treated with chemoradiotherapy after neoadjuvant, triplet chemotherapy (95%), compared to 99% in the standard of care arm (p = 0.019) [32]. Moreover, the addition of both doublet and triplet chemotherapy to neoadjuvant (chemo)radiotherapy did not result in less patients proceeding to surgery compared to standard treatment [32, 33].
In conclusion, personalised medicine in rectal cancer is warranted to improve surgical and oncological outcomes and to prevent both over- and undertreatment. The primary aim of this single-arm, phase II study is to evaluate whether neoadjuvant chemotherapy with FOLFOXIRI prior to chemoradiotherapy provides an increased CR rate in a homogeneous group of patients with hr-LARC, fulfilling the MEND criteria, compared to current literature.
Methods/design
Aim, design, and study setting
This is a multicentre, single-arm, open-label, phase II trial. All included patients will be treated with induction chemotherapy, consisting of FOLFOXIRI, prior to chemoradiotherapy. The study is registered in Clinicaltrials.gov (NCT04838496) and in the Netherlands Trial Register (NL9790), where a list of participating centres can be obtained. The primary aim of this study is to evaluate whether the addition of neoadjuvant chemotherapy with FOLFOXIRI prior to chemoradiotherapy results in a higher pCR rate or sustained clinical complete response (cCR) rate at 1 year in patients with hr-LARC, facing a particularly poor prognosis.
Eligibility criteria
Patients, at least 18 years of age, with histopathologically confirmed, (deemed) resectable hr-LARC, with the low border of the tumour at or below the sigmoidal take-off as established on MRI, with a World Health Organization (WHO) performance status of ≤ 1, and fit for (modified dose) triplet chemotherapy are eligible for inclusion [59, 60]. An expected gross incomplete resection with overt tumour remaining in the patient after resection, tumour invasion in the neuroforamina, encasement of the ischiadic nerve and invasion of the cortex from S2 upwards are considered unresectable. Patients will be excluded in case of metastatic disease at inclusion, a homozygous DPD (dihydropyrimidine dehydrogenase) deficiency, any chemotherapy within the past 6 months, prior radiotherapy in the pelvic area interfering with the planned study treatment, any contraindication for the planned treatment, or concurrent malignancies that interfere with the planned study treatment or prognosis. Enlarged iliac or inguinal lymph nodes and aspecific lung nodules do not result in exclusion. All patients will be discussed during a multidisciplinary team (MDT) meeting to ensure their eligibility.
Definition of hr-LARC
To ensure a study population with the most unfavourable prognosis in terms of disease-free survival, the definition of hr-LARC is based on the previously described, imaging-based characteristics that represent LARC at particularly high risk of treatment failure. Hr-LARC is therefore defined as LARC with on baseline MRI at least one of the following characteristics; indisputable tumour invasion into the MRF, EMVI grade 4, bilateral LLN with a SA of ≥ 7 mm, or extensive LLN involving pelvic side wall structures such as vessels, nerves or the ureter, at high risk of treatment failure, or tumour deposits. The presence of an “involved” or “threatened” MRF, or EMVI grade 3 is insufficient for inclusion in this study [55]. Moreover, consensus should be reached about the eligibility during a MDT meeting. A syllabus containing information and images of eligible hr-LARC will be provided to all participating hospitals.
Interventions
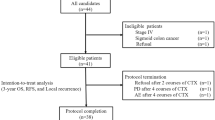
Figure 1 provides a general flowchart of the study.
Neoadjuvant chemotherapy
All included patients will receive neoadjuvant (induction) chemotherapy within 4 weeks after inclusion. Neoadjuvant chemotherapy consists of six two-weekly cycles of FOLFOXIRI (irinotecan 165 mg/m2 Body Surface Area (BSA), oxaliplatin 85/m2 BSA, leucovorin 400 mg/m2 BSA, 5-flourouracil 3200 mg/m2 BSA). Dose reductions are permitted on discretion of the medical oncologist.
