Abstract
Background
This study assesses the use of hormonal therapy to treat high-risk localized prostate cancer (HRLPCa) cases diagnosed between 2005 and 2015.
Methods
All N0-XM0 with ≥T3a, or PCa cases with poorly differentiated feature (equivalent to Gleason score ≥ 8), diagnosed between 2005 and 2015 were extracted from German population-based cancer registries. Cases treated by surgery or chemotherapy were excluded. Description of hormonal therapy use by HRLPCa cases’ profile was presented. Relative risk (RR) was computed with a log-link function to identify factors associated with hormonal therapy use among radiotherapy-treated HRLPCa cases.
Results
A total of 5361 HRLPCa cases were analyzed. Only 27.6% (95% confidence interval [CI]: 26.4–28.8%) of the HRLPCa cases received hormonal therapy in combination with radiotherapy. The use of combined hormonal therapy and radiotherapy varied from 19.8% in Saxony to 47.8% in Schleswig-Holstein.
Application of hormonal therapy was higher for the locally advanced cases compared to the poorly differentiated cases (relative risk [RR] = 1.28; 95%CI: 1.19, 1.37). Older patients showed a slightly increased use of hormonal therapy (RR for a 10-year age increase = 1.09; 95%CI: 1.02, 1.16). Compared to PCa cases from the most affluent residential areas, cases from the least affluent (RR = 0.71; 95%CI: 0.55, 0.92) and medium (RR = 0.75; 95%CI: 0.58, 0.96) areas had decreased use of hormonal therapy. The introduction of the German S3-guideline did not make a marked difference in the uptake of both hormonal therapy and radiotherapy (RR = 1.02; 95%CI: 0.95, 1.09).
Conclusion
This study found a low use of hormonal therapy among HRLPCa patients treated without surgery. The introduction of the German S3-guideline for prostate cancer treatment does not seem to have impacted hormonal therapy use.
Similar content being viewed by others
Background
Prostate cancer (PCa) is a malignant neoplasm of the prostate gland characterized by heterogeneous features and a variable natural history [1, 2]. PCa accounted for 22.7% (58,800) of the estimated 258,500 diagnosed cancer cases among German men in 2016. During the same year, with a predicted age-standardized incidence rate of 91.6 and a mortality rate of 19.5 per 100,000, PCa was an important cause of health problem in Germany [3]. Its treatment is costly, and adds a substantial economic burden to the German healthcare budget [4]. By 2030, PCa is projected to be the most frequent cancer in Germany, exceeding breast cancer [5]. An estimate of 15% PCa diagnoses are of high-risk disease, but what constitutes “high-risk” localized PCa (HRLPCa) varies in the literature [1, 6, 7]. In common with D’Amico et al., the “German S3-Guideline for Prostate Cancer” and the European Association of Urology (EAU)-guideline (see Additional file 11) define HRLPCa as having prostate specific antigens (PSA) at a level of > 20 ng/ml, a Gleason score (GS) ≥8, or clinical stage ≥ T2c [8, 9]. Discrepant with this definition, stage T2c cases without other high-risk features have shown better treatment outcomes than HRLPCa cases so classified in other ways; it has therefore been suggested that they be classified as intermediate-risk [10]. In line with these findings, the National Comprehensive Cancer Network (NCCN) guideline modified D’Amico’s definition of HRLPCa to include one or more of the following features: PSA > 20 ng/ml, biopsy GS ≥8, or clinical stage of > T2c [11]. In both the German S3-guideline and EAU, the term “locally advanced” PCa has been used to refer to a subgroup of HRLPCa with T3-4N0M0 clinical features [9, 12]. Since the data underlying this study lack records of PSA values, the focus of our investigation is only on HRLPCa cases with GS ≥8 or clinical stage ratings higher than T2c.
HRLPCa has a high chance of developing distant metastases or of not responding to treatment, either of which increases the risk of PCa-specific mortality [1, 8]. In the context of multimodal therapeutic intervention, a combination of long-term hormonal therapy (HT) and external-beam radiation therapy (EBRT) is the standard treatment for men with HRLPCa disease, although radical prostatectomy is also an optional major mode of treatment [1, 12,13,14,15,16,17,18,19,20,21]. Long-term androgen HT synergistically potentiates EBRT, and their combination is superior to radiotherapy (RT) or HT alone [13, 14, 17]. According to the evidence- and consensus-based interdisciplinary German S3-Guideline and the EAU-guideline, HRLPCa cases should be treated by a combination of long-term HT and RT, or surgery [9]. Similarly, the European guideline recommends external irradiation in combination with long-term HT as the standard treatment modality for high-risk localized and locally advanced PCa patients [12]. Additional file 11 summarizes the definition of HRLPCa and the detailed treatment recommendations of both the German S3-guideline (2009 to 2021) and EAU guideline (2005 to 2020). Despite the well-substantiated evidence and guideline recommendations that HT should be the mainstay adjuvant treatment for HRLPCa-treated with RT [9, 11, 12, 14, 21], under-treatment of high-risk PCa cases is a concern [6, 22, 23]. In addition to having direct negative consequences for patients, clinical practices which diverge from guidelines have been reported to incur unnecessary expenses [24].
