Abstract
Purpose
The purpose of this study is to analyze the effect of body mass index (BMI) on patients with concurrent colorectal cancer (CRC) and type 2 diabetes mellitus (T2DM).
Methods
Patients who underwent primary radical CRC surgery from Jan 2011 to Jan 2020 were retrospectively collected. The perioperative information, overall survival (OS) and disease-free survival (DFS) were compared between the higher BMI group and the lower BMI group.
Results
A total of 574 patients with concurrent CRC and T2DM were included in this study. The higher BMI group had higher portion of hypertension (p < 0.01) and coronary heart disease (CHD) (p < 0.01). Furthermore, the higher BMI group had better OS (p = 0.016) and DFS (p = 0.040) than the lower BMI group in stage II CRC. In multivariate analysis, age (OS: p = 0.002, HR = 2.016, 95% CI = 1.307–3.109/ DFS: p = 0.003, HR = 1.847, 95% CI = 1.230–2.772), TNM stage (OS: p < 0.01, HR = 1.667, 95% CI = 1.281–2.169/ DFS: p = 0.001, HR = 1.545, 95% CI = 1.207–1.977), overall complications (OS: p = 0.004, HR = 1.837, 95% CI = 1.218–2.880/ DFS: p = 0.006, HR = 1.783, 95% CI = 1.184–2.686) and major complications (OS: p = 0.005, HR = 2.819, 95% CI = 1.376–5.774/ DFS: p = 0.014, HR = 2.414, 95% CI = 1.196–4.870) were independent factors of OS and DFS. Moreover, BMI (p = 0.019, HR = 0.413, 95% CI = 0.197–0.864) was an independent factor of OS in stage II CRC.
Conclusion
Higher BMI was associated with better OS in diabetic patients with stage II CRC.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer-related deaths worldwide. In 2018, there were nearly 1.8 million new cases of CRC and 881,00 CRC related deaths [1]. The incidence of CRC in China is increasing, especially in economically developed areas [2]. Although there are many treatments for CRC including surgery, radiotherapy, and chemotherapy. Radical surgery is still the cornerstone of the treatment of CRC [3,4,5].
Type 2 Diabetes mellitus (T2DM) is a metabolic disease characterized by high blood sugar caused by insulin deficiency or resistance [6]. The global burden of T2DM is increasing recently. There are nearly 500 million patients with T2DM worldwide, and it is expected to reach to 629 million by 2045 [7, 8]. T2DM is one of the most common causes of death in the world as well.
Body mass index (BMI) is a commonly used scale for assessing obesity [9]. BMI is not only associated with T2DM, but also has an impact on CRC. Previous studies reported conflicting results about the impact of BMI on CRC. Some studies reported that higher BMI decreased the overall survival (OS) and disease-free survival (DFS) of CRC [10, 11], however, other studies reported that BMI did not affect the prognosis on CRC [12, 13].
Ye Z et al. [14] reported that low preoperative BMI was a poor prognostic marker for T2DM patients with gastric cancer. However, the role of preoperative BMI on prognosis of diabetic patients with CRC was unclear. Therefore, the purpose of this study is to analyze the effect of BMI on patients with concurrent CRC and T2DM.
Methods
Study design
This is a retrospective study and the results are reported with consideration to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) [15].
Ethical approval
This study was in accordance with the World Medical Association Declaration of Helsinki. The study was approved by the ethical review board (2021–336), and all patients signed informed consents.
Patients
We retrospectively collected diabetic patients who underwent primary radical CRC surgery in a single clinical center from Jan 2011 to Jan 2020.
Inclusion and exclusion criteria
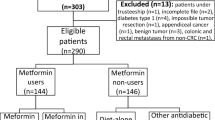
The inclusion criteria were as follows: 1, patients who underwent primary radical CRC surgery; and 2, patients were diagnosed with concurrent CRC and T2DM. According to the inclusion criteria, a total of 702 patients were identified in the current study. The exclusion criteria were as follows: 1, incomplete medical records (n = 103); 2, palliative CRC surgery (n = 25). Finally, 574 patients with concurrent CRC and T2DM were included in the study.