Restaging will be performed with a pelvic MRI and a thoraco-abdominal computed tomography (CT) scan after four cycles of FOLFOXIRI. The timing of restaging should not interfere with the potential continuation of neoadjuvant chemotherapy. Radiological evaluation will be performed by a radiologist with expertise in abdominal imaging conform a standard operating procedure. The results will be discussed during a MDT meeting in one of the participating centres in the attendance of at least a surgical oncologist, a medical oncologist, a radiation oncologist, and a radiologist with expertise in abdominal imaging. If a patient has stable or responsive disease, neoadjuvant chemotherapy will be continued with two additional cycles of FOLFOXIRI. Subsequently, chemoradiotherapy will start within 3–6 weeks after the last cycle of neoadjuvant chemotherapy. If progressive, but still resectable disease is assumed during restaging, no further systemic therapy will be administered, and the patient will start with chemoradiotherapy within 3–6 weeks. If restaging suggests progressive, unresectable disease or the presence of distant metastases, the best palliative treatment will be offered according to standard of care.
Chemoradiotherapy
After neoadjuvant chemotherapy, all patients with (deemed) resectable disease will receive concomitant chemoradiotherapy according to standard of care within 3–6 weeks after the first day of the last cycle of FOLFOXIRI. Chemoradiotherapy will consist of 50 Gy or 50.4 Gy delivered in 25 or 28 fractions, respectively, with concomitant capecitabine (825 mg/m2 BSA) twice daily on radiotherapy days.
Treatment evaluation
Six to eight weeks after the last day of the chemoradiotherapy, restaging will be performed with a pelvic MRI and a thoraco-abdominal CT scan. The results will be discussed during a MDT meeting. A surgical resection will be planned for all patients with resectable disease after neoadjuvant treatment. In case of a cCR, a W&W strategy with close surveillance may be discussed with the patient by the treating physician according to standard of care. The presence of distant metastases or unresectable local disease will result in the best palliative treatment according to standard of care.
Surgery
Surgery will be performed according to the standard of care by a surgical team with experience in rectal cancer surgery, 10–14 weeks after the completion of chemoradiotherapy. The type and extent of the surgery and the possible addition of intraoperative radiotherapy (IORT) will be left to the discretion of the surgeon.
Follow-up
Patients’ follow-up will be performed according to standard of care every 3 months in the first 3 years and every 6 months thereafter up to 5 years postoperatively [1]. The level of carcinoembryonic antigen (CEA) will be determined at every follow-up moment. If during the follow-up the CEA level increases, a thoraco-abdominal CT scan will be performed according to the Dutch guidelines. At 6, 12, 18, 24, 30, 36, 48- and 60-months post-operatively, a thoraco-abdominal CT scan will be performed. A FDG-PET/CT scan is allowed, but not mandatory. In case of a W&W approach, the aforementioned follow-up will be expanded according to Dutch standard of care [23].
Questionnaires
All patients participating in the study will receive validated quality of life questionnaires after informed consent. Patients will be asked to complete the EORTC QLQ-C30, QLQ-CR29 (from the European Organisation for Research and Treatment of Cancer Quality of Life Group), and EQ-5D-5L (from the EuroQoL Group, Rotterdam, the Netherlands) at inclusion, 3, and 12 months post-operatively [61,62,63]. Patients receive questionnaires either by email or on paper, according to their own preferences.
Translational research
All patients will be asked for informed consent to collect blood samples for future translational research. An additional 20 ml blood is drawn during regular blood draws before start of the neoadjuvant chemotherapy, before chemoradiotherapy, before surgery, three months after surgery, and once a year during three years of follow-up, resulting in 7 samples. In addition, all patients will be asked for informed consent to collect fresh frozen tumour tissue obtained during surgery for translational research.
Outcomes
The primary outcome of this study is the CR rate, defined as the presence of a pCR or a cCR. A pCR is defined as the absence of viable tumour cells at pathological examination of the resected specimen [22, 64]. A cCR is defined as the sustained absence of tumour tissue 1 year after treatment as assessed during clinical evaluations [16, 17]. Since the MEND-IT study is a phase II study, the CR rate observed in this trial will be compared to the CR rate as described in current literature for patients with hr-LARC treated with chemoradiotherapy alone (i.e. 10%) [32, 33]. In addition, the results of the MEND-IT trial will be compared to results of comparable cohorts treated with different treatment regimens.
