Abstract
Background
Osimertinib is a standard first-line treatment for advanced non-small-cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) mutations. Although malignant pleural effusion (PE) is a common clinical problem in NSCLC, information about the efficacy of osimertinib in patients with PE is limited, especially regarding its efficacy in EGFR T790M-negative patients with PE remains unclear.
Methods
We retrospectively reviewed the medical records of patients with NSCLC harboring EGFR mutations who were treated with osimertinib in our institution between May 2016 and December 2020.
Results
A total of 63 patients with EGFR mutated NSCLC were treated with osimertinib; 33 (12 with PE) had no EGFR T790M mutation, while 30 (12 with PE) had EGFR T790M mutation. In EGFR T790M-negative NSCLC, the progression-free survival (PFS) of the patients with PE was comparable to that of the patients without PE (median PFS 19.8 vs. 19.8 months, p = 0.693). In EGFR T790M- positive NSCLC, the PFS and overall survival (OS) of the patients with PE were significantly shorter than those of the patients without PE (median PFS 16.8 vs. 8.3 months, p = 0.003; median OS 44.9 vs. 14.2 months, p = 0.007). In the multivariate analysis, the presence of PE was independently associated with shorter PFS and OS in EGFR T790M-positive NSCLC patients, but not EGFR T790M-negative patients.
Conclusions
These data suggest the efficacy of osimertinib may differ between EGFR T790M-positive and -negative NSCLC patients with PE.
Similar content being viewed by others
Introduction
Malignant pleural effusion (PE) is a common clinical problem in non-small-cell lung cancer (NSCLC). Previous studies have reported that malignant PE is present in 15% to 20% of patients with NSCLC, and that it is associated with a poor prognosis in patients with advanced NSCLC [1,2,3]. Even minimal PE (defined as thickness < 10 mm on chest computed tomography [CT] scan) is an independent prognostic factor of a worse survival among patients with NSCLC, and the survival of patients with minimal PE is as short as that of patients with malignant PE in stage IV disease [3].
The introduction of epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) into the treatment paradigm of NSCLC harboring EGFR mutations dramatically improved clinical outcomes. For advanced NSCLC patients with EGFR-activating mutations involving deletions in exon 19 (exon 19 deletion) or a substitution mutation in exon 21, specifically Leu858Arg (L858R), EGFR-TKIs are the standard first-line therapies. The presence of EGFR mutations has been reported to be significantly associated with PE and may play an important role in the formation of malignant PE [4, 5]. The studies examining EGFR-mutated NSCLC patients treated with erlotinib, first-generation EGFR-TKIs, have shown that the presence of malignant PE is associated with a shorter progression-free survival (PFS) and overall survival (OS) [6, 7].
Osimertinib is a third-generation EGFR-TKI that potently and selectively inhibits both EGFR-TKI-sensitizing and T790M-resistant mutations. The phase III study (AURA 3) showed that osimertinib improved PFS over platinum combined chemotherapy in patients with EGFR T790M-positive NSCLC whose disease had progressed after EGFR-TKI treatment [8, 9]. In Japan, osimertinib has been approved for NSCLC harboring an EGFR Thr790Met mutation in exon 20 (T790M mutation) since 2016. The phase III study (FLAURA) found that osimertinib led to a significant improvement in the PFS and OS over first-generation EGFR-TKIs in untreated advanced NSCLC patients with EGFR mutation [10, 11]. Since then, osimertinib has become a standard first-line treatment for advanced NSCLC harboring EGFR mutations.
A few retrospective studies have investigated the efficacy of osimertinib in patients with EGFR T790M-positive NSCLC with PE [12,13,14]. Patients with PE were reported to have a significantly shorter median time to treatment failure (TTF) than those without PE, as well as a shorter median OS [12]. Conversely, it has been shown that the median PFS with osimertinib treatment did not significantly differ between patients with and without PE [13, 14]. Therefore, the efficacy of osimertinib in EGFR T790M-positive patients with PE remains unclear.
Recently, osimertinib is usually administered as a first-line treatment to patients with EGFR T790M-negative NSCLC, and the efficacy of osimertinib in EGFR T790M-negative patients with PE is unknown. Thus, we conducted a retrospective study to investigate the efficacy of osimertinib in the treatment of EGFR T790M-negative NSCLC patients with PE.