The objective of our study is to assess the use of HT to treat patients with HRLPCa diagnosed between 2005 and 2015, using data from the German population-based cancer registries. In addition to presenting HT use in relation to the clinical characteristics of incident cases, we examine the predictors of HT use among the RT-treated subgroup. The effects of area-based socio-economic position and the introduction of the German S3-guideline for prostate cancer on the use of HT are also assessed.
Methods
Data source description and study population
Population-based cancer registries are crucial sources of information for cancer epidemiology and health services research. Following the enactment of the Federal Cancer Register Data Act (Bundeskrebsregisterdatengesetz, BKRG) in 2009, the German Center for Cancer Registry Data (Zentrum für Krebsregisterdaten, ZfKD) was set up at the Robert Koch-Institute [25]. By law, all federal states were obliged to collect cancer registry data.
In brief, the state cancer registries collect data on key case demographics such as gender, month and year of birth, and area of residence; data about the tumor at time of diagnosis including date of diagnosis, tumor topography and morphology, and tumor grading and stage; and data on delivered treatments, death events, and cause of death for deceased cases. Comprehensive data are also collected from clinical cancer registries (CCRs). The population-based state cancer registries are currently responsible in most cases for both the population-based and clinical cancer registries [26], although only the population-based, and not the CCRs, data are transferred to the ZfKD. On receiving data from the state population-based cancer registries, the ZfKD checks the data quality, pools the data, and produces nationwide and regional reports. The ZfKD also provides anonymized data to external users upon request [3]. Details on techniques of data quality assessment, and procedures for data request can be found on the web page [27].
This study used pooled nationwide HRLPCa data, representing all diagnoses from 2005 to 2015. Similar to Hager et al. [28], we included only state cancer registries which had diagnosis and basic treatment data for at least 70% of their registered cases since 2005. Only seven federal states (Schleswig-Holstein, Berlin, Brandenburg, Mecklenburg-Vorpommern, Saxony, Saxony-Anhalt, and Thuringia) met these inclusion criteria. Data from Berlin and Saxony-Anhalt were not included in the main analysis since both states had relatively low numbers of HRLPCa cases. All cases treated by surgery and/or chemotherapy, or for which diagnosis was only by death certificate or autopsy, were also excluded. Specific conditions of high-risk PCa cases, like cases with limited life expectancy (< 10 years), could have led to their being under- or over-treated, which could affect our analysis [29]. Because of this likely problem, only those cases with sufficient life expecancy (> 10 years) were included [30]. Estimation of life expectancy (stratified by age and calendar period) for the German male population was obtained from the Human Mortality Database [30]. We assumed that the HRLPCa cases would have comparable life expectancy with the age group- and calendar period- matched German population, had they received the recommended treatment standard. Because of the limited life expectancy, all HRLPCa cases in individuals over 79 years old were excluded from this study. These excluded cases are also likely to suffer from comorbidities, such as cardiovascular diseases, a clinical scenario that may prevent prescription of HT in clinical practice. The exclusion of these cases was, therefore, methodologically relevant.
Measurements and statistical analysis
All N0-XM0 PCa (ICD-10 C61) cases with ≥T3a cases or histopathological tumor grades 3, 4, and 7 according to the coding system of the registries, which were equivalent to a Gleason’s score of eight or higher [31], were included. The term ‘locally advanced PCa’ refers to PCa cases that harbored ≥T3a feature [9, 12]. Cases with both poor differentiation and locally advanced characteristics were considered locally advanced. Those cases, which were diagnosed before and after September 2009, were considered as “diagnosed before the era of the German S3-guideline for prostate cancer treatment”, and “diagnosed during the era of the German S3-guideline” respectively [32].
In this study, non-treatment was constituted by receiving neither RT nor HT in the included HRLPCa cases. The German Indicator for socio-economic deprivation (GISD) was used to measure regional socio-economic deprivation [33]. GISD data were collected at German municipal, administrative district, and regional levels. We extracted data from the recommended, 2018-updated version, which covers the period 1998 and 2014 [34]. This GISD version did not include data for 2015. Therefore, district-level GISD data, covering 2005 to 2014, were linked with the prostate cancer registry data to model factors of HT use. Comprehensive methodological approaches are detailed elsewhere [33]. Calendar year-specific quintile classifications were created from the total GISD score. The degree of socio-economic deprivation increases with quantile increase; quantile 1 represents the least deprived group, whereas quantile 5 represents the least affluent group. In our model, the lowest quintile was classified as “most affluent”, the middle three quintiles (quintiles 2, 3 and 4) as “medium”, and the highest quintile as the “least affluent” [33].