Secondary objectives comprise the number of patients proceeding to surgery, the 3- and 5-year local recurrence-free, distant metastasis-free, progression-free, disease-free and overall survival. In addition, radiological response, pathological response, R0 resection rate, toxicity, treatment compliance rate, surgical morbidity, quality of life, and costs will be evaluated. Local recurrence-free survival is defined as the interval between surgery and a local recurrence. Metastasis-free survival is defined as the interval between inclusion and the detection of distant metastases. Progression-free survival will be calculated from inclusion onwards to the date of clinically or histopathologically proven distant metastases, a local recurrence, or the date of death from any cause, whichever occurs first. Disease-free survival will be calculated from the date of surgery onwards or the date of the second restaging MRI in case of a cCR until the date of a local recurrence, distant metastasis, or death from any cause, whichever occurs first. Overall survival will be calculated from the date of inclusion until the date of death from any cause. Toxicity from neoadjuvant treatment will be presented according to the NCI (US National Cancer Institute) Common Terminology Criteria for Adverse Events (CTCAE) v5.0 [65]. Perioperative complications will be presented according to the Clavien-Dindo grading system [66]. Pathological response will be presented according to the Mandard grading system [64].
Sample size
A 10% CR rate is assumed in patients diagnosed with hr-LARC, treated with chemoradiotherapy alone prior to surgery [30, 32]. Based on the available literature and a retrospective analysis of patients from our own institution a CR rate of 20% is hypothesised after neoadjuvant chemotherapy and chemoradiotherapy for hr-LARC [30, 32, 33]. A 5% significance level and a 90% power, resulted in a total of 121 required patients. A drop-out of 5% is expected, resulting in a total sample size of 128 patients.
Recruitment
All eligible patients with hr-LARC presenting in one of the study centres will be identified by their physician and reviewed for eligibility during a MDT meeting.
Data collection and management
Central data management will be performed by the research team of the Catharina Hospital Eindhoven. Local data management will be performed by the coordinating investigator or an in-hospital qualified local data management team. Data will be collected in a central study database with an electronic case report form (eCRF) according to the Good Clinical Practice guidelines and the Dutch legal requirements. Major protocol violations will be recorded.
Statistical methods
Demographics, patient, and tumour characteristics will be presented for all patients. Continuous data will be reported as mean with a standard deviation or as median with an interquartile range or 95% confidence interval, depending on the distribution. Categorical data will be reported as count, including a percentage. All statistical tests will be two-sided and a P-value of less than 0.05 will be considered as statistically significant.
The proportion of patients with a CR, defined as a pCR or a sustained cCR, will be compared to the CR rate of a comparable group of patients treated with chemoradiotherapy alone before surgery, as described in the available literature (i.e. 10%), performing a chi-squared goodness-of-fit-test.
The Kaplan–Meier method will be used to display survival curves. In addition, Hazard Ratio’s will be calculated using the Cox proportional hazard regression model and will be accompanied by 95% confidence intervals. Perioperative morbidity, surgical characteristics, and histopathological findings will be presented for all patients treated with a surgical resection. The incidence of toxicity related to neoadjuvant treatment will be presented separately for neoadjuvant chemotherapy and chemoradiotherapy. Health-related quality of life is graphically presented across all time points. Moreover, quality of life will be compared during those different time points using the ‘repeated measures ANOVA’. Incremental costs are calculated for the extra costs per additional patient alive and the extra costs per additional quality adjusted life year, respectively. The 95% confidence intervals for (differences in) costs and health outcomes will be generated by non-parametric bootstrapping, drawing samples of the same size as the original samples.
Data monitoring
A data safety monitoring board (DSMB) has been appointed to monitor patients’ safety and to advice the study steering committee on the continuation of the study after the interim analysis. The interim analysis will be performed when 50 patients have undergone surgery or entered a W&W approach. The number of patients not able to complete chemoradiotherapy and the number of patients with major postoperative morbidity (i.e. Clavien-Dindo ≥ 3) will be analysed [66]. Results will be discussed during a meeting of the DSMB, subsequently resulting in a recommendation about the continuation of the study. A premature termination of the study may be advised if more than 35% of the patients is unable to complete chemoradiotherapy due to treatment-related toxicity, or if more than 50% of the patients experience severe postoperative complications (i.e. Clavien-Dindo ≥ 3).
Harms
All serious adverse events (SAEs) and/or suspected unexpected serious adverse reactions (SUSARs) will be reported to the coordinating investigator within 24 h after detection of the SAE. SAEs will be reported by the coordinating investigator to the accredited medical research ethics committee that approved the MEND-IT study protocol, using the web portal ToetsingOnline (https://toetsingonline.nl).
