Abstract
Background
Advances in hematopoietic cell transplantation (HCT) have led to marked improvements in survival. However, adolescents and young adults (AYAs) who undergo HCT are at high risk of developing sarcopenia (loss of skeletal muscle mass) due to the impact of HCT-related exposures on the developing musculoskeletal system. HCT survivors who have sarcopenia also have excess lifetime risk of non-relapse mortality. Therefore, interventions that increase skeletal muscle mass, metabolism, strength, and function are needed to improve health in AYA HCT survivors. Skeletal muscle is highly reliant on mitochondrial energy production, as reflected by oxidative phosphorylation (OXPHOS) capacity. Exercise is one approach to target skeletal muscle mitochondrial OXPHOS, and in turn improve muscle function and strength. Another approach is to use “exercise enhancers”, such as nicotinamide riboside (NR), a safe and well-tolerated precursor of nicotinamide adenine dinucleotide (NAD+), a cofactor that in turn impacts muscle energy production. Interventions combining exercise with exercise enhancers like NR hold promise, but have not yet been rigorously tested in AYA HCT survivors.
Methods/design
We will perform a randomized controlled trial testing 16 weeks of in-home aerobic and resistance exercise and NR in AYA HCT survivors, with a primary outcome of muscle strength via dynamometry and a key secondary outcome of cardiovascular fitness via cardiopulmonary exercise testing. We will also test the effects of these interventions on i) muscle mass via dual energy x-ray absorptiometry; ii) muscle mitochondrial OXPHOS via an innovative non-invasive MRI-based technique, and iii) circulating correlates of NAD+ metabolism via metabolomics. Eighty AYAs (ages 15-30y) will be recruited 6–24 months post-HCT and randomized to 1 of 4 arms: exercise + NR, exercise alone, NR alone, or control. Outcomes will be collected at baseline and after the 16-week intervention.
Discussion
We expect that exercise with NR will produce larger changes than exercise alone in key outcomes, and that changes will be mediated by increases in muscle OXPHOS. We will apply the insights gained from this trial to develop individualized, evidence-supported precision initiatives that will reduce chronic disease burden in high-risk cancer survivors.
Trial registration
ClinicalTrials.gov, NCT05194397. Registered January 18, 2022, https://clinicaltrials.gov/ct2/show/NCT05194397 {2a}.
Similar content being viewed by others
Background
Advances in hematopoietic cell transplantation (HCT) have led to 10% improvements in survival each decade since the 1980s [1]. Research in the growing population of long-term HCT survivors has highlighted the chronic and debilitating co-morbidities associated with toxicity from pre-HCT exposures, HCT conditioning, immunosuppression, and graft versus host disease (GvHD) [2]. Adolescents and young adults (AYAs) who undergo HCT are at an especially high risk of developing sarcopenia (loss of skeletal muscle mass), a consequence of HCT-related exposures on the developing musculoskeletal system [3]. HCT-associated risk factors for sarcopenia include inflammation related to GvHD, treatment-related myopathy (e.g., from glucocorticoids), undernutrition, physical inactivity, and/or de novo co-morbidities (e.g., hypogonadism, growth hormone deficiency) that develop during or shortly after HCT [4]. In AYAs undergoing HCT, we have shown that sarcopenia occurs earlier than would be expected from advancing age alone, likely because the AYA age range (15-30y) represents a critical window for attainment of peak muscle mass. We have also shown that HCT survivors who have sarcopenia have twice the risk of non-relapse mortality as compared to survivors without sarcopenia, which may be mediated in part by an excess incidence of premature cardiovascular disease (CVD) [5]. Therefore, evidence-based interventions are needed that will increase skeletal muscle mass, metabolism, strength, and function and ultimately improve health outcomes in AYA HCT survivors (Table 1).
Strategies to improve skeletal muscle health
Skeletal muscle is highly reliant on mitochondrial energy production, as reflected by oxidative phosphorylation (OXPHOS) capacity. Thus, one strategy to improve skeletal muscle function is to increase muscle OXPHOS. Exercise (aerobic and resistance training) is a well-established approach to target skeletal muscle mitochondrial OXPHOS, as well as mass, strength, and function, though responsiveness to exercise training interventions may vary in both individuals who are healthy and those who have chronic disease. Moreover, individuals who survive HCT often do not meet minimum public health recommendations for exercise, and also demonstrate significant declines in daily physical activity from pre- to post-HCT [6]. In addition, individuals who develop GvHD are even more likely to be inactive because the disproportionate atrophy that develops in lower extremity and back extensor muscles makes activity more challenging to pursue. The combination of inactivity plus decreased muscle mass further compromises muscle capillary transport and utilization of oxygen, leading to additional impairments in cardiovascular reserve [7]. Thus, targeting physical inactivity along with treatment-related muscle metabolic deficits may produce benefits that are more than additive in sedentary HCT survivors.
Another approach to improve skeletal muscle health is to use an “exercise enhancer” such as precursors of nicotinamide adenine dinucleotide (NAD+), a cofactor with myriad roles in cellular metabolism, to recapitulate and/or add to the physiologic benefits of exercise [8, 9]. Nicotinamide riboside (NR) is an NAD+ precursor that is consumed as part of the diet and can be administered orally in humans [8]. In preclinical models, NR has been shown to increase muscle OXPHOS capacity and may improve exercise performance [10]. NAD+ precursor supplementation with NR has also been shown to increase oxidative metabolism in both muscle [11, 12] and liver [13], and its therapeutic potential is being explored in the wide range of conditions where disordered mitochondrial metabolism occurs, including aging and CVD [14].
Although NR is a niacin analog, unlike niacin, NR is unlikely to produce flushing because it does not activate the niacin receptor (GPR109A) [15]. The pharmacokinetics and pharmacodynamics of NR have been measured in humans [16,17,18], and safety and toxicity studies in both animals and humans indicate a favorable safety profile that is similar to or better than niacin [18]. Two recent studies in humans found that NR, which is metabolized to nicotinamide, was well tolerated without serious adverse events [19, 20]. Pediatric participants (n = 163) who received 1.2 g/m2/day of nicotinamide for 5 years had a similar number of serious adverse events as participants receiving placebo (n = 166) [21]. A safety study of higher dose (up to 8 g daily) of nicotinamide for up to 8 weeks in individuals with complex neuromuscular disease found it to be generally well-tolerated, with nausea being the predominant side effect observed at the highest doses that resolved spontaneously after dose reduction and/or with anti-nausea treatment [22]. In 12 older men receiving 1 g daily of NR for 21 days, NR was well tolerated, and produced an increase in skeletal muscle NAD+ and a decrease in circulating inflammatory cytokines [23]. A longer 12-week trial tested 1,000 mg po twice daily (that is, a total daily dose of 2,000 mg NR) in N = 40 healthy sedentary men, and found NR to be well-tolerated over this duration with sustained increases in urinary NAD+ metabolites [19] {6b}.