The dependent variable was use of hormonal therapy among RT-treated HRLPCa cases. Age at time of diagnosis, state, GISD, stage, tumor grade, and era of diagnosis (before or during S3-German guideline era) were the independent variables. Multivariable log-binomial model was used to identify factors associated with HT use among 2349 RT-treated HRLPCa cases from the five federal states (Schleswig-Holstein, Brandenburg, Mecklenburg-Vorpommern, Saxony, and Thuringia) whose data met our criteria, see Additional file 1. In order to assess the robustness of the estimated relative risks, we performed a sensitivity analysis on the data that additionally included Berlin and Saxony-Anhalt (Additional file 3). Predictors of missing treatment and tumor grade information were assessed using multivariable binary logistic regression (Additional file 6 and Additional file 8). The statistical analyses were carried out using Stata 15.1 (Stata Corp, College Station, TX, USA). Maps of regional HT use were plotted in R.
Results
Description of the incident case population by treatment
A total of 5361 HRLPCa cases were included from five federal states, of which 3546 (66.1%) and 1815 (33.9%) were poorly differentiated and locally advanced PCa cases, respectively. The median age was 73 years (range, 42 to 79; interquartile range, 69 to 76). Majority of the cases, 3306 (61.7%), were from Saxony (35.7%), and Brandenburg (26%), together. GISD information was available for 4933 cases (92%), and just over half, 2566 (52%), of them were living in the least affluent residential areas (Table 1).
The mean proportion of hormonal therapy (HT) use, regardless of RT treatment status, was 57.8%, varying from 41.2% in Thuringia to 68.1% Brandenburg. As shown in Table 1, only 27.6% (95% confidence interval [CI]: 26.4–28.8%) of the HRLPCa cases received HT in combination with radiotherapy (RT). This proportion varied among states, ranging from about one-fifth, 378 (19.8%), in Saxony to about half, 335 (47.8%), in Schleswig-Holstein. Compared to other states, Schleswig-Holstein and Brandenburg had the highest proportions of cases treated using combined hormonal and radiation treatment, 47.8 and 29.6% respectively (P < 0.001).
Nearly one-fifth, 19.7% (95% CI: 18.6–20.8%), of HRLPCa cases did not receive either RT or HT. The proportion of cases that received neither treatment was higher during the guideline era (post 2009) than the pre-guideline era (15.5% vs. 21.9%, P < 0.001). Non-treatment was slightly higher in the poorly differentiated cases compared with locally advanced cases (20.5% vs. 18.1%, P < 0.041). It was also higher for cases from Saxony (26.2%) and Mecklenburg-Vorpommern (25.8%), whereas Schleswig-Holstein (3.3%) documented the lowest proportion. The proportion of non-treatment was 7.2% lower for the most affluent group compared to the least affluent group with (12.8% vs. 20%, P = 0.034) (data not shown).
Treatment patterns
Figure 1 shows the proportion of HT used for both RT-treated and -untreated HRLPCa cases in five federal states of Germany from 2005 to 2015. The use of HT among RT-treated, poorly differentiated group ranges from 31.1% in Thuringia to 66% in Saxony. Poorly differentiated PCa cases were more likely to receive HT alone than a combination of HT and RT, except those poorly differentiated cases from Mecklenburg-Vorpommern and Saxony (Fig. 1A). A similar pattern was also observed for the locally advanced cases, but not in Thuringia (Fig. 1B). In all the five states, the locally advanced group, compared to the poorly differentiated group, more frequently received HT. On average, the percentage use of HT among the RT-treated, locally advanced cases was 14% higher than the poorly differentiated cases of the same treatment group. Similarly, the mean percentage was 12.7% higher for the RT-untreated, locally advanced cases than the same treatment group in the poorly differentiated cases. The highest proportion of use of combined HT and RT to treat locally advanced cases was observed in Saxony and Brandenburg, while the lowest was in Thuringia (Fig. 1B).
Figure 2 presents the proportions of HT use in HRLPCa cases of the five federal states, stratified by PCa German S3-Guideline era and RT treatment status. The share of cases for which HT was used in combination with RT was highest in Schleswig-Holstein in both PCa groups, regardless of guideline implementation era. Use of both treatments in combination was consistently lowest in Saxony, with the single exception of the poorly differentiated group during the guideline era, where Saxony (19.1%) had the second lowest rank next to Thuringia (16.8%). For the poorly differentiated group, the mean percentage use of combined HT and RT before and during the guideline era were 34.6 and 26.9%, respectively. However, there was a 4.5% increase (from 28.4 to 32.9%) in the mean percentage use of combined HT and RT during the guideline era by the locally advanced group (Fig. 2). The proportion of untreated HRLPCa cases was higher during the guideline era, compared to the pre-guideline era. Schleswig-Holstein had the lowest proportion of untreated cases (Fig. 2).