Auditing
The study will be monitored by a qualified monitor from the Netherlands Comprehensive Cancer Organization (IKNL) based on a predetermined monitoring plan.
Protocol amendments
All substantial protocol amendments will be presented to the competent authority, the medical research ethics committee, the institutional review boards of all study centres, the (principal) investigators and the trial registers.
Confidentiality
Individual patient information obtained as a result of this study will be handled conform the Dutch guidelines regarding General Data Protection Regulation (in Dutch: AVG). Moreover, the use of study numbers will ensure patients’ confidentiality.
Ancillary and post-trial care
There is no provision for ancillary or post-trial care in the MEND-IT study.
Dissemination policy
The results of this study will be published in an international peer-reviewed journal. Moreover, results will be presented by offering an abstract to (inter)national congresses. Any presentation, publication, or abstract based on the results of this study must be approved by the trial steering committee and coordinating investigator.
Discussion
Despite encouraging results of neoadjuvant chemotherapy in patients diagnosed with LARC, the clinical applicability remains a topic of debate due to varying inclusion criteria, varying treatment strategies, and the lack of improvement in overall survival in previous studies [31]. The addition of (intensified) neoadjuvant chemotherapy to the current treatment regimen may improve surgical and oncological outcomes in some patients, but may also prove unfeasible in many patients and even induce unnecessary risks [32, 33]. This highlights the need for uniform guidelines regarding patient selection and treatment regimens in LARC [26, 27, 31]. The aim of this multicentre, single-arm phase II study is to evaluate the CR rate of intensified neoadjuvant treatment, consisting of triplet chemotherapy (FOLFOXIRI) prior to chemoradiotherapy, in a select, homogeneous group of rectal cancer patients meeting the MEND criteria, and who are therefore facing the worst prognosis. We refer to this subgroup of locally advanced rectal cancer as “hr-LARC”.
Triplet chemotherapy has been shown to be feasible and effective in patients with rectal cancer [32, 56]. Moreover, an improved tumour response has been described with FOLFOXIRI (triplet) chemotherapy compared to doublet chemotherapy in metastatic colorectal cancer [56,57,58]. The PRODIGE 23 trial observed an enhanced CR rate and 3-year DFS for patients treated with neoadjuvant triplet chemotherapy, consisting of FOLFIRINOX, prior to standard treatment with chemoradiotherapy and surgery [32]. Despite the intensity of triplet chemotherapy with the occurrence of serious adverse events in 20% of the patients, the vast majority of the patients in the experimental arm was able to proceed to chemoradiotherapy (95%) and surgery (92%) afterwards [32]. Nevertheless, patients with LARC facing the worst prognosis were underrepresented. We hypothesised that patients with hr-LARC, meeting the MEND criteria, may benefit most from intensive treatment in an early stage of the disease. Hence, the addition of triplet chemotherapy consisting of FOLFOXIRI to current standard treatment has been suggested.
With regard to this trial protocol, it could be questioned if chemotherapy should be administered after instead of prior to chemoradiotherapy given the beneficial results on CR rate and organ preservation in the OPRA and the CAO/ARO/AIO-12 trials [19, 31, 67]. However, no difference in DFS was reported in these trials. Definite recommendations for clinical practice regarding the timing of neoadjuvant chemotherapy are still awaited. Therefore, it has been suggested that the optimal timing of neoadjuvant chemotherapy might differ among patients and treatment strategies, highlighting the need for personalised treatment regimens [31, 67]. Considering the increased intensity of triplet chemotherapy compared to doublet chemotherapy, the administration of FOLFOXIRI prior to chemoradiotherapy will offer participants sufficient time to optimise their fitness ahead of surgery. Moreover, an improved effect of IORT, which is considered standard of care for many of these patients in the Netherlands, has been described with a limited time-interval between preoperative (chemo)radiotherapy and IORT [68, 69]. In addition, patients with hr-LARC have a particularly high risk of developing distant metastases. It has been hypothesised that the early eradication of micrometastatic disease by systemic therapy might contribute to a decreased distant metastasis rate [32].