Rationale for combining exercise with NR to improve muscle health in AYA HCT survivors
Exercise and NAD+ precursor supplementation via NR are each independently expected to improve muscle mass and strength in HCT survivors. When combined, exercise and NR may even produce benefits that are synergistic (Fig. 1). Exercise requires energy, and NAD+ availability promotes mitochondrial ATP production. Indeed, one of the known physiologic adaptations to exercise training in humans is up-regulation of NAD+ salvage via increased expression of NAMPT [24]. Studies in preclinical models demonstrate the relevance of this response. For example, mice who have increased NAD+ availability gain more exercise capacity in response to exercise training than their counterparts with typical NAD+ availability [25]. Also, increased NAD+ availability leads to more sirtuin-dependent PGC-1α related mitochondrial biogenesis with training [25]. Recently released physical activity guidelines emphasize the universal benefits of exercise, including for individuals with health problems [26]. However, exercise can require a disproportionate investment of time and effort for those with chronic conditions. Strategies such as NAD+ precursor supplementation that enhance the benefits of training would increase the yield of this important investment and could be a valuable addition to our therapeutic arsenal. Therefore, exercise combined with NAD+ precursor supplementation may yield additional benefits compared to either alone, but this approach has not been tested in AYA HCT survivors {6a}.
An improvement of overall skeletal muscle health via combination intervention of exercise and NAD + precursor supplementation. Cancer has both direct and indirect effects that compromise cardiovascular reserve capacity, and thus contribute to premature mortality. Both exercise and NAD + precursor therapy (nicotinamide riboside, NR) may increase skeletal muscle mass, mitochondrial OXPHOS capacity, strength, and aerobic capacity. This, in turn, will lead to a lower rate of premature mortality caused by a significant decline in cardiovascular reserve capacity. The figure was generated using Adobe Illustrator (version 22.0)
Methods/design
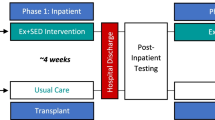
In this randomized controlled trial, we will prospectively enroll 80 participants status-post HCT at two sites, the Children’s Hospital of Philadelphia (CHOP) (Philadelphia, PA, USA) and the City of Hope Medical Center (COH) (Duarte, CA, USA). There will be 4 arms: exercise intervention with NR supplementation, exercise intervention with placebo, no exercise intervention with NR supplementation, and no exercise intervention with placebo. The individualized exercise intervention will occur in the home, overseen remotely via Telehealth by exercise intervention physiologists from St. Jude Children’s Research Hospital (Memphis, TN, USA) {9}. Participants who are randomized to the exercise intervention will be given the necessary equipment to participate either before they depart the site or by mail. Each participant will take part in the study for 16 weeks, with a total of three onsite in-person visits: one at baseline, one interim visit at week 8, and a final visit at the end of the 16-week intervention.
The primary objective of this study is to measure the effect of combination administration (NR + exercise) on skeletal muscle quality (strength along with mitochondrial OXPHOS capacity) in AYA HCT survivors {7}. Skeletal muscle quality will be measured by dynamometry, while OXPHOS capacity will be assessed through creatine chemical exchange saturation magnetic resonance imaging (CrCEST MRI). The secondary objective of this study is to measure the effect of combination administration (NR + exercise) on aerobic capacity (VO2max), as determined by cardiopulmonary exercise testing (CPET).
Participants and randomization
Potentially eligible participants will be identified and recruited both at CHOP and COH through existing databases and physician referrals. Study staff will pre-screen potential participants by reviewing the medical charts and exclude those who do not meet inclusion/exclusion criteria {15}. Individuals who meet the eligibility criteria (Table 2) will be invited to enroll. All participants will be enrolled when they provide written consent to the principal investigator(s) either virtually or in-person and all the initial eligibility requirements are met {26a}. Once eligibility is confirmed via in-person screening procedures, participants will be randomized into one of four arms, with randomization stratified by age (≥ 18y or < 18y) and site (CHOP or COH) by a designated study member using REDCAP randomization scheme (Fig. 2) {16b} {16c}. Balance between arms will be maintained through randomly permuted blocks of size four or eight {16a}. The research team, outcomes assessors, care providers, and participants will be blinded with respect to whether participants are receiving NR vs. placebo. NR and placebo are dispensed in identical capsules to avoid un-blinding. The outcomes assessor (but not the study team, care providers, or participants) will be blinded with respect to whether individuals are receiving the in-home exercise intervention vs. no exercise intervention {17a}. The Investigator will be unblinded only if an AE occurs that is determined to be serious, and treatment for the adverse event (AE) depends on knowing the group assignment. Participants/families will be unblinded at the completion of the entire study {17b}. The current study has been approved by the Institutional Review Board of Children’s Hospital of Philadelphia (approval number: IRB #17,320) and has been pre-registered at www.clinicaltrials.gov (NCT05194397). The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guideline was used to generate the report [27].
Overview of the study scheme. During their baseline visit, participants will be randomized into either exercise or non-exercise group and either NR supplementation or placebo. After randomization, participants who were randomized into exercise group will receive their equipment and be oriented to the in-home exercise intervention. Participants who were randomized into NR group will receive NR daily at a dose based on body weight: 300 mg for individuals with weight 24 to < 48 kg, 600 mg for those with weight 48 to < 72 kg, and 900 mg for those with weight 72 kg or greater. After 8 weeks (\(\pm 1.5\) weeks), all the participants will have their interim visit. After 16 weeks from the baseline visit, participants will have their final study visit. The figure was generated using Microsoft PowerPoint (Version 16.62)
Study assessments {18a}
All study participants will complete a review of medical history, have their vital signs measured, and have laboratory assessments (CBC, CMP, HbA1c, lipid profile, blood to be banked for future ancillary metabolic and/or hormonal studies) (Table 3). Activity monitoring will be reviewed for seven days via an activity monitor (ActiGraph wGT3X-BT; ActiGraph Corp., Pensacola, FL) and, if assigned, the exercise training orientation will occur. After all study procedures are completed, supplement/placebo (NR/placebo) will be provided to the participant along with instructions and administration log. Specific to the primary and secondary outcomes, all participants will undergo the following assessments at the baseline and follow-up visits:
Dynamometry (hand grip, lower extremities)
Trained personnel will perform testing at CHOP and COH. A dynamometer (Takei Scientific Instruments Co., Ltd., Japan) will be used to measure instantaneous hand grip strength because in epidemiological studies this index of muscle strength has been shown to be associated with morbidity and mortality [28]. Three trials will be performed using each hand, and the maximum value will be recorded (N). The Biodex Dynamometer (Biodex Corp., Shirley, NY) will be used to measure muscular strength in the ankle and knee. Participant will perform isometric strength tests, with three repetitions performed at each position. A 5 s rest is given between repetitions. At each position, peak torque (N) will be recorded. References will be used to generate age- and sex-specific Z-scores [29].