Treatment trends of high-risk PCa cases by state and German S3-Guideline era, 2005-2015. (A) Treatment trend of poorly differentiated cases before guideline era. B Treatment trend of poorly differentiated cases during guideline era. C Treatment trend of locally advanced cases before guideline era. D Treatment trend of locally advanced cases during guideline era
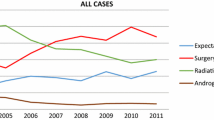
The use of HT among RT-treated, poorly differentiated cases was lower than the use of HT in cases which were not treated by RT across all the years, with the two annual exceptions of 2010 and 2015. Additionally, the proportion of HT use for both the RT-treated and -untreated poorly differentiated cases appears to decline starting from 2011 (Fig. 3A). In contrast to the poorly differentiated group, in locally advanced cases the use of combined HT and RT has been relatively higher since 2010, compared to the RT-untreated cases (Fig. 3B).
Use of HT was higher in older cases (age at diagnosis) in both poorly differentiated and locally advanced cases, as well as among RT-untreated cases within the same age group (Fig. 4A-B). The locally advanced cases had higher use of HT compared to the poorly differentiated cases (Fig. 4A-B).
The proportions of HT use in the five federal states, stratified by RT treatment status, are summarized in Fig. 5. In general, use of HT was higher for treatment of locally advanced cases. For poorly differentiated cases, the use of HT in combination with RT was highest in Saxony (Fig. 5B), and Saxony and Brandenburg also showed highest proportions of use of HT in combination with RT for the locally advanced cases (Fig. 5D). Data from the seven federal states also showed similar results (Additional file 2).
Proportion of HT use among HRLPCa cases by RT treatment status in five German states. (A) All poorly differentiated cases among RT-treated and -untreated cases (B) Poorly differentiated cases that received RT (C) All locally advanced among RT-treated and -untreated cases (D) Locally advanced cases that received RT
Predictors of hormonal therapy use among HRLPCa cases treated by radiotherapy
Table 2 presents the univariable and multivariable regression results. The multivariable log-binomial model identified that locally advanced PCa cases compared to the poorly differentiated cases (RR = 1.28: 95%CI: 1.19, 1.37) were associated with increased use of HT. For every 10-year increase in the patients’ age, there was a slight increase in the use of HT (RR = 1.09; 95%CI: 1.02, 1.16).
Based on the German Index for Socioeconomic-Deprivation classification, HRLPCa cases from medium (RR = 0.75; 95%CI: 0.58, 0.96), and least affluent (RR = 0.71; 95%CI: 0.55, 0.92) residential areas had decreased HT use compared to those from the most affluent areas (Table 2).
The model from the sensitivity analysis, based on the seven German states, generally showed similar results demonstrating the robustness of the main estimates (Additional file 3). Compared to Schleswig-Holstein, the use of HT was lower in Berlin (RR = 0.68; 95%CI: 0.54, 0.84) and Thuringia (RR = 0.72; 95%CI: 0.62, 0.83).
Additional file 4 shows that higher age was inversely associated with non-treatment in both the poorly differentiated (RR = 0.79; 95%CI: 0.71, 0.88), and locally advanced cases (RR = 0.84; 95%CI: 0.71, 0.99); patients were less likely to undergo treatment the older they were, regardless of how sick they were. Poorly differentiated cases diagnosed during the guideline-era were 1.42 times more likely (RR = 1.42; 95%CI: 1.22, 1.65) to risk of non-treatment, compared with cases diagnosed before the guideline-era, but no evidence of a strong association was observed for the locally advanced cases (Additional file 4).
Sensitivity analysis
A sensitivity analysis based on 778 PCa cases, excluding cases with tumor code grade 3 in the registry regardless of their tumor stage, showed that the proportion of use of HT in combination with RT was 24.3% (95%CI: 21.4–27.4%). The relative risk estimates from the sensitivity analysis performed on 2648 RT-treated HRLPCa cases were similar to the estimates from the main model (Additional file 3). This reflected the stability of the estimated risk-ratio from the main model. Moreover, increasing age, year of diagnosis (2011–2015 vs. 2005–2010), and missing RT data were predictors of missing hormonal treatment data (Additional file 6). Generally, increasing age was a strong predictor for missing TNM-stage and histopathological tumor grade (Additional files 8 and 9). While year of diagnosis (2011–2015 vs. 2005–2010) was associated with missing tumor grade data, it was inversely associated with missing TNM-stage data (Additional files 8 and 9). Unfortunately, the state-specific odds ratio estimates for missing PCa stage data were not precise due to few numbers of missing observations in each state (Additional file 8). Additional file 5 summarizes the proportions of missing treatment data, stratified by German federal states, for all the non-metastatic PCa cases.