The primary endpoint, i.e. CR rate, will evaluate treatment response in this select, relatively rare group of patients with hr-LARC. To observe a substantial improvement in long-term outcomes, i.e. disease-free and overall survival, larger study populations are required. Considering the intensity of treatment, the evaluation of a clinically relevant, treatment-related effect, i.e. CR rate in a smaller cohort, seems justified. Additionally, long-term outcomes will be evaluated. Moreover, extending the current phase II to a phase III trial could be considered based on the results. Therefore, results from this study will contribute to the personalisation of rectal cancer treatment and the development of an adequate and uniform treatment strategy for patients with hr-LARC.
Availability of data and materials
Access to the final trial dataset will be preserved for the central data manager, study statistician, coordinating investigator, and trial steering committee. No contractual agreements have been drawn limiting the access to data for these investigators. The dataset supporting the conclusions of this trial will be available on reasonable request.
Abbreviations
- AE:
-
Adverse Event
- AR:
-
Adverse Reaction
- c:
-
Clinically staged
- CR:
-
Complete response
- cCR:
-
Clinical complete response
- CEA:
-
Carcinoembryonic antigen
- CR:
-
Complete response (pCR and cCR)
- CRM:
-
Circumferential resection margin
- (PET) CT:
-
(Positron emission tomography) computed tomography
- DPD:
-
Dihydropyrimidine dehydrogenase
- DSMB:
-
Data Safety Monitoring Board
- eCRF:
-
Electronic case report form
- EMVI:
-
Extramural venous invasion
- FOLFOXIRI:
-
5-Fluorouracil, leucovorin, oxaliplatin, irinotecan
- GDPR:
-
General Data Protection Regulation;
- Gy:
-
Gray
- hr-LARC:
-
High-risk locally advanced rectal cancer
- IORT:
-
Intraoperative radiotherapy
- IV:
-
Intravenously
- LARC:
-
Locally advanced rectal cancer
- LLN:
-
Lateral lymph nodes
- MDT:
-
Multidisciplinary team
- METC :
-
Medical research ethics committee (MREC); in Dutch: medisch-ethische toetsingscommissie (METC)
- MRF:
-
Mesorectal fascia
- MRF + :
-
Mesorectal fascia invasion
- MRI:
-
Magnetic resonance imaging
- R0 resection:
-
A resection with clear resection margins
- pCR:
-
Pathological complete response
- SA:
-
Short-axis
- (S)AE:
-
(Serious) Adverse Event
- SUSAR:
-
Suspected unexpected serious adverse reaction
- TD:
-
Tumour deposits
- WHO:
-
World Health Organization
- WMO:
-
Medical Research Involving Human Subjects Act; in Dutch: Wet Medisch-wetenschappelijk Onderzoek met Mensen
- W&W:
-
Watch and wait
References
Federation of Medical Specialists. Dutch National Guidelines Colorectal Cancer. 2020. https://richtlijnendatabase.nl/richtlijn/colorectaal_carcinoom_crc/primaire_behandeling_rectumcarcinoom_bij_crc/neoadjuvante_chemo_radiotherapie_bij_het_lokaal_gevorderd_rectumcarcinoom_rc.html. Accessed 1 Apr 2022.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4.
Oronsky B, Reid T, Larson C, Knox SJ. Locally advanced rectal cancer: The past, present, and future. Semin Oncol. 2020;47:85–92.
Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28 suppl_4:iv22-40.
van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–82.
Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–46.
Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–33.
Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–23.
Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620–5.
Ngan SY, Burmeister B, Fisher RJ, Solomon M, Goldstein D, Joseph D, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol. 2012;30:3827–33.
Swedish Rectal Cancer Trial, Cedermark B, Dahlberg M, Glimelius B, Pahlman L, Rutqvist LE, et al. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980–7.
Schaap DP, Voogt ELK, Burger JWA, Cnossen JS, Creemers GM, van IL, et al. Prognostic Implications of MRI-Detected EMVI and Tumor Deposits and Their Response to Neoadjuvant Therapy in cT3 and cT4 Rectal Cancer. Int J Radiat Oncol Biol Phys. 2021;111:816–25.
Radwan RW, Jones HG, Rawat N, Davies M, Evans MD, Harris DA, et al. Determinants of survival following pelvic exenteration for primary rectal cancer. Br J Surg. 2015;102:1278–84.
Feeney G, Sehgal R, Sheehan M, Hogan A, Regan M, Joyce M, et al. Neoadjuvant radiotherapy for rectal cancer management. World J Gastroenterol. 2019;25:4850–69.