CrCEST MRI is a non-invasive way to test skeletal muscle OXPHOS capacity [30]. With CrCEST, creatine content can be simultaneously measured in every muscle of the leg [31, 32]. CrCEST is used to determine the exponential time constant for the decline in creatine post-exercise (τCr) in each muscle of the calf (lateral gastrocnemius, medial gastrocnemius, and soleus) after an in-magnet standardized exercise on an MR-compatible. Prolonged τCr is suggestive of decreased OXPHOS capacity. In a “proof-of-principle” study using CrCEST at a 7 T MRI field strength [33], our team found that individuals with genetic diseases affecting mitochondria had significantly prolonged post-exercise τCr in the medial gastrocnemius muscle, indicating decreased OXPHOS capacity. Using CrCEST, we also found that habitual exercise duration is positively associated with muscle OXPHOS in individuals with genetic mitochondrial disorders [33]. In a separate study using CrCEST at 3 T, we found that decreased physical activity in individuals with Friedreich’s Ataxia, a neurological disorder, accounted for some of the difference in τCr in lateral gastrocnemius between individuals with Friedreich’s Ataxia and healthy controls [34]. We will use the same 3 T CrCEST to examine the effects of mitochondria-targeted interventions in the present study.
CPET
Maximal oxygen uptake (VO2max) derived from CPET represents the integrative efficiency with which oxygen is delivered (by the heart and vascular system) and used (primarily by skeletal muscle) to produce ATP to sustain exercise. Using near infrared spectroscopy, an association has been found between muscle oxygen extraction and CPET performance, specifically implicating muscle mitochondrial deficits in individuals with low VO2max [35]. Study participants will undergo an incremental symptom-limited CPET. After a 1-min warm-up at 0-Watt (W) workload, a ramp protocol of 5 or 10 W/min will be started and continued until maximal volition. Participants will be monitored for any potentially exercise limiting symptoms such as chest pain, signs of ischemia, or arrhythmias to develop or other indications for exercise termination to appear. Respiratory gas exchange measurements will be obtained breath by breath by a commercially available system at CHOP and at COH (Vmax 29C; Sensormedics, Yorba Linda, CA). VO2max will be recorded as the mean value of VO2 during the last 20 s of the test. Gas exchange will be measured continuously throughout exercise and for the first 2 min of recovery. The ventilatory anaerobic threshold will be detected by use of the V-slope and dual criteria methods. Musculoskeletal aerobic work efficiency will be calculated as the change in VO2 versus the change in work rate (ml O2/min/Watt) prior to anaerobic threshold (AT).
Intervention components
Exercise
Participants randomized to exercise intervention group will be provided with the results of their CPET and dynamometry and receive an individually tailored exercise prescription, written by the exercise physiologist. The participant will be given a set of weights (varying depending on their weight and physical ability), a foldable exercise bike, (FB350 Folding Exercise Bike; XTERRA Fitness, Jonesboro, AR), resistance bands, a tablet (iPad 9th Generation; Apple Inc., Cupertino, CA) to interact with the exercise physiologist, and a heart rate monitor (Polar Verity Sense; Polar Electro Oy, Kempele, Finland). Those randomized to the “no exercise” intervention group will be given the option to receive a similar individually tailored exercise prescription after the 16-week intervention is complete. The exercise prescription will be based on the results of their baseline assessments and account for any functional impairments. Each prescription will include aerobic and strengthening components designed to progress persons gradually towards the 150–300 min of aerobic activity (with duration depending on intensity) and twice weekly strengthening over 16 weeks that are recommended as part of public health guidance [36].
The exercise physiologist will supervise and provide guidance for the participants six times for the first two weeks, and then taper to twice a week for weeks 3–4, once for weeks 5–7, every other week for weeks 8–11, and to one time midway between weeks 12–16, each visit expecting to last one hour. During each session, participants will be asked to answer general physical and mental health questions. For aerobic training, the eventual intensity and duration goal will be for participants to train at 70–80% of peak heart rate 20–45 min 3–5 days/week [37]. Participants who have blunted heart rate responses to exercise will train using the Borg Rating of Perceived Exertion (RPE) scale [37]. For resistance exercises the goal will be a load/weight that results in fatigue after 3 sets of 10–12 repetitions on 8–10 exercises 2 days/week [38]. As many of these individuals will have chronic conditions and/or will be sedentary at baseline, the initial intensity and duration of the training may be much lower than typical sedentary individuals. In addition, progression toward and achievement of this goal will be heterogeneous. However, our tailored approach has great potential to impact our outcomes of interest since individuals who are sedentary [39] and those who are the most impaired may be positioned to achieve the largest incremental change [40]. Tailoring will be designed to allow each participant to start at a level reflecting their initial capacity, with personalized modifications as the study continues. Progression will also be tailored and be modified during the study to accommodate response to the intervention {11b}.
NR Supplement dose and rationale for dose selection
We will use 300 mg tablets of the supplement nicotinamide riboside (NR) (Tru Niagen®, ChromaDex, Irvine CA) which is the formulation used in previous well-controlled pharmacokinetic/pharmacodynamic and interventional studies [19, 41]. NR is a form of vitamin B3, a precursor for NAD+ [14]. NR is available as a dietary supplement and is generally recognized as safe (GRAS) by the FDA.
The “no adverse effect level” of NR proposed by the European Commission Scientific Committee on Food was 25 mg/kg per day, and the conservative tolerable upper intake level proposed was 12.5 mg/kg per day, or 900 mg for adults [42]. In adults, doses of 100, 300, and 1,000 mg of NR achieve dose dependent increases in the blood NAD+ metabolome [41], thus we propose to use the tolerable upper intake level, 900 mg, as the maximum dose for adults, and corresponding doses (in proportion to body weight) in children. We will use the following doses:
-
For individuals with weight 72 kg: 900 mg po qd × 16 wks.
-
For individuals with weight 48 to < 72 kg: 600 mg po qd × 16 wks.
-
For individuals with weight 24 to < 48 kg: 300 mg po qd × 16 wks.
This regimen achieves a maximum dose of 12.5 mg/kg/day (24 kg is ~ 3rd%ile for weight for a 10yo child, thus this weight threshold is more than sufficient for adolescents ages 15y and older to enroll). In a previous pediatric study, ~ 1,200 mg/m2 of nicotinamide for 5y was well-tolerated, more than what is proposed here [21] {11a}.
A concurrent use of any medication that likely increases the risk of NR toxicity is not permitted for all participants during the study period. Furthermore, those who are using such medications will be excluded from the study {11d}.