Discussion
In this study, we assessed the clinical practice of prescribing HT for localized high-risk PCa cases in the context of prostate cancer treatment in Germany. We have found that only 27.6% (95%CI: 26.4–28.8%) of the 5361 HRLPCa cases not treated by surgery or chemotherapy received HT in combination with RT. Older age and non-affluent residential area were associated with increased risk of non-use of HT among HRLPCa cases which also received RT. However, locally advanced tumors were associated with increased use of HT compared to the poorly differentiated tumors. Another key finding was that nearly one in five cases were untreated.
Evidence-based guidelines, if successfully implemented and regularly revised with up-to-date evidence, may play a crucial role in improving clinical practice and treatment outcomes [35, 36]. Following objectively defined procedures, Germany developed its evidence and consensus based guideline, and published its first version in 2009 [9, 32]. This guideline recommends that HRLPCa cases be treated either by radical prostatectomy and adjuvant RT, or long-term HT in combination with EBRT [9, 18, 19]. This study assessed the use of HT in relation to the second treatment option. Multiple randomized control trials have demonstrated that long-term HT in combination with RT for treating high-risk localized and locally advanced PCa patients showed superior oncological outcomes such as improved overall survival, reduced diseases progression and biochemical failure [13, 17,18,19,20, 37, 38]. In our study, however, only approximately one quarter of the surgically untreated HRLPCa cases received guideline-recommended treatment. This finding was far below those reported in studies in the U.S.A. [22, 39,40,41] and in the Netherlands [42]. Contrary to our study, data from German Prostate Cancer Centers found high guideline-adherence (85.4% in 2017) in terms of delivering RT with HT for locally advanced (T3-4N0M0) PCa cases [43]. Similarly, a study based on 70,683 patients treated in certified prostate cancer treatment centers between 2010 and 2013, found high fulfilment of quality requirement for more than 80% of the certified treatment centers [44]. Our population-based cancer registry data suggest that the target of achieving more than 90% for the use of RT with HT [43], an important quality indicator of PCa treatment, appears to be off track. In particular, the fact that HT use did not increase after the introduction of the German S3-guideline for Prostate Cancer treatment was highly unanticipated. Before the German S3-guideline became available, the EAU-guideline also recommended that HRLPCa cases receive a combination of HT and RT, although the duration of HT for the localized high-risk cases was mostly restricted to 6 months (Additional file 11). Regrettably, desired clinical outcomes are not achieved simply by publishing clinical guidelines [24]. A key finding of the current study, suboptimal HT use, may suggest low adherence to the guideline. Whether the observed HT underutilization was related to poor guideline adherence or to treatment underreporting remains unanswered by the data underlying our results, and further study may need to be undertaken. Prior studies indicate that adherence to guideline recommendations has been a concern, and several barriers may affect guideline use in clinical practice [24, 35]. It has been shown that discordance from PCa guideline may cause unfavorable effects at the patient- and health system-levels [24].
Comorbidity, patient refusal, advanced age and, in some cases, failure to initiate by physicians were mentioned as causes of non-prescription of HT among eligible PCa cases treated in certified prostate cancer treatment centers in Germany [45]. Since patients with PCa are a generally older population, the likelihood of comorbidity could also be higher in our study population. If this holds true, we could suppose that occurrence of comorbidities might have contributed for the decreased uptake of HT in the HRLPCa cases. While some studies on the association of comorbidity and HT use showed conflicting results [23, 42, 46], absence of comorbidity data in this study makes the interpretation of our results difficult. Wang et al. found a decrease in the duration of HT was certainly related to comorbidities, but it was mentioned that the degree of HT underutilization was not fully explained by comorbidities alone [40]. On the other hand, regional differences in the translation of evidence into clinical practice, rather than patient-related factors such as comorbidity, were deemed to be a possible cause of regional variation in the U.S. [46]. For instance, prescription of HT was more affected by the practices of individual urologists than by tumor- or patient-related characteristics [47]. HT prescription was also shown to vary by institutional factors, like differences in institutional policy, or whether the treating institutions are public or private [42, 48]. Similar to our study, decreasing patterns of HT use were observed in the U.S. [22, 23], but a reverse pattern was found in Australia [48]. Another important factor that could influence uptake of HT is the modality of radiation used for treatment. High-risk PCa cases which received brachytherapy were observed to experience lower odds of receiving HT [39]. However, the overall use of brachytherapy in Germany has been less than 2% [49], and thus brachytherapy is unlikely to influence the observed underutilization of HT.
In this study, the threat of underutilization of HT among HRLPCa is the most important clinically relevant finding, and this did not show improvement after the introduction of the German S3-guideline. It is also important to point out that the German S3-guideline suggests that localized, intermediate-risk PCa cases should be treated with a combination of EBRT and short-term HT [9, 45]. However, further classification of the intermediate-risk PCa cases into favorable and unfavorable groups has clinical importance [50]. A combination of RT and short-term HT is the optimal treatment for unfavorable intermediate-risk PCa cases, but not necessarily for favorable cases. The German S3-guideline did not introduce this classification scheme during the treatment period now studied (2005 to 2015) and adoption of this scheme could be beneficial for PCa patients, at least by avoiding HT overtreatment among the favorable intermediate-risk PCa cases [50].