PelvEx Collaborative. Surgical and Survival Outcomes Following Pelvic Exenteration for Locally Advanced Primary Rectal Cancer: Results From an International Collaboration. Ann Surg. 2019;269:315–21.
van der Valk MJM, Hilling DE, Bastiaannet E, Meershoek-Klein KE, Beets GL, Figueiredo NL, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391:2537–45.
Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U Jr, Silvae e Sousa Jr. AH, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711–7.
Al-Sukhni E, Attwood K, Mattson DM, Gabriel E, Nurkin SJ. Predictors of Pathologic Complete Response Following Neoadjuvant Chemoradiotherapy for Rectal Cancer. Ann Surg Oncol. 2016;23:1177–86.
Garcia-Aguilar J, Patil S, Kim JK, Yuval JB, Thompson H, Verheij F, et al. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. J Clin Oncol. 2020;38(15_suppl):4008.
Pucciarelli S, Toppan P, Friso ML, Russo V, Pasetto L, Urso E, et al. Complete pathologic response following preoperative chemoradiation therapy for middle to lower rectal cancer is not a prognostic factor for a better outcome. Dis Colon Rectum. 2004;47:1798–807.
Janjan NA, Khoo VS, Abbruzzese J, Pazdur R, Dubrow R, Cleary KR, et al. Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M.D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys. 1999;44:1027–38.
Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–44.
Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29:4633–40.
Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15:184–90.
Hong YS, Nam B-H, Kim K-P, Kim JE, Park SJ, Park YS, et al. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2014;15:1245–53.
Petrelli F, Trevisan F, Cabiddu M, Sgroi G, Bruschieri L, Rausa E, et al. Total Neoadjuvant Therapy in Rectal Cancer: A Systematic Review and Meta-analysis of Treatment Outcomes. Ann Surg. 2020;271:440–8.
Kong JC, Soucisse M, Michael M, Tie J, Ngan SY, Leong T, et al. Total Neoadjuvant Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Metaanalysis of Oncological and Operative Outcomes. Ann Surg Oncol. 2021;28:7476–86.
Jing J, Yuan T, Chen H, Yong C, Yuan Z, Guanghui C, et al. A multicenter, randomized, phase III trial of short-term radiotherapy plus chemotherapy versus long-term chemoradiotherapy in locally advanced rectal cancer (STELLAR): The final reports. J Clin Oncol. 2021;39 15_suppl A:3510.
Fernandez-Martos C, Garcia-Albeniz X, Pericay C, Maurel J, Aparicio J, Montagut C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial. Ann Oncol. 2015;26:1722–8.
Voogt ELK, Schaap DP, van den Berg K, Nieuwenhuijzen GAP, Bloemen JG, Creemers GJ, et al. Improved response rate in patients with prognostically poor locally advanced rectal cancer after treatment with induction chemotherapy and chemoradiotherapy when compared with chemoradiotherapy alone: A matched case-control study. Eur J Surg Oncol. 2021;47:2429–35.
Giunta EF, Bregni G, Pretta A, Deleporte A, Liberale G, Bali AM, et al. Total neoadjuvant therapy for rectal cancer: Making sense of the results from the RAPIDO and PRODIGE 23 trials. Cancer Treat Rev. 2021;96: 102177.
Conroy T, Bosset JF, Etienne PL, Rio E, Francois É, Mesgouez-Nebout N, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:702–15.
Bahadoer RR, Dijkstra EA, van EB, Marijnen CAM, Putter H, Kranenbarg EM, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29–42.
Bahadoer R, Dijkstra E. Patterns of locoregional failure and distant metastases in patients treated for locally advanced rectal cancer in the RAPIDO trial. Eur J Surg Oncol. 2022;48:E34–E34.
Ciseł B, Pietrzak L, Michalski W, Wyrwicz L, Rutkowski A, Kosakowska E, et al. Long-course preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer: long-term results of the randomized Polish II study. Ann Oncol. 2019;30:1298–303.
Fernández-Martos C, Pericay C, Aparicio J, Salud A, Safont M, Massuti B, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined. J Clin Oncol. 2010;28:859–65.
Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16:957–66.
Tang J, Wu X, Bai Y, Gao Y, Jiang W, Kong L, et al. Long-Term Outcome of Oxaliplatin and Capecitabine (XELOX) Concomitant with Neoadjuvant Radiotherapy and Extended to the Resting Period in High Risk Locally Advanced Rectal Cancer. J Cancer. 2018;9:1365–70.