Data management {19}
The study team will work with Data Management CHOP CHPS Informatics Core to generate case report forms and design a REDCap database for data capture. REDCap is an NIH-supported web-based data management software designed by Vanderbilt University investigators.
The PI is responsible for the accuracy and completeness of data collection and management. The PI may designate qualified individual(s) to collect data and manage data. Only investigators and research staff that have completed appropriate IRB training and approval and are listed on the IRB approved protocol are eligible to collect and work on information from the study. Future studies that may use patients or data collected from this study must have separate approved IRB protocols and consent forms, if applicable. Partial or complete datasets will be provided depending on the stated purpose of each data request. Only de-identified data will be shared. We will make the data available to users only under a data-sharing agreement that provides for:
-
commitment to use the data only for not-for-profit research purposes;
-
commitment to maintain the data in a secure environment;
-
commitment to protect the privacy and identity of study participants and not to identify any individual participant;
-
commitment to securing the data using appropriate computer technology;
-
commitment to destroying or returning the data after analyses are completed;
-
commitment that the data will not be transferred to other users;
-
commitment to maintain all appropriate regulatory requirements;
-
commitment to acknowledge the source of the data (NIH grant support and research team) in all publications and presentations.
It is the goal of this research team to translate the study findings into clinical care in order to impact the health and well-being of children. To that end, it is the intention of the research team to communicate the results of this study to the scientific community as rapidly as possible, and to share data as openly as possible {31a}.
Recruitment data will be recorded onto the screening questionnaire. Original data will be recorded directly onto CRFs by the study coordinator or a study investigator. Copies of testing results will be received through inter-office mailing, picked up directly, and sometimes through email. This information also will be recorded onto CRFs while the originals may be kept at the testing site. CRFs will be kept in a locked filing cabinet in a locked room at all times (study coordinator and/or PI office). All information will be transferred to REDCap, a secure, web-based application supported by the CHOP Research Institute. The password to log onto the database will be unique to each member of the study team. Information may also be stored in Oncore Clinical Trials Management System, a secure electronic data capture system with access controls and a data back-up plan. The system is password protected. Only study team members will have access to study data and case report forms stored in OnCore. Access to the system is monitored and logged for review, if needed {29}. The identifiable information collected as part of this study will be retained for a duration that is compliant with CHOP Data Retention Policy A-3–9 {31c}. Blood specimens will be retained indefinitely, as subjects will consent to during informed consent. The PHI linked to these specimens will remain coded and accessible only to IRB-approved study staff {27}.
Once a year, the division of Oncology at the CHOP will audit the study. Additionally, the CHOP IRB will, at random, select the study for an internal audit. The study will follow the institutional guideline to ensure that it meets the regulations and is prepared for both an internal and an external audit {23}.
Harms management {22}
Safety will be monitored through the collection of laboratory assessments at baseline and follow-up. Participants will also be monitored using a standardized assessment of symptoms. We will use the Common Terminology Criteria for Adverse Events (CTCAE, version 5.0, U.S. Department of Health and Human Services) to grade AEs. Individuals who experience a new Grade 3 or higher AE that is at least possibly related to the study intervention may require cessation of study participation at the discretion of the investigative team. Grade 4 AEs will require study cessation of study participation.
If any unanticipated problems related to the research involving risks to subjects or others happen during the course of this study including serious adverse events (SAE), they will be reported to the IRB in accordance with CHOP IRB SOP 408: Unanticipated Problems Involving Risks to Subjects. AEs that are not serious but that are notable and could involve risks to subjects will be summarized in narrative or other format and submitted to the IRB at the time of continuing review. All AEs will be noted in the study records and on the case report form with a full description including the nature, date and time of onset, determination of non-serious versus serious, intensity (mild, moderate, severe), duration, causality, and outcome of the event. An AE is defined as any untoward medical occurrence in a subject who has received an intervention (drug, biologic, or other intervention), and an SAE is defined as any adverse drug experience occurring at any dose that results in death, in a life-threatening event, in a persistent or significant disability/incapacity, or a congenital anomaly/birth defect in the offspring of a subject. The occurrence does not necessarily have to have a causal relationship with the treatment. An AE can therefore be any unfavorable or unintended sign (including an abnormal laboratory finding, for example), symptom, or disease temporally associated with the use of a medicinal product, whether or not considered related to the medicinal product.
The Safety Monitoring Uniform Report Form (SMURF) will also be used to assess NR specific side effects and will be administered at each in-person visit. The full SMURF contains a general inquiry, several questions about daily activities (e.g., sleep, appetite, energy level, bowel and bladder function), and modified queries specific to NR side effects. The general inquiry includes an open-ended question about any problems or complaints, as well as questions regarding the need for other medications and doctor or health care encounters since the last study visit. The next section includes 25 system-specific queries to ensure completeness. All new AEs will be documented. We will also check on the status of any previously reported possible AEs.
Statistical considerations
Linear mixed-effects modeling will be used to evaluate effect of interventions over time on the primary and key secondary outcomes. The models incorporate subject-specific random effects to account for within-subject correlation due to repeated measures. Group averages, as well as subject-specific intercepts and slopes, will be estimated to capture potential variations in the baseline values and slopes among individuals. Linear mixed-effects models accommodate missing data due to dropout, such that all randomized participants can be included. We will compare participants who complete the study with those who do not and identify potential factors that are associated with dropout {20c}. In the mixed-effects models, we will adjust for time-invariant covariates (age at randomization, sex) and time-varying covariates (muscle strength, lean mass, muscle OXPHOS capacity, VO2max). By including these indices of “muscle quality” as covariates in the models, we will be able to assess whether intervention effects are explained by these effects. To assess other potential factors influencing outcomes, we will also analyze the incremental effect of adding each of the other measured covariates (final dose of NR achieved, final training intensity achieved) to the models.
The primary outcome for our study is the change in muscle strength (quadriceps extension, Z-score) from baseline to 16 weeks. We expect exercise and NR to each produce an increase (relative to control/placebo) with respect to the primary outcome of muscle strength from baseline to week 16. We posit that the effects of exercise and NR in combination will be additive and possibly even synergistic. Despite our strategies to track and optimize adherence, it is possible that the change in Z-score will vary across participants in response to one or both interventions. We will fit a linear model with the outcome of change in Z-score of muscle strength and covariates of treatment arm and baseline Z-score. Using a modification of previous strategies for the analysis of factorial designs, the family-wise Type I error rate is maintained at < 5% by using a two-stage approach: a Holm-Bonferroni correction is applied in Stage 1, and a single contrast is pre-specified in Stage 2 [43]. In Stage 1, we will test if the mean Z-score in each of the 3 active treatment arms differs from control (no-exercise + placebo). In Stage 2, if the exercise + NR arm differs from the control arm, we will then compare exercise + NR to exercise alone, to determine whether NR increases mean Z-score beyond the effect of exercise alone; 95% confidence intervals will be determined for each effect of interest.