Methods
Patients
We retrospectively reviewed the medical records of all patients, who were diagnosed with NSCLC harboring EGFR mutation and who were treated with osimertinib in Tokushima University Hospital between May 2016 and December 2020. The end of the follow-up period was June 30, 2021.
This study was performed in accordance with the Declaration of Helsinki and was approved by the institutional review board.
Assessments
We used either an Oncomine Dx Target Test or Cobas EGFR Mutation Test version 2 [15] for the EGFR mutations analyses of tissue or cytology samples at the time of the diagnosis. After treatment with first- or second-generation EGFR-TKIs, patients were confirmed to have the EGFR T790M mutation in tissue, cytology or blood samples using the Cobas EGFR Mutation Test kit version 2. These tests were performed at SRL, Inc. (Tokyo, Japan) in a clinical practice setting.
Patients with positive pleural fluid cytology results, pleural effusion requiring drainage, or presenting with multiple pleural nodules and nodular pleural thickening with pleural fluid on a CT scan was diagnosed as having PE. According to the thickness of the pleural fluid (judged relative to a criterion of 10 mm on chest CT scans), patients with PE were classified into two groups: minimal PE (thickness < 10 mm) and malignant PE [3]. We diagnosed the presence of PE before beginning with osimertinib treatment.
The tumor response to osimertinib was categorized as either complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), or not evaluated (NE), according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [16]. PFS was defined as the period from the start of treatment with osimertinib to the date of clinical or radiographic disease progression or death from any cause, and in the absence of confirmation of disease progression or death data were censored at the last date the patient was known to be alive. OS was defined as the period from the commencement of osimertinib treatment to the date of death from any cause, and in the absence of confirmation of death data were censored at the last date the patient was known to be alive.
Statistical analyses
Baseline characteristics were compared between patients with and without PE using the Mann-Whitney U test or Fisher’s exact test for categorical variables. The PFS and OS were estimated by the Kaplan-Meier method, and their statistical differences were analyzed by the log-rank test. Univariate and multivariate analyses were performed using Cox proportional hazards regression models. In these analyses, p-values of < 0.05 were considered to indicate a statistically significant difference. The statistical analyses were performed using EZR version 1.54 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [17].
Results
Patient disposition and characteristics
A total of 63 patients with EGFR mutated NSCLC who were treated with osimertinib were identified (Additional file 1: Figure A1). Among 63 patients, 33 patients had no EGFR T790M mutation (with PE, n = 12; maximum thickness < 10 mm, n = 4; maximum thickness ≥ 10 mm, n = 8; cytologically confirmed malignant cells, n = 6). Thirty patients had EGFR T790M mutation (with PE, n = 12; maximum thickness < 10 mm, n = 7; maximum thickness ≥ 10 mm, n = 5; cytologically confirmed malignant cells, n = 3) including a patient with both L858R and T790M (de novo) at the time of diagnosis. The baseline characteristics are shown in Table 1. Among 33 EGFR T790M-negative patients, one patient relapsed after curative chemoradiotherapy, three relapsed after adjuvant chemotherapy, and EGFR mutations was confirmed in one patient after the start of platinum-based chemotherapy. One patient discontinued first-generation EGFR-TKI treatment because of adverse events, and received osimertinib without confirmation of the EGFR T790M mutation status. The baseline characteristics of the patients with and without PE did not differ to a statistically significant extent in EGFR T790M-positive or negative patients.
Efficacy of osimertinib in the patients with PE
The objective response rate (ORR) in the patients with PE was lower than that in the patients without PE in both EGFR T790M-negative (58.3% vs. 71.4%, p = 0.443) and in EGFR T790M-positive (66.7% vs. 83.3%, p = 0.290) patients, although the result did not reach statistical significance (Table 2). EGFR T790M-negative patients with and without PE showed a similar disease control rate (DCR) (91.7% vs. 95.2%) (Table 2).