Surgery and follow-up
The surgical resection of CRC was according to the clinical guideline. Total mesorectal excision or complete mesocolic excision was performed, and the pathology confirmed R0 resection. Patients were followed up every three months for the first three years and every six months for the following two years. The follow-up items included computed tomography (CT), magnetic resonance imaging (MRI), carcinoembryonic antigen (CEA) or colonoscopy.
Definitions
Tumor nodes metastasis (TNM) stage was defined according to the AJCC 8thEdition [16]. Complications were defined according to the Clavien-Dindo classification [17], and major complications were defined as ≥ III classification complications including patients who needed surgery, endoscopy or interventional operation. OS was defined as the time from surgery to death or last follow-up time. DFS was defined as the time from surgery to recurrence, death or last follow-up time.
Data collection
The perioperative information was collected from the inpatient system. We collected the perioperative information such as sex, age, BMI, drinking, smoking, coronary heart disease (CHD), family history, tumor location, TNM stage, vessel invasion, perineural invasion, adjuvant therapy, operation time, blood loss, retrieved lymph nodes, overall complications, major complications and hospital stay. The follow-up information including OS and DFS were collected from the outpatient system and telephone interviews.
Statistical analysis
Patients were divided into two groups according to the median of BMI (the higher BMI group and the lower BMI group), and the cut-off of BMI was 23.4 kg/m2. Continuous variables were expressed as the mean ± SD, and independent-sample t test was used to compare the difference between the two groups. Frequency variables were expressed as n (%), and Chi-square test exact test was used. The Kaplan–Meier curve was conducted to compare the difference of BMI on each TNM stage, and cox regression analyses were performed to identify independent predictive factors for OS and DFS. Interaction analyses was conducted in the COX regression model between the baseline information. Data were analyzed using SPSS (version 22.0) statistical software. A bilateral p value of < 0.05 was considered statistically significant.
Results
Baseline information
A total of 574 patients with concurrent CRC and T2DM were included in this study, and the flow chart of inclusion and exclusion was shown in Fig. 1. There were 336 males and 238 females, and the average BMI was 23.6 ± 3.3 kg/m2. The age, tumor site, TNM stage, family history, smoking, drinking, hypertension and CHD were shown in Table 1.
Comparison between higher BMI group and lower BMI group
The BMI was divided in two groups according to the median, and there were 288 patients in the higher BMI group and 286 patients in the lower BMI group. The perioperative information was compared between the two groups. The higher BMI group had higher portion of hypertension (p < 0.01) and CHD (p < 0.01), however, there were no difference of other perioperative information (p > 0.05) (Table 2).
Univariate and multivariate analysis of OS/ DFS
The medium follow-up time was 31 (1–113) months. In univariate analysis, age (p = 0.001, HR = 1.039, 95% CI = 1.016–1.062), BMI (p = 0.049, HR = 0.665, 95% CI = 0.443–0.999), TNM stage (p < 0.01, HR = 1.672, 95% CI = 1.294–2.162), vessel invasion (p < 0.01, HR = 3.517, 95% CI = 1.760–7.026), overall complications (p < 0.01, HR = 2.312, 95% CI = 1.554–3.439) and major complications (p < 0.01, HR = 4.398, 95% CI = 2.278–8.493) were significant risk factors. In multivariate analysis, age (p = 0.001, HR = 2.089, 95% CI = 1.353–3.227), TNM stage (p < 0.01, HR = 1.601, 95% CI = 1.229–2.086), vessel invasion (p = 0.006, HR = 2.759, 95% CI = 1.339–5.683), overall complications (p = 0.003, HR = 1.936, 95% CI = 1.258–2.980) and major complications (p = 0.019, HR = 2.412, 95% CI = 1.157–5.029) were independent factors of OS. Interaction analysis of age and BMI revealed no significant difference (p = 0.374 > 0.05) (Table 3).
In terms of DFS, age (p = 0.002, HR = 1.876, 95% CI = 1.250–2.814), TNM stage (p = 0.001, HR = 1.502, 95% CI = 1.173–1.924), vessel invasion (p = 0.010, HR = 2.515, 95% CI = 1.243–5.087), overall complications (p = 0.004, HR = 1.834, 95% CI = 1.217–2.765) and major complications (p = 0.032, HR = 2.185, 95% CI = 1.070–4.462) were independent factors as well (Table 4).