In secondary analyses, we will seek to identify disease covariates and physiological factors (e.g., low muscle OXPHOS capacity at baseline) that are associated with treatment response [44]. With respect to additional outcomes, we anticipate that strength in other muscle groups, lower extremity muscle mass (measured via DXA), and muscle OXPHOS capacity (measured via CrCEST), will all increase in response to both interventions, and in statistical models will demonstrate independent contributions to the observed increases in muscle strength (primary outcome) and other key outcomes. In addition, blood metabolomics will show correlates of increased OXPHOS capacity (e.g., decreased acylcarnitine related to improved fatty acid oxidation capacity, organic acid profiles consistent with increased TCA cycle anaplerosis, and increased CoA species). Even if we do not detect changes in response to either of these interventions, our mechanistic assessments will produce an integrated understanding of the factors associated with low muscle mass, strength and OXPHOS in HCT survivors. The safety data will be an ancillary benefit to guide clinicians caring for HCT survivors who may be considering taking this supplement. The primary analysis will be repeated with each secondary outcome. Additional models will explore whether change from baseline can be explained by either baseline demographic or clinical covariates (age at randomization, sex, key clinical characteristics such as anthracycline and/or steroid exposure, age at HCT, TBI, GvHD grades II-IV, interval since HCT, and hypogonadism) or features of the intervention (final dose of NR, final training intensity in Mets per week). We will explore interactions between key variables and treatment arm. With respect to sex, we will include sex by treatment interactions in the model and explore the results of individual sex-stratified analyses, though we may lack adequate power to detect sex-specific differences {12}.
In addition, we expect exercise and NR to each produce an increase (relative to control/placebo) with respect to the secondary outcome of VO2max. We expect that the effects of exercise and NR in combination will be additive, and possibly even synergistic. The effect of sex will also be assessed. The analysis for VO2max Z-score (Aim 2) will be the same as for Aim 1, since both outcomes are expressed as a Z-score. We will fit a linear model with the outcome of change in Z-score of VO2max and covariates of treatment arm and baseline Z-score for VO2max {8} {20a}.
Sample size and power
With the proposed design, we have > 80% power to detect four scenarios of treatment-related changes in mean Z-score of 0.93–1.66 accounting for multiple testing with a family-wise Type I error rate < 5%. Notably, we have 80% power to detect a difference between exercise and exercise + NR (Scenario 1) of 0.73 Z-score. We have demonstrated that increases in muscle group specific strength of up to 100% (absolute amount) over 24 weeks are plausible, depending on the degree of initial deficits and muscle group tested [45]. In a 12-week pilot study, a mean increase of quadriceps strength of 14% was observed [46]. In a previous meta-analysis in adults, effect sizes of 0.53–0.79 were reported with supervised aerobic exercise [47, 48]. We anticipate that increases in the present study may be even larger for several reasons. First, these relatively young participants will have substantial muscle deficits, thus there is a clear opportunity to demonstrate benefit. Our preliminary data indicate muscle deficits in HCT survivors, with low Z-scores for muscle area (mean -0.9, SD 1.4). Also, we have chosen to intervene at the soonest potential opportunity post-HCT, thus we expect muscle deficits will be less established and participants will be more responsive to the interventions. Our team has considerable experience in supervising, individualizing, and adjusting the exercise regimen to achieve maximum benefit. Also, we will randomize 80 participants to achieve 64 (n = 16 in each of 4 arms) with complete data (i.e., conservatively allowing 20% attrition) {14}.
Discussion
To our knowledge, our study represents the first intervention clinical trial to use combination of exercise and NR in AYA HCT survivors to address sarcopenia. Additionally, this trial will set the stage for the development of future studies that leverage the promise of a mitochondrial bioenergetic approach to the metabolic complications of cancer therapy. With mechanistic insights from this study, we can pursue trials to enhance clinically relevant metabolic function (e.g., glucose homeostasis that impacts risk for diabetes mellitus) in affected individuals after cancer therapy. The growing population of AYA cancer survivors (estimated to be 633,000 in the U.S. alone), makes the development of such interventions imperative, to optimize their long-term health in decades after completion of their cancer treatment [49].
We have considered a number of potential limitations and barriers that may arise in performing this study and have developed solutions in anticipation. It is possible that despite having an adequate number of potentially eligible participants (anticipated N = 185), we will not accrue the 80 HCT survivors over the course of the study. To address this concern, we will review and report accrual quarterly, and if recruitment is slower than anticipated, we will take several steps. Specifically, we will: 1) work with the primary treatment team to perform as many study procedures as possible during regularly scheduled clinic visit days; 2) minimize the time required for study measurements (total time commitment 6 h); and 3) provide compensation ($200) to each participant for each visit. If necessary, we can also expand the age range to 12-39y, with continued stratification of randomization by pediatric/adult status since the results may not vary significantly for those in peri-pubertal stages and those in late young adulthood.
With regards to ensuring adherence and compliance to the intervention, our team will be monitoring study participants in real-time via regular telephone check-ins and for those participating in exercise, via remote oversight, and thus be well-positioned to troubleshoot any barriers that may emerge during the conduct of the study. For NR/placebo, the investigative team will interview the adherence and intervene where necessary to ensure consistent administration of NR/placebo. Barriers to adherence can be reviewed at weekly check-ins and the interim visit. With regards to the exercise intervention, in-person orientation at the study site to exercise training using the in-home equipment will occur on the day of randomization, thus any functional or logistical limitations can be addressed in real time. To improve retention, we will offer participants randomized to the “no exercise” condition the opportunity to participate in in-home training after completion of the study {30}. Subjects who withdraw from the study will be recommended to update their medical history/concomitant medications, undergo physical exam, measure vital signs, test their clinical labs, get a DXA scan, get a 60 min MRI, perform a CPET, provide used/unused NR or placebo, and review adverse events as the early termination visit if both safe and feasible {18b}. In our team’s previous two studies of remotely supervised exercise interventions, we have demonstrated adherence rates exceeding 80% using similar procedures {11c}.
We will apply the insights gained from this trial to develop individualized, evidence-supported precision initiatives that will reduce chronic disease burden in high-risk cancer survivors. Our proposed trial is an important first step and will guide future interventions. We may also find that the benefits that accrue to skeletal muscle in response to skeletal muscle-focused interventions are recapitulated in other organ systems. For example, NAD+ precursors have impact on cardiac muscle, tissue that can also be adversely affected in cancer patients; in a preclinical model of dilated cardiomyopathy, NAD+ precursor attenuated the development of heart failure [50]. Additional metrics, such as circulatory power at peak exercise, can be measured to provide a non-invasive estimate of the circulatory system (i.e., heart rate, stroke volume, and systemic blood pressure) at peak exercise to explore such potential benefits.