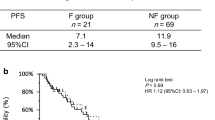
The median follow-up period was 17.1 months (range, 6.9 to 31.1 months) for all EGFR T790M-negative patients and 19.8 months (range, 2.6 to 56.6 months) for all EGFR T790M-positive patients. The median PFS and OS were 19.8 months (95% confidence interval [CI] 9.0 to 25.5) and not reached (NR) (95%CI 29.1 to NR), respectively, in EGFR T790M-negative patients, and 13.1 months (95%CI 9.1 to 19.0) and 30.7 months (95% CI 14.2 to 44.9) in EGFR-T790M positive patients (Fig. 1). In EGFR-T790M negative NSCLC, the PFS and OS of the patients with PE were comparable to those of the patients without PE (median PFS 19.8 vs. 19.8 months, p = 0.693; median OS NR vs. NR, p = 0.712) (Figs. 2A and 3A). In EGFR T790M-positive NSCLC, the PFS and OS of the patients with PE were significantly shorter in comparison to the patients without PE (median PFS 16.8 vs. 8.3 months, p = 0.003; median OS 44.9 vs. 14.2 months, p = 0.007) (Figs. 2B, 3B). In EGFR-mutated patients with PE, the PFS and OS did not significantly differ between the patients with exon 19 deletion and those with L858R (median PFS NR vs. 8.3 months, p = 0.056; median OS 28.9 vs. 25.8 months, p = 0.777).
The multivariate analysis showed that the presence of PE was significantly and independently associated with shorter PFS (hazard ratio [HR] 7.31, 95% CI 2.05 to 26.03, P = 0.002) and OS (HR 4.11, 95% CI 1.08 to 15.62, P = 0.038) in EGFR T790M-positive patients treated with osimertinib, while in EGFR T790M-negative patients, the presence of PE did not significantly influence PFS or OS (Table 3). Only 3 of the 33 EGFR T790M-negative patients had a performance status of 2 or 3; these patients had no disease progression and were alive at the end of the follow-up period. As is well known, in EGFR T790M-negative patients, EGFR mutation (L858R or other) was significantly associated with shorter PFS (hazard ratio [HR] 5.90, 95% CI 1.22 to 28.54, P = 0.027) (Table 3).
Discussion
In current study, we found that in EGFR T790M-negative NSCLC patients treated with osimertinib the PFS of the patients with PE were comparable to that of the patients without PE. On the other hand, the PFS and OS of EGFR T790M-positive patients with PE were significantly shorter in comparison to the patients without PE. The presence of PE was an independent negative predictor affecting the PFS and OS in the patients with EGFR T790M-positive NSCLC, but not those with EGFR T790M-negative NSCLC. To the best of our knowledge, this is the first report to investigate the efficacy of osimertinib in EGFR T790M-negative NSCLC patients with PE.
Osimertinib has been shown to be effective in untreated advanced NSCLC patients with EGFR-TKI-sensitizing mutation without T790M. The median PFS and OS of patients treated with osimertinib were reported to be 18.9 months and 38.6 months, respectively [10, 11]. The median PFS (19.8 months, 95% CI 9.0 to 25.5) in our study was consistent with that in the previous report (FLAURA study). In patients with EGFR T790M-positive NSCLC whose disease had progressed after EGFR-TKI treatment, the median PFS was shown to be 10.1 months [8] and the median OS was 26.8 months [9]. In our study, the median PFS (13.1 months, 95% CI 9.1 to 19.0) and the median OS (30.7 months, 95% CI 14.2 to 44.9) were similar to the results of the phase III AURA3 study [8, 9].
Previous reports showed that the efficacy of gefitinib or erlotinib were limited in patients with PE [6, 7]. In addition, Masuhiro et al. reported that similarly to first-generation EGFR-TKIs, osimertinib monotherapy appears to be less effective in patients with EGFR T790M-positive NSCLC with PE [12], this is consistent with our results in EGFR T790M-positive patients. In contrast, Kawamura et al. and Ohe et al. reported that in EGFR T790M-positive NSCLC treated with osimertinib, PFS did not differ to significant extent between the patients with and without PE [13, 14]. In the study reported by Kawamura et al. [13], because the patients with minimal PE (thickness < 10 mm on CT scan) were included in the group of patients without PE, the difference in PFS according PE status may be diminishing. The PFS of the patients with EGFR T790M mutation that were detected via malignant effusion was significantly shorter than in the patients in whom EGFR T790M mutation were detected by other methods [13]. In the large post-marketing study reported by Ohe et al. [14], osimertinib was effective for EGFR T790M-positive NSCLC, regardless of the PE status. However, the Kaplan–Meier curve for PFS in the patients with PE is slightly lower than that in the patients without PE. In this study, because the diagnosis of PE depended on the clinical judgment of the investigators, the patients with minimal PE may be included in the patients without PE. In our study, in EGFR T790M- positive patients, but not in EGFR T790M-negative patients, the PFS and OS of the patients with minimal PE were as short as those of the patients with malignant PE (Additional file 1: Figure A2, A3).