OS/ DFS in different TNM stages
We conducted Kaplan–Meier curve to analyze the specific effect of BMI on different TNM stages. The higher BMI group had better OS (p = 0.016) and DFS (p = 0.040) than the lower BMI group in terms of stage II CRC, however, no significant difference was found in other TNM stages in terms of OS and DFS (p > 0.05) (Figs. 2 and 3).
Therefore, we conducted univariate and multivariate analysis of OS/ DFS of stage II CRC. As for OS, age (p = 0.025, HR = 2.392, 95% CI = 1.113–5.140), BMI (p = 0.019, HR = 0.413, 95% CI = 0.197–0.864) and major complications (p = 0.046, HR = 3.461, 95% CI = 1.025–11.686) were independent factors of stage II CRC. Interaction analysis of age and BMI revealed no significant difference (p = 0.501 > 0.05) (Table 5). In terms of DFS, age (p = 0.035, HR = 2.069, 95% CI = 1.053–4.066) was an independent prognostic factor of stage II CRC. Interaction analysis of age and BMI revealed no significant difference (p = 0.934 > 0.05). (Table 6).
Discussion
A total of 574 patients with concurrent CRC and T2DM were included in this study. The higher BMI group had higher portion of hypertension and CHD, however, there were no difference of other perioperative information. Furthermore, the higher BMI group had better OS and DFS than the lower BMI group in stage II CRC, however, no significant difference was found in other TNM stages in terms of OS or DFS. Age, TNM stage, overall complications and major complications were independent factors of OS and DFS. Moreover, BMI was an independent factor of OS in stage II CRC.
Higher BMI could increase the risk of CRC [18, 19], and BMI might have an impact on the outcomes and prognosis of CRC surgery [20]. BMI was also related to T2DM and metabolic diseases [21, 22]. Therefore, it is necessary to analyze the exact effect of BMI on diabetic patients with CRC.
However, there were no studies reporting the association of BMI with diabetic CRC patients. Only one study reported the effect of BMI on diabetic patients with gastric cancer [14]. To our knowledge, this is the first study to report the effect of BMI on diabetic patients with CRC.
In this study, we found that the ratio of hypertension and CHD was higher in the higher BMI group compared with the lower BMI group. The probable reason was that BMI was associated with metabolic and cardiovascular diseases [21, 22]. However, there was no difference between the two groups in terms of surgical outcomes. Kwak HD et al. [10] reported that obese patients would cause less lymph nodes harvesting and more blood loss. However, another studies reported that there was no difference in surgical outcomes which was consistent with our study [23, 24]. More studies are needed to analyze the effect of BMI on the surgical outcomes in the future.
The OS and DFS of CRC were affected by some factors including the TNM stage, postoperative complications and age [25,26,27,28]. In this study, we found similar independent factors of OS and DFS.
Although BMI was not an independent factor of OS or DFS, it was found statistically different in univariate analysis. Therefore, we hypnotized that BMI might have potential effects on different TNM stages. Therefore, we analyzed the impact of BMI on different TNM stages. It was found that the higher BMI was associated with better OS and DFS in stage II CRC patients. The mechanism was unclear, and few studies had reported the impact of BMI on different TNM stages. Shahjehan F et al. [29] reported that higher BMI had better OS in stage III and IV CRC patients, and another study reported that higher BMI increased the recurrence rate of stage III CRC patients [30]. The possible reason in this study was that higher BMI patients might have more muscle and fat mass, allowing them to cope with the metabolic demands of tumor progression and treatment [31, 32]. Other studies reported lower BMI was associated with cancer-related cachexia and underlying biology in late stage disease patients, which might cause worse prognosis in lower BMI CRC patients [29]. Among patients with higher BMI and CRC, the survival benefit in higher BMI patients might be related to better nutritional status, more optimized drug therapy, more prominent endothelial progenitor cells, lower thromboxane production, higher ghrelin sensitivity and lower TNF-α [33].
Some limitations were existed in this study. First, this was a single-center retrospective study with a relatively short follow-up time; Second, the number of included diabetic patients in stage I and stage II were small, which might result in selection bias; Third, the clinical course and severity of T2DM were not included, which needed to be analyzed in the future. Fourth, pathological factors (lymphatic l invasion, budding, desmoplastic reaction) were lacking in this study. Therefore, larger sample size and multi-center studies with more detailed patients’ information should be conducted in the following experiments.