Trial status
The study recruitment has not commenced. The approximate date of the final participant recruitment is January 2025.
Availability of data and materials
Study enrollment has not yet commenced; thus, there is no available data or materials.
Abbreviations
- AE:
-
Adverse event
- ATP:
-
Adenosine triphosphate
- AYA:
-
Adolescents and young adults
- CBC:
-
Complete blood count
- CHOP:
-
Children’s Hospital of Philadelphia
- CMP:
-
Complete metabolic panel
- CrCEST:
-
Creatine chemical exchange saturation transfer
- COH:
-
City of Hope Medical Center
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- CVD:
-
Cardiovascular disease
- DXA:
-
Dual energy x-ray absorptiometry
- eGFR:
-
Estimated glomerular filtration rate
- FDA:
-
Food and Drug Administration
- GRAS:
-
Generally recognized as safe
- GvHD:
-
Graft versus host disease
- HCT:
-
Hematopoietic cell transplant
- HbA1c:
-
Hemoglobin A1c
- HUP:
-
Hospital of the University of Pennsylvania
- MRI:
-
Magnetic resonance imaging
- NAD+ :
-
Nicotinamide adenine dinucleotide
- NIH:
-
National Institutes of Health
- NR:
-
Nicotinamide riboside
- OXPHOS:
-
Oxidative phosphorylation
- PGC-1α:
-
PPARG coactivator 1 alpha
- RPE:
-
Borg Rate of Perceived Exertion
- RR:
-
Respiratory rate
- SAE:
-
Serious adverse event
- SMURF:
-
Safety Monitoring Uniform Report Form
- ULN:
-
Upper limit of normal
- VO2max:
-
Peak oxygen uptake/maximal aerobic capacity
References
Penack O, Peczynski C, Mohty M, Yakoub-Agha I, Styczynski J, Montoto S, et al. How much has allogeneic stem cell transplant–related mortality improved since the 1980s? A retrospective analysis from the EBMT. Blood Adv. 2020;4(24):6283–90.
Seneviratne AK, Wright C, Lam W, Lipton JH, Michelis FV. Comorbidity profile of adult survivors at 20 years following allogeneic hematopoietic cell transplantation. Eur J Haematol. 2021;106(2):241–9.
Armenian SH, Xiao M, Berano Teh J, Lee B, Chang HA, Mascarenhas K, et al. Impact of sarcopenia on adverse outcomes after allogeneic hematopoietic cell transplantation. JNCI J Natl Cancer Inst. 2019;111(8):837–44.
Baker KS, Chow E, Steinberger J. Metabolic syndrome and cardiovascular risk in survivors after hematopoietic cell transplantation. Bone Marrow Transpl. 2012;47(5):619–25 2011/06/07 ed.
Mostoufi-Moab S, Ginsberg JP, Bunin N, Zemel BS, Shults J, Thayu M, et al. Body composition abnormalities in long-term survivors of pediatric hematopoietic stem cell transplantation. J Pediatr. 2012;160(1):122–8 2011/08/16 ed.
Zhang FF, Saltzman E, Must A, Parsons SK. Do childhood cancer survivors meet the diet and physical activity guidelines? A review of guidelines and literature. Int J Child Health Nutr. 2012;1(1):44–58.
Smith SR, Haig AJ, Couriel DR. Musculoskeletal, neurologic, and cardiopulmonary aspects of physical rehabilitation in patients with chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21(5):799–808.
Yoshino J, Baur JA, Imai SI. NAD(+) Intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab. 2018;27(3):513–28.
Amjad S, Nisar S, Bhat AA, Shah AR, Frenneaux MP, Fakhro K, et al. Role of NAD+ in regulating cellular and metabolic signaling pathways. Mol Metab. 2021;49:101195.
Frederick DW, Loro E, Liu L, Davila A Jr, Chellappa K, Silverman IM, et al. Loss of NAD homeostasis leads to progressive and reversible degeneration of skeletal muscle. Cell Metab. 2016;24(2):269–82.
van de Weijer T, Phielix E, Bilet L, Williams EG, Ropelle ER, Bierwagen A, et al. Evidence for a direct effect of the NAD+ precursor acipimox on muscle mitochondrial function in humans. Diabetes. 2015;64(4):1193–201.
McCormack S, Polyak E, Ostrovsky J, Dingley SD, Rao M, Kwon YJ, et al. Pharmacologic targeting of sirtuin and PPAR signaling improves longevity and mitochondrial physiology in respiratory chain complex I mutant Caenorhabditis elegans. Mitochondrion. 2015;22:45–59.
Mitchell SJ, Bernier M, Aon MA, Cortassa S, Kim EY, Fang EF, et al. Nicotinamide improves aspects of healthspan, but not lifespan, Mice. Cell Metab. 2018;27(3):667-676 e4.
Rajman L, Chwalek K, Sinclair DA. Therapeutic potential of NAD-boosting molecules: the in vivo evidence. Cell Metab. 2018;27(3):529–47.
Benyo Z, Gille A, Kero J, Csiky M, Suchankova MC, Nusing RM, et al. GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing. J Clin Invest. 2005;115(12):3634–40.
Conze DB, Crespo-Barreto J, Kruger CL. Safety assessment of nicotinamide riboside, a form of vitamin B3. Hum Exp Toxicol. 2016; Available from: https://www.ncbi.nlm.nih.gov/pubmed/26791540
Dellinger RW, Santos SR, Morris M, Evans M, Alminana D, Guarente L, et al. Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD(+) levels in humans safely and sustainably: a randomized, double-blind, placebo-controlled study. NPJ Aging Mech Dis. 2017;3:17.
Airhart SE, Shireman LM, Risler LJ, Anderson GD, Nagana Gowda GA, Raftery D, et al. An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS One. 2017;12(12):e0186459.
Dollerup OL, Christensen B, Svart M, Schmidt MS, Sulek K, Ringgaard S, et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am J Clin Nutr. 2018;108(2):343–53.
Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD(+) in healthy middle-aged and older adults. Nat Commun. 2018;9(1):1286.
Gale EA, Bingley PJ, Emmett CL, Collier T, European Nicotinamide Diabetes Intervention Trial G. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet. 2004;363(9413):925–31.
Libri V, Yandim C, Athanasopoulos S, Loyse N, Natisvili T, Law PP, et al. Epigenetic and neurological effects and safety of high-dose nicotinamide in patients with Friedreich’s ataxia: an exploratory, open-label, dose-escalation study. Lancet. 2014;384(9942):504–13.
Elhassan YS, Kluckova K, Fletcher RS, Schmidt MS, Garten A, Doig CL, et al. Nicotinamide riboside augments the aged human skeletal muscle NAD(+) metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Rep. 2019;28(7):1717-1728 e6.