Yu X, Wang QX, Xiao WW, Chang H, Zeng ZF, Lu ZH, et al. Neoadjuvant oxaliplatin and capecitabine combined with bevacizumab plus radiotherapy for locally advanced rectal cancer: results of a single-institute phase II study. Cancer Commun (Lond). 2018;38:24.
Tuta M, Boc N, Brecelj E, Omejc M, Anderluh F, Ermenc AS, et al. Total neoadjuvant treatment of locally advanced rectal cancer with high risk factors in Slovenia. Radiol Oncol. 2019;53:465–72.
Balyasnikova S, Brown G. Optimal Imaging Strategies for Rectal Cancer Staging and Ongoing Management. Curr Treat Options Oncol. 2016;17:32.
Nagtegaal ID, Knijn N, Hugen N, Marshall HC, Sugihara K, Tot T, et al. Tumor Deposits in Colorectal Cancer: Improving the Value of Modern Staging-A Systematic Review and Meta-Analysis. J Clin Oncol. 2017;35:1119–27.
Ogura A, Konishi T, Beets GL, Cunningham C, Garcia-Aguilar J, Iversen H, et al. Lateral Nodal Features on Restaging Magnetic Resonance Imaging Associated With Lateral Local Recurrence in Low Rectal Cancer After Neoadjuvant Chemoradiotherapy or Radiotherapy. JAMA Surg. 2019;154: e192172.
Nagtegaal ID, Marijnen CA, Kranenbarg EK, van VD, Svan Krieken JH. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002;26:350–7.
Al-Sukhni E, Milot L, Fruitman M, Beyene J, Victor JC, Schmocker S, et al. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19:2212–23.
Schaap DP, Ogura A, Nederend J, Maas M, Cnossen JS, Creemers GJ, et al. Prognostic implications of MRI-detected lateral nodal disease and extramural vascular invasion in rectal cancer. Br J Surg. 2018;105:1844–52.
Tan JJ, Carten RV, Babiker A, Abulafi M, Lord AC, Brown G. Prognostic Importance of MRI-Detected Extramural Venous Invasion in Rectal Cancer: A Literature Review and Systematic Meta-Analysis. Int J Radiat Oncol Biol Phys. 2021;111:385–94.
Ogura A, Konishi T, Cunningham C, Garcia-Aguilar J, Iversen H, Toda S, et al. Neoadjuvant (Chemo)radiotherapy With Total Mesorectal Excision Only Is Not Sufficient to Prevent Lateral Local Recurrence in Enlarged Nodes: Results of the Multicenter Lateral Node Study of Patients With Low cT3/4 Rectal Cancer. J Clin Oncol. 2019;37:33–43.
Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003;90:355–64.
Chand M, Swift RI, Tekkis PP, Chau I, Brown G. Extramural venous invasion is a potential imaging predictive biomarker of neoadjuvant treatment in rectal cancer. Br J Cancer. 2014;110:19–25.
Bates DDB, Homsi ME, Chang KJ, Lalwani N, Horvat N, Sheedy SP. MRI for Rectal Cancer: Staging, mrCRM, EMVI, Lymph Node Staging and Post-Treatment Response. Clin Color Cancer. 2021;21(1):10–8.
Lord AC, Graham MC, D’Souza N, Pucher PH, Brown G, Nagtegaal ID. The significance of tumour deposits in rectal cancer after neoadjuvant therapy: a systematic review and meta-analysis. Eur J Cancer. 2019;122:1–8.
Beets-Tan RG, Beets GL, Vliegen RF, Kessels AG, Van BH, De BA, et al. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet. 2001;357:497–504.
Dahlbäck C, Korsbakke K, Alshibiby BT, Zaki J, Zackrisson S, Buchwald P. Accuracy of magnetic resonance imaging staging of tumour and nodal stage in rectal cancer treated by primary surgery: a population-based study. Color Dis. 2021;00:1–7.
Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95:229–36.
Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306–15.
Cremolini C, Loupakis F, Antoniotti C, Lonardi S, Masi G, Salvatore L, et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol. 2015;26:1188–94.
Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nor. J Clin Oncol. 2007;25:1670–6.