In conclusion, higher BMI was associated with better OS in diabetic patients with stage II CRC.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due [The database from our clinical center were relatively private] but are available from the corresponding author on reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Liu HR, Yang P, Han S, et al. The application of enhanced recovery after surgery and negative-pressure wound therapy in the perioperative period of elderly patients with colorectal cancer. BMC Surg. 2021;21(1):336. https://doi.org/10.1186/s12893-021-01331-y.
Ji X, Zhao Y, Zhu X, et al. Outcomes of stereotactic body radiotherapy for metastatic colorectal cancer with oligometastases, oligoprogression, or local control of dominant tumors. Front Oncol. 2021;29(10):595781. https://doi.org/10.3389/fonc.2020.595781.
Tharin Z, Blanc J, Alaoui IC, et al. Influence of first line chemotherapy strategy depending on primary tumor location in metastatic colorectal cancer. J Gastrointest Oncol. 2021;12(4):1509–17. https://doi.org/10.21037/jgo-20-593.
Peng D, Cheng YX, Cheng Y. Improved Overall Survival of Colorectal Cancer under Multidisciplinary Team: A Meta-Analysis. Biomed Res Int. 2021;1(2021):5541613. https://doi.org/10.1155/2021/5541613.
Hu X, Wu J, Xiong H, et al. Type 2 diabetes mellitus promotes the proliferation, metastasis and suppresses the apoptosis in oral squamous cell carcinoma. J Oral Pathol Med. 2021. https://doi.org/10.1111/jop.13244.
Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. https://doi.org/10.1016/j.diabres.2018.02.023.
Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. https://doi.org/10.1016/j.diabres.2019.107843.
Harriss DJ, Atkinson G, George K, et al. Lifestyle factors and colorectal cancer risk (1): systematic review and meta-analysis of associations with body mass index. Colorectal Dis. 2009;11(6):547–63. https://doi.org/10.1111/j.1463-1318.2009.01766.x.
Kwak HD, Ju JK, Kang DW, et al. Outcomes according to body mass index following laparoscopic surgery in patients. J Minim Access Surg. 2018;14(2):134–9. https://doi.org/10.4103/jmas.JMAS_68_17.
Baade PD, Meng X, Youl PH, et al. The impact of body mass index and physical activity on mortality among patients with colorectal cancer in Queensland Australia. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1410–20. https://doi.org/10.1158/1055-9965.EPI-11-0079.
Toiyama Y, Hiro J, Shimura T, et al. The impact of body mass index on oncological outcomes in colorectal cancer patients with curative intent. Int J Clin Oncol. 2016;21(6):1102–10. https://doi.org/10.1007/s10147-016-1016-7.
Schlesinger S, Siegert S, Koch M, et al. Postdiagnosis body mass index and risk of mortality in colorectal cancer survivors: a prospective study and meta-analysis. Cancer Causes Control. 2014;25(10):1407–18. https://doi.org/10.1007/s10552-014-0435-x.
Ye Z, Wei S, Zeng Y, et al. Prognostic value of preoperative body mass index for diabetic patients with non-metastasis gastric cancer: a single center experience. BMC Surg. 2021;21(1):320. https://doi.org/10.1186/s12893-021-01316-x.
von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–8. https://doi.org/10.1136/bmj.39335.541782.AD.
Weiser MR. AJCC 8th Edition: Colorectal Cancer. Ann Surg Oncol. 2018;25(6):1454–5. https://doi.org/10.1245/s10434-018-6462-1.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96. https://doi.org/10.1097/SLA.0b013e3181b13ca2.
Li H, Boakye D, Chen X, et al. Association of body mass index with risk of early-onset colorectal cancer: systematic review and meta-analysis. Am J Gastroenterol. 2021. doi: https://doi.org/10.14309/ajg.0000000000001393.
Li H, Boakye D, Chen X, et al. Associations of body mass index at different ages with early-onset colorectal cancer. Gastroenterology. 2022;162(4):1088-1097.e3. https://doi.org/10.1053/j.gastro.2021.12.239.
Lee S, Lee DH, Lee JH, et al. Association of body mass index with survival in Asian patients with colorectal cancer. Cancer Res Treat. 2021. https://doi.org/10.4143/crt.2021.656.