Costford SR, Brouwers B, Hopf ME, Sparks LM, Dispagna M, Gomes AP, et al. Skeletal muscle overexpression of nicotinamide phosphoribosyl transferase in mice coupled with voluntary exercise augments exercise endurance. Mol Metab. 2018;7:1–11.
Brouwers B, Stephens NA, Costford SR, Hopf ME, Ayala JE, Yi F, et al. Elevated nicotinamide phosphoribosyl transferase in skeletal muscle augments exercise performance and mitochondrial respiratory capacity following exercise training. Front Physiol. 2018;9:704.
Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The Physical Activity Guidelines for Americans. JAMA. 2018; Available from: https://www.ncbi.nlm.nih.gov/pubmed/30418471
Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;9(346):e7586.
Cawthon PM, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA, et al. Establishing the link between Lean Mass and grip strength cut points with mobility disability and other health outcomes: Proceedings of the sarcopenia definition and outcomes consortium conference. J Gerontol A. 2019;75(7):1317–23.
McKay MJ. Normative reference values for strength and flexibility of 1,000 children and adults. Neurology. 2017;88(1):36–43.
Haris M, Nanga RPR, Singh A, Cai K, Kogan F, Hariharan H, et al. Exchange rates of creatine kinase metabolites: feasibility of imaging creatine by chemical exchange saturation transfer MRI. Nmr Biomed. 2012;25(11):1305–9.
Kogan F, Haris M, Debrosse C, Singh A, Nanga RP, Cai K, et al. In vivo chemical exchange saturation transfer imaging of creatine (CrCEST) in skeletal muscle at 3T. J Magn Reson Imaging. 2014;40(3):596–602.
Kogan F, Hariharan H, Reddy R. Chemical Exchange Saturation Transfer (CEST) imaging: description of technique and potential clinical applications. Curr Radiol Rep. 2013;1(2):102–14 2013/06/05 ed.
DeBrosse C, Nanga RP, Wilson N, D’Aquilla K, Elliott M, Hariharan H, et al. Muscle oxidative phosphorylation quantitation using creatine chemical exchange saturation transfer (CrCEST) MRI in mitochondrial disorders. JCI Insight. 2016;1(18):e88207.
Schur GM, Dunn J, Nguyen S, Dedio A, Wade K, Tamaroff J, et al. In vivo assessment of OXPHOS capacity using 3 T crcest MRI in Friedreich’s ataxia. J Neurol. 2021;269(5):2527–38.
Lanfranconi F, Pollastri L, Ferri A, Fraschini D, Masera G, Miserocchi G. Near infrared spectroscopy (NIRS) as a new non-invasive tool to detect oxidative skeletal muscle impairment in children survived to acute lymphoblastic leukaemia. PLoS One. 2014;9(6):e99282.
U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans 2nd edition. Washington, DC: U.S.; 2018 [cited 17 Feb 2019]. Available from: https://health.gov/paguidelines/second-edition/pdf/Physical_Activity_Guidelines_2nd_edition.pdf
Pina IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, et al. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107(8):1210–25.
American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Philadelphia: Walters Kluwer; 2017.
Ekelund U. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet. 2016;388(10051):1302–10.
U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion. (2018). 2018 Physical Activity Guidelines Advisory Committee Scientific Report. https://health.gov/paguidelines/second-edition/report/. Accessed 17 Feb 2019.
Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016;10(7):12948.
Food ECSC on. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Levels of Nicotinic Acid and Nicotinamide (Niacin). 2018 Apr 2; Available from: http://europa.eu.int/comm/food/fs/sc/scf/index_en.html
Korn EL, Freidlin B. Non-factorial analyses of two-by-two factorial trial designs. Clin Trials. 2016;13(6):651–9.
Stephens NA, Brouwers B, Eroshkin AM, Yi F, Cornnell HH, Meyer C, et al. Exercise response variations in skeletal muscle PCr recovery rate and insulin sensitivity relate to muscle epigenomic profiles in individuals with type 2 diabetes. Diabetes Care. 2018;41(10):2245–54.
Huang TT, Ness KK. Exercise interventions in children with cancer: a review. Int J Pediatr. 2011;27(2011):e461512.
Smith WA, Ness KK, Joshi V, Hudson MM, Robison LL, Green DM. Exercise training in childhood cancer survivors with subclinical cardiomyopathy who were treated with anthracyclines. Pediatr Blood Cancer. 2013. 2013; Available from: https://www.ncbi.nlm.nih.gov/pubmed/24623535
Fuller JT, Hartland MC, Maloney LT, Davison K. Therapeutic effects of aerobic and resistance exercises for cancer survivors: a systematic review of meta-analyses of clinical trials. Br J Sports Med. 2018;52(20):1311 2018/03/20 ed.
Stout NL, Baima J, Swisher A, Winters-Stone KM, Welsh J. A systematic review of exercise systematic reviews in the cancer literature. (2005–2017). PM R. 2017;9(9 Suppl 2):S347-84.
Health Problems Common among AYA Cancer Survivors - National Cancer Institute. 2020 [cited 29 Mar 2022]. Available from: https://www.cancer.gov/news-events/cancer-currents-blog/2020/aya-cancer-survivors-health-problems
Diguet N, Trammell SAJ, Tannous C, Deloux R, Piquereau J, Mougenot N, et al. Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circulation. 2018;137(21):2256–73.
Acknowledgements
The authors would like to thank the study teams for supporting the study and philanthropic supporters for making this work possible.
Funding
This study is supported by the National Institutes of Health/National Cancer Institute (NIH/NCI) under Award Number R01CA254955 (PIs: Mostoufi-Moab, McCormack). Funding for this trial covers salaries and wages (years 1–5); fringe benefits (years 1–5); personnel costs (years 1–5); materials and supplies (years 1–5); facilities and administrative costs (years 1–5); and publication costs (year 5). An external grant (Richard and Sheila Sanford Endowed Chair in Pediatric Oncology Fund) was used to cover part of the personnel and equipment costs for year 1. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH {4}. The funding source had no role in the study design and will not have any role during the analyses, interpretation of the data, and decision to submit results. {5c}.
Author information
Authors and Affiliations
Contributions
MS: study coordination, study concept and design, manuscript preparation, review and revision. SHA: co-investigator, study concept and design, manuscript preparation, review and revision. RB: study concept and design, manuscript review and revision. KL: study concept and design, manuscript preparation, review and revision. KN: co-investigator, study concept and design, manuscript review and revision. MP: co-investigator, study concept and design, manuscript preparation, review and revision. LL: study coordination, manuscript preparation, review and revision. SM: study coordination, manuscript preparation, review and revision. KW: study coordination, manuscript preparation, review and revision. AD: study coordination, manuscript preparation, review and revision. TG: study coordination, manuscript preparation, review and revision. IH: study coordination, manuscript preparation, review and revision. KL: co-investigator, study concept and design, manuscript preparation, review and revision. JB: co-investigator, study concept and design, manuscript preparation, review and revision. SM: principal investigator, study concept and design, manuscript review and revision. SMM: corresponding and principal investigator, study concept and design, manuscript preparation, review and revision. All authors have read and approved the final version of this manuscript. All authors have approved the submitted version and have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature {5a}. No professional writers were used in writing the manuscript {31b}.