Vascular endothelial growth factor (VEGF) promotes the development of PE by increasing vascular permeability and promoting angiogenesis, and is a critical mediator in the formation of PE in lung cancer patients [18]. The serum level of VEGF was associated with the VEGF level in PE in NSCLC patients with malignant PE, and the serum level of VEGF was relatively high in the patients with malignant PE [19]. VEGF receptor 2 (VEGFR2) inhibition was reported to enhance the anti-tumor effects of EGFR-TKI in EGFR-mutated NSCLC models by inhibiting not only tumor angiogenesis but also oncogenic signaling in cancer cells, implying a potent role of VEGFR2 signaling in EGFR-mutated NSCLC cell proliferation [20]. The high level of VEGF may reduce the efficacy of EGFR-TKI in the treatment of EGFR-mutated NSCLC. Furthermore, the activation of EGFR signaling can upregulate the production of VEGF in human cancer cells [21], and EGFR and VEGF share a common downstream pathway, suggesting an important role of VEGF in resistance to EGFR-TKIs [22, 23]. In a preclinical study, Naumov et al. reported that EGFR-TKI resistant (primary resistant or T790M positive) cells highly secreted VEGF and that EGFR-TKI resistance could be associated with VEGF elevation in both the tumor cells and host stroma [23]. Thus, the efficacy of osimertinib for patients with PE may depend on the presence of EGFR T790M mutation affecting the production of VEGF.
Several clinical trials have shown that combination therapy of erlotinib plus VEGF/VEGFR blockade improves the PFS or OS in comparison to erlotinib alone in patients with EGFR-positive NSCLC [24,25,26]. However, the efficacy of EGFR-TKI combined with anti-VEGF/VEGFR antibody for patients with PE remains unclear. An exploratory subgroup analysis of the results from the JO25567 study [24] showed that PFS was significantly longer with erlotinib plus bevacizumab (n = 30) than with erlotinib alone (n = 36) in patients with pleural or pericardial effusion (15.4 vs. 5.7 months, HR 0.45, 95% CI 0.25 to 0.82) [27]. These results suggested that combination therapy with EGFR-TKI and anti-VEGF/VEGFR antibody may be a beneficial strategy for EGFR-mutated NSCLC with PE. A single arm phase II trial of osimertinib combined with bevacizumab for patients with EGFR-mutated NSCLC and malignant pleural and/or pericardial effusion is ongoing [28].
The present study was associated with some limitations. Firstly, data were obtained from a single institution and the sample size was relatively small. Thus, the difference in efficacy according to the presence of PE may have not been detected in patients with EGFR T790M-negative NSCLC who were treated with osimertinib. The discrepancy seen between EGFR T790M-positive and EGFR T790M-negative patients may reflect the small sample size rather than a true differential impact. Secondly, because this was a retrospective study, a collection bias may have been present. We used either an Oncomine Dx Target Test or Cobas EGFR Mutation Test for the EGFR mutations analyses at the time of the diagnosis. Many EGFR T790M-negative patients were diagnosed by Oncomine Dx Target Test, while almost all EGFR T790M-positive patients were diagnosed by Cobas EGFR Mutation Test. There might be difference of gene profile including uncommon mutations and compound mutations between EGFR T790M-positive and EGFR T790M-negative patients. Thirdly, the median follow-up period was 17.1 months (range, 6.9 to 31.1) for all EGFR T790M-negative patients. Some EGFR T790M-negative patients had no disease progression and were alive at the end of the follow-up period; thus the follow-up period may have been insufficient. Finally, PE has been reported to be a poor prognostic factor in patients with advanced NSCLC [1,2,3], PE may be a poor prognostic factor rather than a predictive factor for osimertinib.