There are additional limitations that should be considered when using the results of this study. The data had high proportions of missing diagnostic and treatment information (Additional files 1, 5 and 7). We therefore tried to assess the potential impact of these missing data on our main estimates. Additional file 3 shows that the main relative risk estimates presented in Table 2 are robust. It is important to note that missing hormonal treatment data depended on age, missing RT data, and year of diagnosis. On the other hand, the cancer registries in the former East Germany did not have missing values. This is because only delivered treatments were actively recorded by the respective cancer registries, and hence “no therapy” became the default record value. It is possible that HRLPCa cases might actually have received RT or HT, but treatment data were not submitted to the registries, and hence their treatment status was recorded as “no therapy”. On the other hand, possible side effects of HT might have been a barrier to its uptake [20]. In this study, all federal states with more than 30% missing diagnostic and treatment data were excluded. The five states we included for the main analysis had higher mean socio-economic deprivation compared to the excluded states (Additional file 10). That being the case, selection bias could be a potential limitation of this study, and our results may not reflect the situation of HT use in the excluded German states.
Conclusions
In conclusion, this study assessed the status of HT use in surgically-untreated HRLPCa cases using population-based cancer registry data in selected states of Germany. Despite its limitations, our investigation showed a high likelihood of underutilization of HT in the non-metastatic HRLPCa cases between 2005 and 2015. The introduction of the German S3 treatment guideline for prostate cancer did not markedly affect HT use. This may reflect evidence of sub-optimal guideline adherence.
Availability of data and materials
The data that support the findings of this study, epidemiological data from selected German population-based cancer registries, were obtained from Robert Koch Institute (RKI). As the authors do not own the dataset, they cannot make it publicly available.
Abbreviations
- BKRG:
-
Bundeskrebsregisterdatengesetz (Federal Cancer Register Data Act)
- CCRs:
-
Clinical cancer registries
- EAU:
-
European Association of Urology
- EBRT:
-
External-beam radiation therapy
- GISD:
-
German indicator for socio-economic deprivation
- GS:
-
Gleason score
- HRLPCa:
-
High-risk localized prostate cancer
- HT:
-
Hormonal therapy
- ICD-10:
-
International classification of diseases 10th version
- IQR:
-
Interquartile range
- NCCN:
-
National Comprehensive Cancer Network
- PCa:
-
Prostate cancer
- PSA:
-
prostate specific antigen
- RT:
-
Radiotherapy
- TNM-stage:
-
Tumor, node, metastasis stage
- U.S.:
-
United states
- ZfKD:
-
Zentrum für Krebsregisterdaten (Center for Cancer Registry Data)
References
Chang AJ, Autio KA, Roach M, Scher HI. High-risk prostate cancer-classification and therapy. Nat Rev Clin Oncol. 2014;11(6):308–23.
Sebesta EM, Anderson CB. The surgical Management of Prostate Cancer. Semin Oncol. 2017;44(5):347–57.
Robert Koch Institute (ed.) and the Association of Population-based Cancer Registries in Germany (ed.). Cancer in Germany 2015/2016. 12th edition. Berlin; 2020. https://www.krebsdaten.de/Krebs/EN/Content/Publications/Cancer_in_Germany/cancer_chapters_2015_2016/cancer_germany_2015_2016.pdf?__blob=publicationFile
Fourcade RO, Benedict Á, Black LK, Stokes ME, Alcaraz A, Castro R. Treatment costs of prostate cancer in the first year after diagnosis: a short-term cost of illness study for France, Germany, Italy. Spain and the UK BJU Int. 2010;105(1):49–56.
Quante AS, Ming C, Rottmann M, Engel J, Boeck S, Heinemann V, et al. Projections of cancer incidence and cancer-related deaths in Germany by 2020 and 2030. Cancer Med. 2016;5(9):2649–56.
Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28(7):1117–23.
Bastian PJ, Boorjian SA, Bossi A, Briganti A, Heidenreich A, Freedland SJ, et al. High-risk prostate cancer: from definition to contemporary management. Eur Urol. 2012;61(6):1096–106.
D’Amico AV, Whittington R, Bruce Malkowicz S, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. J Am Med Assoc. 1998;280(11):969–74.
“Interdisciplinary guideline of the quality S3 for the early detection, diagnosis and therapy of the different stages of prostate carcinoma“.https://www.leitlinienprogramm-onkologie.de/leitlinien/prostatakarzinom/. Accessed 19 Nov2020.
Klaassen Z, Singh AA, Howard LE, Feng Z, Trock B, Terris MK, et al. Is clinical stage T2c prostate cancer an intermediate- or high-risk disease? Cancer. 2015;121(9):1414–21.