D’Souza N, MPM de NTB, D’Hoore A, Tiret E, Xynos E, Beets-Tan RGH, et al. Definition of the Rectum: An International. Expert-based Delphi Consensus Ann Surg. 2019;270:955–9.
Hazen SJA, Sluckin TC, Horsthuis K, Lambregts DMJ, Beets-Tan RGH, Tanis PJ, et al. Evaluation of the implementation of the sigmoid take-off landmark in the Netherlands. Color Dis. 2022;24:292–307.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76.
Whistance RN, Conroy T, Chie W, Costantini A, Sezer O, Koller M, et al. Clinical and psychometric validation of the EORTC QLQ-CR29 questionnaire module to assess health-related quality of life in patients with colorectal cancer. Eur J Cancer. 2009;45:3017–26.
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–36.
Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations Cancer. 1994;73:2680–6.
Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. 2014;106:dju244.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96.
Fokas E, Allgäuer M, Polat B, Klautke G, Grabenbauer GG, Fietkau R, et al. Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J Clin Oncol. 2019;37:3212–22.
Holman FA, Haddock MG, Gunderson LL, Kusters M, Nieuwenhuijzen GA, van den Berg HA, et al. Results of intraoperative electron beam radiotherapy containing multimodality treatment for locally unresectable T4 rectal cancer: a pooled analysis of the Mayo Clinic Rochester and Catharina Hospital Eindhoven. J Gastrointest Oncol. 2016;7:903–16.
Holman FA, Bosman SJ, Haddock MG, Gunderson LL, Kusters M, Nieuwenhuijzen GA, et al. Results of a pooled analysis of IOERT containing multimodality treatment for locally recurrent rectal cancer: Results of 565 patients of two major treatment centres. Eur J Surg Oncol. 2017;43:107–17.
Acknowledgements
Not applicable.
Funding
This study is part of the ‘highly specialized care and research programme (TZO programme)’, funded by the Netherlands Organisation for Health Research and Development (ZonMw). The research proposal was assessed on quality by external referees during the assessment procedure. ZonMW will not benefit financially from the results of this study.
Author information
Authors and Affiliations
Contributions
KB and DPS, coordinating investigators of the study, contributed to setting-up the trial, the design of the trial and writing the original draft of the protocol and the manuscript. ELKV, contributed to setting-up the trial and revised the manuscript. TEB, local investigator, contributed to the design of the trial, writing the original draft of the protocol and revised the manuscript. AGJA, CV, JM, JMLR, JHWW, HLW, MB, NAJBP, LHJS, JMWEW and JV are local investigators, they contributed to the design of the trial and revised the protocol and the manuscript. GJMC, HMUP, CAMM, JWBG and HMWV are involved in the study steering committee, contributed to the design of the trial, and revised the manuscript. MV advised on statistical analyses and revised the protocol and manuscript. IMW revised the protocol and manuscript. PS and IGL are involved as pathologists and contributed to the design of the trial, and revised the protocol and manuscript. JN is involved in the trial as radiologist and contributed to the design of the trial, and revised the protocol and manuscript. MJR, GAPN, JGB contributed in the design of the trial and revised the protocol and manuscript. HJTR contributed to the design of the trial, and writing the original draft of the protocol and manuscript. JWAB, principal investigator, contributed to the design of the trial, setting-up the trial, and writing the original draft of the protocol and manuscript. All authors gave final approval for submission of the trial protocol.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Dutch competent authority (CCMO, The Hague, the Netherlands) and the Medical Research Ethics Committees United (MEC-U, Nieuwegein, the Netherlands). The reference number is R20.106. The study will be submitted to all institutional review boards to obtain approval prior to the start of the trial in the participating centres. Information regarding the study will be provided by the treating physician, a research nurse or a local investigator. A written informed consent is required of all study participants before inclusion in the study. Separate informed consent can be provided for the collection of blood and/or tissue for translational research and for quality of life questionnaires.
Consent for publication
This trial protocol does not contain any individual person’s data.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
van den Berg, K., Schaap, D.P., Voogt, E.L.K. et al. Neoadjuvant FOLFOXIRI prior to chemoradiotherapy for high-risk (“ugly”) locally advanced rectal cancer: study protocol of a single-arm, multicentre, open-label, phase II trial (MEND-IT). BMC Cancer 22, 957 (2022). https://doi.org/10.1186/s12885-022-09947-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09947-w