In conclusion, this study showed that the presence of PE was a negative predictor of the efficacy of osimertinib in EGFR T790M-positive NSCLC patients, but not EGFR T790M-negative NSCLC patients. These findings suggest that among NSCLC patients with PE, the efficacy of osimertinib might differ between EGFR T790M-positive and EGFR T790M-negative patients.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Morgensztern D, Waqar S, Subramanian J, Trinkaus K, Govindan R. Prognostic impact of malignant pleural effusion at presentation in patients with metastatic non-small-cell lung cancer. J Thorac Oncol. 2012;7:1485–9. https://doi.org/10.1097/JTO.0b013e318267223a.
Sugiura S, Ando Y, Minami H, Ando M, Sakai S, Shimokata K. Prognostic value of pleural effusion in patients with non-small cell lung cancer. Clin Cancer Res. 1997;3:47–50.
Ryu JS, Ryu HJ, Lee SN, Memon A, Lee SK, Nam HS, Kim HJ, Lee KH, Cho JH, Hwang SS. Prognostic impact of minimal pleural effusion in non-small-cell lung cancer. J Clin Oncol. 2014;32:960–7. https://doi.org/10.1200/JCO.2013.50.5453.
Zou J, Bella AE, Chen Z, Han X, Su C, Lei Y, Luo H. Frequency of EGFR mutations in lung adenocarcinoma with malignant pleural effusion: Implication of cancer biological behaviour regulated by EGFR mutation. J Int Med Res. 2014;42:1110–7. https://doi.org/10.1177/0300060514539273.
Tsai MF, Chang TH, Wu SG, Yang HY, Hsu YC, Yang PC, Shih JY. EGFR-L858R mutant enhances lung adenocarcinoma cell invasive ability and promotes malignant pleural effusion formation through activation of the CXCL12-CXCR4 pathway. Sci Rep. 2015;5:13574. https://doi.org/10.1038/srep13574.
Atagi S, Goto K, Seto T, Yamamoto N, Tamura T, Tajima K, Inagaki N. Erlotinib for Japanese patients with activating EGFR mutation-positive non-small-cell lung cancer: combined analyses from two Phase II studies. Future Oncol. 2016;12:2117–26. https://doi.org/10.2217/fon-2016-0163.
Taniguchi Y, Tamiya A, Nakahama K, Naoki Y, Kanazu M, Omachi N, Okishio K, Kasai T, Atagi S. Impact of metastatic status on the prognosis of EGFR mutation-positive non-small cell lung cancer patients treated with first- generation EGFR-tyrosine kinase inhibitors. Oncol Lett. 2017;14:7589–96. https://doi.org/10.3892/ol.2017.7125.
Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, Lee CK, Sebastian M, Templeton A, Mann H, Marotti M, Ghiorghiu S, Papadimitrakopoulou VA, AURA3 Investigators. AURA3 Investigators. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med. 2017;376:629–40. https://doi.org/10.1056/NEJMoa1612674.
Papadimitrakopoulou VA, Mok TS, Han JY, Ahn MJ, Delmonte A, Ramalingam SS, Kim SW, Shepherd FA, Laskin J, He Y, Akamatsu H, Theelen WSME, Su WC, John T, Sebastian M, Mann H, Miranda M, Laus G, Rukazenkov Y, Wu YL. Osimertinib versus platinum-pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann Oncol. 2020;31:1536–44. https://doi.org/10.1016/j.annonc.2020.08.2100.
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, Cho BC, Cheng Y, Cho EK, Voon PJ, Planchard D, Su WC, Gray JE, Lee SM, Hodge R, Marotti M, Rukazenkov Y, Ramalingam SS, FLAURA Investigators. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:113–25. https://doi.org/10.1056/NEJMoa1713137.
Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y, Chewaskulyong B, Shah R, Cobo M, Lee KH, Cheema P, Tiseo M, John T, Lin MC, Imamura F, Kurata T, Todd A, Hodge R, Saggese M, Rukazenkov Y, Soria JC, FLAURA Investigators. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med. 2020;382:41–50. https://doi.org/10.1056/NEJMoa1913662.