National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/pdf/prostate_blocks.pdf. Accessed 25 Sep 2021.
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 2014; 65(1): 124-37.
Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11(11):1066–73.
Horwitz EM, Bae K, Hanks GE, Porter A, Grignon DJ, Brereton HD, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26(15):2497–504.
Bolla M, Very C, Long JA. High-risk prostate cancer: combination of high-dose, high-precision radiotherapy and androgen deprivation therapy. Curr Opin Urol. 2013;23(4):349–54.
Pignot G, Maillet D, Gross E, Barthelemy P, Beauval JB, Constans-Schlurmann F, et al. Systemic treatments for high-risk localized prostate cancer. Nat Rev Urol. 2018;15(8):498–510.
Zapatero A, Guerrero A, Maldonado X, Ana A, Segundo CGS, Cabeza RMA, et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16(3):320–7.
Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate Cancer significantly reduces risk of metastases and improves survival: Long-term follow up of a randomized clinical trial. J Urol. 2009;181(3):956–62.
Wiegel T, Bottke D, Steiner U, Siegmann A, Golz R, Störkel S, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27(18):2924–30.
Sargos P, Mottet N, Bellera C, Richaud P. Long-term androgen deprivation, with or without radiotherapy, in locally advanced prostate cancer: updated results from a phase III randomised trial. BJU Int. 2020;125(6):810–6.
Bandini M, Fossati N, Gandaglia G, Preisser F, Dell’Oglio P, Zaffuto E, et al. Neoadjuvant and adjuvant treatment in high-risk prostate cancer. Expert Rev Clin Pharmacol. 2018;11(4):425–38.
Dell’Oglio P, Abou-Haidar H, Leyh-Bannurah SR, Tian Z, Larcher A, Gandaglia G, et al. Assessment of the rate of adherence to international guidelines for androgen deprivation therapy with external-beam radiation therapy: a population-based study. Eur Urol. 2016;70(3):429–35.
Swisher-Mcclure S, Pollack CE, Christodouleas JP, Guzzo TJ, Haas NB, Vapiwala N, et al. Variation in use of androgen suppression with external-beam radiotherapy for nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2012;83(1):8–15.
Simonato A, Varca V, Gacci M, Gontero P, De Cobelli O, Maffezzini M, et al. Adherence to guidelines among Italian urologists on imaging preoperative staging of low-risk prostate cancer: results from the MIRROR (multicenter Italian report on radical prostatectomy outcomes and research) study. Ther Adv Urol. 2012;2012:651061.
“Federal Cancer Registry Data Act (BKRG) 2009.” http://www.gesetze-im-internet.de/bkrg/index.html . Accessed 24 Mar 2021.
Arndt V, Holleczek B, Kajüter H, Luttmann S, Nennecke A, Zeissig SR, et al. Data from population-based Cancer registration for secondary data analysis: methodological challenges and perspectives. Gesundheitswesen. 2020;82(S 01):S62–71.
Center for Cancer Registry Data (ZfKD). https://www.krebsdaten.de/Krebs/DE/Content/Scientific_Use_File/scientific_use_file_node.html . Accessed 21 Nov 2021.
Hager B, Kraywinkel K, Keck B, Katalinic A, Meyer M, Zeissig SR, et al. Increasing use of radical prostatectomy for locally advanced prostate cancer in the USA and Germany: a comparative population-based study. Prostate Cancer Prostatic Dis. 2017;20(1):61–6.
Bratt O, Folkvaljon Y, Eriksson MH, Akre O, Carlsson S, Drevin L, et al. Undertreatment of men in their seventies with high-risk nonmetastatic prostate cancer. Eur Urol. 2015;68(1):53–8.
Human Mortality Database. https://www.mortality.org/hmd/DEUTNP/STATS/mltper_5x1.txt . Accessed 3 Dec 2020.
Hermann S, Kraywinkel K. Epidemiology of prostate cancer in Germany. Der Onkol. 2019;25:294–303.
Röllig C, Nothacker M, Wöckel A, Weinbrenner S, Wirth M, Kopp I, et al. Development of the interdisciplinary evidence-based S3 guideline for the diagnosis and treatment of prostate cancer: methodological challenges and solutions. Onkologie. 2010;33(7):396–400.
Kroll LE, Schumann M, Hoebel JLL. Regional health differences – developing a socioeconomic deprivation index for Germany. J Heal Monit. 2017;2(2):98–114.
GISD-The German Index of Socioeconomic Deprivation 2018. https://github.com/lekroll/GISD . Accessed 29 Jul 2020.
Francke AL, Smit MC, De Veer AJE, Mistiaen P. Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta-review. BMC Med Inform Decis Mak. 2008;8:38.
Dahm P, Yeung LL, Chang SS, Cookson MS. A critical review of clinical practice guidelines for the management of clinically localized prostate Cancer. J Urol. 2008;180(2):451–9 discussion 460.