Authors’ information {5d}
Principal investigator and research physicians: Saro H. Armenian, Kirsten Ness, Mary Putt, Kimberly Lin, Joseph Baur, Shana McCormack, and Sogol Mostoufi-Moab. Their roles include but are not limited to the preparation of protocol and revisions, the design of the study concept, publication of study reports, and organization of the overall study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol, CHOP and COH informed consent forms, recruitment materials, and other requested documents have been reviewed and approved by the Children’s Hospital of Philadelphia Institutional Review Board with respect to scientific content and compliance with applicable research and human subjects’ regulations {24}. The study protocol has undergone full external peer review by the National Health Institute/National Cancer Institute (NIH/NCI) as part of the peer review process. Subsequent to initial review and approval, Children’s Hospital of Philadelphia Institutional Review Boards will review the protocol at least annually. Any modifications to the protocol or the informed consent form that may include but are not limited to the procedure of the study, the study design, the conduct of the study, and the changes to the study staff members, will be made through a formal amendment submitted through and approved by the site IRB {25}. The written informed consent will be obtained from a parent or guardian for participants under 18 years old. The written informed consent will be obtained from the participants who are 18 years old or older. The protocol is the 4th edition and was last updated in February 22nd, 2022 {3}.
Consent for publication
Not applicable as study enrollment has not commenced.
Competing interests
There is no competing interest to declare on the part of any named author {28}.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Appendix II. Informed Consent Form {32}.
Appendix
Appendix
Data and Safety Monitoring Board charter and responsibilities {21a}
The Data and Safety Monitoring Board (DSMB) will act in an advisory capacity to the CHOP IRB to monitor participant safety, data quality and evaluate the progress of the study. It includes Scott Baker (Chair), Wassim Chemaitilly, Bonnie Ky, and Garry Cutter {5d}.
DSMB responsibilities
The DSMB responsibilities are to:
-
review the research protocol, informed consent documents and plans for data safety and monitoring;
-
evaluate the progress of the trial, including periodic assessments of data quality and timeliness, recruitment, accrual and retention, participant risk versus benefit, and other factors that can affect study outcome;
-
consider factors external to the study when relevant information becomes available, such as scientific or therapeutic developments that may have an impact on the safety of the participants or the ethics of the trial;
-
review study performance, make recommendations and assist in the resolution of problems reported by the Principal Investigator (s);
-
protect the safety of the study participants;
-
make recommendations to the Principal Investigators, the IRB, and, if required, to the Food and Drug Administration (FDA) concerning continuation, termination or other modifications of the trial based on the observed beneficial or adverse effects of the supplement under study; and
-
ensure the confidentiality of the study data and the results of monitoring.
The DSMB will discharge itself from its duties when the last participant completes the study.
Membership
The DSMB will include (1) a pediatric oncologist with expertise in bone marrow transplantation who will serve as Chairperson, (2) pediatric endocrinologist, (3) a statistician, and (4) a cardiologist with expertise in cardiac late effects after cancer therapy. In the event of a “tie,” the DSMB Chair (pediatric oncologist) will make the determination.
Board process
At the first meeting, prior to subject enrollment, the DSMB will discuss the protocol, suggest any needed modifications, and establish guidelines to study monitoring by the Board. The DSMB Chairperson in consultation with the Principal Investigators will prepare the agenda to address the review of study materials, modifications to the study protocol and informed consent document, initiation of the trial, reporting of adverse events, statistical analysis plan including stopping rules, etc.
Meetings of the DSMB will be held initially every 6 months at the discretion of the Chairperson and/or the PIs and IRB; frequency of meetings may be modified as per the initial experience. An emergency meeting of the DSMB may be called at any time by the Chair other others should participant safety questions or other unanticipated problems arise.
Meetings are closed to the public because discussions may address confidential participant data. Meetings are attended by the Principal Investigators and members of their staff. Meetings may be convened as conference calls as well as in-person.
Meeting format
DSMB meetings will consist of open sessions and, if needed, closed sessions. Discussion held in all sessions is confidential. The Principal Investigators and key members of the study team attend the open sessions. Open session discussion will focus on the conduct and progress of the study, including participant accrual, protocol compliance, and problems encountered. Unblinded data are not presented in the open session.
Any closed session, if needed, will be attended by the DSMB members. The study statistician may be present, at the request of the DSMB. Any unblinded data, if necessary, are presented during the closed session.
Each meeting must include a recommendation to continue or to terminate the study and whether the DSMB has any concerns about participant safety made by a formal DSMB majority or unanimous vote. Should the DSMB decide to issue a termination recommendation, the full vote of the DSMB is required.
A recommendation to terminate the study may be made by the DSMB at any time by majority vote. The Chair should provide such a recommendation to the PIs immediately by telephone and email.
Meeting materials
DSMB interim report templates will be prepared by the study staff to be reviewed by the DSMB members. Format and content of the reports should be finalized and approved at the initial DSMB meeting, although changes throughout the trial may be requested by the Board.
The reports will list and summarize safety data and describe the status of the study. Open session reports generally include administrative reports by site that describe participants screened, enrolled, completed, and discontinued, as well as baseline characteristics of the study population. Other general information on study status may also be presented. Listings of adverse events and serious adverse events as well as any other information requested by the DSMB may also be in the open session report, but none of the data will be presented in an unblinded manner. In the closed session report, listings of adverse events and serious adverse events as well as any other information will be reported grouped by treatment arm, remaining blinded to details of the intervention. The DSMB may direct additions and other modifications to the reports on a one-time or continuing basis.
Reports from the DSMB
A formal report containing the meeting minutes and recommendations for continuation or modifications of the study will be prepared by the DSMB Chairperson. The draft report will be sent to the DSMB members for review and approval, and then sent to the Principal Investigators. It is the responsibility of the Principal Investigators to distribute the DSMB recommendation to all co-investigators and to ensure that copies are submitted to all the IRBs associated with the study.
Confidentiality
All materials, discussions and proceedings of the DSMB are completely confidential. Members and other participants in DSMB meetings are expected to maintain confidentiality.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Song, M., Armenian, S.H., Bhandari, R. et al. Exercise training and NR supplementation to improve muscle mass and fitness in adolescent and young adult hematopoietic cell transplant survivors: a randomized controlled trial {1}. BMC Cancer 22, 795 (2022). https://doi.org/10.1186/s12885-022-09845-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09845-1