Masuhiro K, Shiroyama T, Suzuki H, Takata SO, Nasu S, Takada H, Morita S, Tanaka A, Morishita N, Okamoto N, Hirashima T. Impact of Pleural Effusion on Outcomes of Patients Receiving Osimertinib for NSCLC Harboring EGFR T790M. Anticancer Res. 2018;38:3567–71. https://doi.org/10.21873/anticanres.12629.
Kawamura T, Kenmotsu H, Kobayashi H, Omori S, Nakashima K, Wakuda K, Ono A, Naito T, Murakami H, Mori K, Endo M, Takahashi T. Negative impact of malignant effusion on osimertinib treatment for non-small cell lung cancer harboring EGFR mutation. Invest New Drugs. 2020;38:194–201. https://doi.org/10.1007/s10637-019-00808-1.
Ohe Y, Kato T, Sakai F, Kusumoto M, Endo M, Saito Y, Baba T, Sata M, Yamaguchi O, Sakamoto K, Sugeno M, Tamura R, Tokimoto T, Shimizu W, Gemma A. Real-world use of osimertinib for epidermal growth factor receptor T790M-positive non-small cell lung cancer in Japan. Jpn J Clin Oncol. 2020;50:909–19. https://doi.org/10.1093/jjco/hyaa067.
Benlloch S, Botero ML, Beltran-Alamillo J, Mayo C, Gimenez-Capitán A, de Aguirre I, Queralt C, Ramirez JL, Ramón y Cajal S, Klughammer B, Schlegel M, Bordogna W, Chen D, Zhang G, Kovach B, Shieh F, Palma JF, Wu L, Lawrence HJ, Taron M. Clinical validation of a PCR assay for the detection of EGFR mutations in non-small-cell lung cancer: retrospective testing of specimens from the EURTAC trial. PLoS One. 2014;e8951:e8951. https://doi.org/10.1371/journal.pone.0089518.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford D, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–8. https://doi.org/10.1038/bmt.2012.244.
Bradshaw M, Mansfield A, Peikert T. The role of vascular endothelial growth factor in the pathogenesis, diagnosis and treatment of malignant pleural effusion. Curr Oncol Rep. 2013;15:207–16. https://doi.org/10.1007/s11912-013-0315-7.
Fafliora E, Hatzoglou C, Gourgoulianis KI, Zarogiannis SG. Systematic review and meta-analysis of vascular endothelial growth factor as a biomarker for malignant pleural effusions. Physiol Rep. 2016;4:e12978. https://doi.org/10.14814/phy2.12978.
Watanabe H, Ichihara E, Kayatani H, Makimoto G, Ninomiya K, Nishii K, Higo H, Ando C, Okawa S, Nakasuka T, Kano H, Hara N, Hirabae A, Kato Y, Ninomiya T, Kubo T, Rai K, Ohashi K, Hotta K, Tabata M, Maeda Y, Kiura K. VEGFR2 blockade augments the effects of tyrosine kinase inhibitors by inhibiting angiogenesis and oncogenic signaling in oncogene-driven non-small-cell lung cancers. Cancer Sci. 2021;112:1853–64. https://doi.org/10.1111/cas.14801.
Goldman CK, Kim J, Wong WL, King V, Brock T, Gillespie GY. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Mol Biol Cell. 1993;4:121–33. https://doi.org/10.1091/mbc.4.1.121.
Viloria-Petit A, Crombet T, Jothy S, Hicklin D, Bohlen P, Schlaeppi JM, Rak J, Kerbel RS. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: a role for altered tumor angiogenesis. Cancer Res. 2001;61:5090–101.
Naumov GN, Nilsson MB, Cascone T, Briggs A, Straume O, Akslen LA, Lifshits E, Byers LA, Xu L, Wu HK, Jänne P, Kobayashi S, Halmos B, Tenen D, Tang XM, Engelman J, Yeap B, Folkman J, Johnson BE, Heymach JV. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res. 2009;15:3484–94. https://doi.org/10.1158/1078-0432.CCR-08-2904.
Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, Yamamoto N, Hida T, Maemondo M, Nakagawa K, Nagase S, Okamoto I, Yamanaka T, Tajima K, Harada R, Fukuoka M, Yamamoto N. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15:1236–44. https://doi.org/10.1016/S1470-2045(14)70381-X.
Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, Tsunezuka Y, Yamaguchi O, Okada M, Yoshimori K, Nakachi I, Gemma A, Azuma K, Kurimoto F, Tsubata Y, Fujita Y, Nagashima H, Asai G, Watanabe S, Miyazaki M, Hagiwara K, Nukiwa T, Morita S, Kobayashi K, Maemondo M. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20:625–35. https://doi.org/10.1016/S1470-2045(19)30035-X.
Nakagawa K, Garon EB, Seto T, Nishio M, Ponce Aix S, Paz-Ares L, Chiu CH, Park K, Novello S, Nadal E, Imamura F, Yoh K, Shih JY, Au KH, Moro-Sibilot D, Enatsu S, Zimmermann A, Frimodt-Moller B, Visseren-Grul C, Reck M. RELAY Study Investigators. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(1655):1669. https://doi.org/10.1016/S1470-2045(19)30634-5.
Hosomi Y, Seto T, Nishio M, Goto K, Yamamoto N, Okamoto I, Tajima K, Inagaki N, Yamamoto M. Erlotinib plus bevacizumab (EB) versus erlotinib alone (E) as first-line treatment for advanced non-squamous non–small-cell lung cancer (NSCLC) with activating EGFR mutation (mt): JO25567 exploratory subgroup analysis. Ann Oncol. 2015;26(Suppl 9):ix125–47.
Hiranuma O, Uchino J, Yamada T, Chihara Y, Tamiya N, Kaneko Y, Yoshimura K, Takayama K. Rationale and Design of a Phase II Trial of Osimertinib combined with bevacizumab in patients with untreated epidermal growth factor receptor-mutated non-small-cell lung cancer and malignant pleural and/or pericardial effusion (SPIRAL II Study). Clin Lung Cancer. 2019;20:e402–6. https://doi.org/10.1016/j.cllc.2019.02.016.
Acknowledgements
We thank all the patients for participating in this study, their families and the staff of the Department of Respiratory Medicine and Rheumatology of Tokushima University for their advice and assistance.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Hiroshi Nokihara: Conceptualization, Methodology, Formal analysis, Investigation, Methodology, Project administration, and Writing—Original Draft. Hirokazu Ogino: Investigation, Writing—review & editing. Atsushi Mitsuhashi: Investigation, Writing—review & editing. Kensuke Kondo: Investigation, Writing—review & editing. Ei Ogawa: Investigation, Writing—review & editing. Ryohiko Ozaki: Investigation, Writing—review & editing. Yohei Yabuki: Investigation, Writing—review & editing. Hiroto Yoneda: Investigation, Writing—review & editing. Kenji Otsuka: Investigation, Writing—review & editing. Yasuhiko Nishioka: Conceptualization, Methodology, Project administration, Supervision, Project administration, Supervision, Validation, Writing—review & editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by Ethics Committee of Tokushima University. Because of its retrospective nature, the need for written informed consent was waived by the Ethics Committee of Tokushima University. All methods were carried out in accordance with Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
Hiroshi Nokihara has received research grant funding from AstraZeneca K.K, Chugai Pharmaceutical Co., Ltd., Nippon Pfizer Japan Inc., and speaker fees as honoraria from AstraZeneca K.K, Chugai Pharmaceutical Co., Nippon Boehringer lngelheim Co., Ltd., Eli Lilly Japan K.K., outside the submitted work. Yasuhiko Nishioka has received research grant funding from Chugai Pharmaceutical Co., Ltd., Nippon Boehringer lngelheim Co., Ltd., donation from Chugai Pharmaceutical Co., Ltd., Nippon Boehringer lngelheim Co., Ltd., Eli Lilly Japan K.K., Pfizer Japan Inc., and speaker fees as honoraria from AstraZeneca K.K, Chugai Pharmaceutical Co., Ltd., Nippon Boehringer lngelheim Co., Ltd., Pfizer Japan Inc., Eli Lilly Japan K.K. outside the submitted work. The other authors have declared no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nokihara, H., Ogino, H., Mitsuhashi, A. et al. Efficacy of osimertinib in epidermal growth factor receptor-mutated non-small-cell lung cancer patients with pleural effusion. BMC Cancer 22, 597 (2022). https://doi.org/10.1186/s12885-022-09701-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09701-2