Çam HH, Ustuner Top F. Prevalence of hypertension and its association with body mass index and waist circumference among adolescents in Turkey: a cross-sectional study. J Pediatr Nurs. 2021;57:e29–33. https://doi.org/10.1016/j.pedn.2020.09.017.
Tang ML, Zhou YQ, Song AQ, et al. The relationship between body mass index and incident diabetes mellitus in Chinese aged population: a cohort study. J Diabetes Res. 2021;24(2021):5581349. https://doi.org/10.1155/2021/5581349.
Yamamoto N, Fujii S, Sato T, et al. Impact of body mass index and visceral adiposity on outcomes in colorectal cancer. Asia Pac J Clin Oncol. 2012;8(4):337–45. https://doi.org/10.1111/j.1743-7563.2011.01512.x.
Collaborative EuroSurg. Body mass index and complications following major gastrointestinal surgery: a prospective, international cohort study and meta-analysis. Colorectal Dis. 2018;20(8):O215–25. https://doi.org/10.1111/codi.14292.
Peng D, Liu XY, Cheng YX, et al. Improvement of diabetes mellitus after colorectal cancer surgery: a retrospective study of predictive factors for type 2 diabetes mellitus remission and overall survival. Front Oncol. 2021;6(11):694997. https://doi.org/10.3389/fonc.2021.694997.
Hisada H, Takahashi Y, Kubota M, et al. Clinical and therapeutic features and prognostic factors of metastatic colorectal cancer over age 80: a retrospective study. BMC Gastroenterol. 2021;21(1):199. https://doi.org/10.1186/s12876-021-01791-9.
Beck C, Weber K, Brunner M, et al. The influence of postoperative complications on long-term prognosis in patients with colorectal carcinoma. Int J Colorectal Dis. 2020;35(6):1055–66. https://doi.org/10.1007/s00384-020-03557-3.
Tamagawa H, Aoyama T, Numata M, et al. Impact of infectious complications on survival and recurrence of patients with stage II/III colorectal cancer: a multicenter retrospective study. Anticancer Res. 2022;42(5):2763–9. https://doi.org/10.21873/anticanres.
Shahjehan F, Merchea A, Cochuyt JJ, et al. Body mass index and long-term outcomes in patients with colorectal cancer. Front Oncol. 2018;17(8):620. https://doi.org/10.3389/fonc.2018.00620.
Sakin A, Samanci NS, Secmeler S, et al. The effect of body mass index on location of recurrence and survival in early-stage colorectal cancer. J Cancer Res Ther. 2020;16(Supplement):S176–82. https://doi.org/10.4103/jcrt.JCRT_326_18.
Chiu CC, Ho CH, Hung CM, et al. Correlation of body mass index with oncologic outcomes in colorectal cancer patients: a large population-based study. Cancers (Basel). 2021;13(14):3592. https://doi.org/10.3390/cancers13143592.
Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114. J Clin Oncol. 2004;22(4):648–57. https://doi.org/10.1200/JCO.2004.07.121.
Kroenke CH, Neugebauer R, Meyerhardt J, et al. Analysis of body mass index and mortality in patients with colorectal cancer using causal diagrams. JAMA Oncol. 2016;2(9):1137–45. https://doi.org/10.1001/jamaoncol.2016.0732.
Acknowledgements
We acknowledge all the authors whose publications are referred in our article. We thank Xun Lei, a statistician, for the substantial work in the statistical methods.
Funding
This study was supported by Chongqing key diseases Research and Application Demonstration Program (Colorectal Cancer Prevention and Treatment Technology Research and Application Demonstration [No. 2019ZX003]).
Author information
Authors and Affiliations
Contributions
Peng D and Liu XY contributed to the conception and design of the study. Kang B organized the database. Peng D performed the statistical analysis. Peng D and Liu XY wrote the first draft of the manuscript. Cheng YX, Yuan C, Tao W Zhang B and Wei ZQ wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of our institution (The First Affiliated Hospital of Chongqing Medical University, 2021–336), and all patients signed informed consent. This study was conducted in accordance with the World Medical Association Declaration of Helsinki as well.
Consent for publication
Not Applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, XY., Kang, B., Cheng, YX. et al. Higher body mass index was associated with better prognosis in diabetic patients with stage II colorectal cancer. BMC Cancer 22, 596 (2022). https://doi.org/10.1186/s12885-022-09691-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09691-1