Pilepich MV, Winter K, Lawton CA, Krisch RE, Wolkov HB, Movsas B, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma - Long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61(5):1285–90.
Bolla M. Current status of combined radiation therapy and androgen suppression in locally advanced prostate cancer: what is the way forward? Eur Urol Suppl. 2010;9(11):788–93.
Chen YW, Muralidhar V, Mahal BA, Nezolosky MD, Beard CJ, Choueiri TK, et al. Factors associated with the omission of androgen deprivation therapy in radiation-managed high-risk prostate cancer. Brachytherapy. 2016;15(6):695–700.
Wang C, Raldow AC, Nickols NG, Nguyen PL, Spratt DE, Dess RT, et al. Underutilization of androgen deprivation therapy with external beam radiotherapy in men with high-grade prostate Cancer. Eur Urol Oncol. 2021;4(2):327–30.
Mohiuddin JJ, Narayan V, Venigalla S, Vapiwala N. Variations in patterns of concurrent androgen deprivation therapy use based on dose escalation with external beam radiotherapy vs. brachytherapy boost for prostate cancer. Brachytherapy. 2019;18(3):322–31.
Rijksen BLT, Pos FJ, Hulshof MCCM, Vernooij RWM, Jansen H, van Andel G, et al. Variation in the prescription of androgen deprivation therapy in intermediate- and high-risk prostate Cancer patients treated with radiotherapy in the Netherlands, and adherence to European Association of Urology guidelines: a population-based study. Eur Urol Focus. 2021;7(2):332–9.
Griesshammer E, Adam H, Sibert NT, Wesselmann S. Implementing quality metrics in European Cancer centers (ECCs). World J Urol. 2021;39(1):49–56.
Kowalski C, Ferencz J, Albers P, Fichtner J, Wiegel T, Feick G, et al. Quality assessment in prostate cancer centers certified by the German Cancer society. World J Urol. 2016;34(5):665–72.
“Annual report of the certified prostate cancer centers 2021”. https://www.krebsgesellschaft.de/jahresberichte.html . Accessed 27 Jul 2021.
Falchook AD, Basak R, Mohiuddin JJ, Chen RC. Use of androgen deprivation therapy with radiotherapy for intermediate- and high-risk prostate cancer across the United States. JAMA Oncol. 2016;2(9):1236–8.
Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Determinants of androgen deprivation therapy use for prostate cancer: role of the urologist. J Natl Cancer Inst. 2006;98(12):839–45.
Ong WL, Foroudi F, Evans S, Millar J. Large institutional variations in use of androgen deprivation therapy with definitive radiotherapy in a population-based cohort of men with intermediate- and high-risk prostate cancer. BJU Int. 2017;120(Suppl 3):35–42.
“Recording the frequency of radiotherapies in Germany - Project 3618S42434”. http://doris.bfs.de/jspui/bitstream/urn:nbn:de:0221-2021010424620/7/2020_BfS_3618S42434.pdf . Accessed 25 Aug 2021.
Beck M, Böhmer D, Aebersold DM, Albrecht C, Flentje M, Ganswindt U, et al. Role of combined radiation and androgen deprivation therapy in intermediate-risk prostate cancer: statement from the DEGRO working group on prostate cancer. Strahlenther Onkol. 2020;196(2):109–16.
Acknowledgements
The authors are grateful to Dr. Klaus Kraywinkel, who works at the Centre for Cancer Registry Data (ZfKD) at the Robert Koch-Institute, for his revision and comments.
Funding
Open Access funding enabled and organized by Projekt DEAL. We acknowledge the financial support within the funding programme Open Access Publishing by the German Research Foundation (DFG). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
SFA designed the study, performed the analyses, and wrote the draft manuscript; AB gave critical review of the manuscript; DM and DV contributed to the data acquisition. AG read the manuscript critically, clinical review; DV participated in the design of the study, contributed to the statistical methods, gave critical review of the manuscript, made clinical review; DM participated in the design of the study, contributed to the statistical methods, wrote part of the manuscript, and made clinical review. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study used secondary epidemiologic data of the German cancer registry, which were centrally pooled and freely shared by the German Center for Cancer Registry Data (Zentrum für Krebsregisterdaten, ZfKD) at the Robert Koch-Institute. Informed consent was therefore not required in this particular case.
Consent for publication
Not applicable.
Competing interests
Professor Dirk Vordermark is a member of the editorial board of BMC Cancer journal.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Inclusion and exclusion criteria
Additional file 2.
Proportion of HT use among poorly differentiated and locally advanced PCa cases by RT treatment status in seven federal states of Germany, 2005–2015. (A) All poorly differentiated cases among RT- treated and -untreated cases (B) Poorly differentiated cases that received RT (C) All locally advanced among RT- treated and -untreated cases (D) Locally advanced cases that received RT.
Additional file 3
Factors associated with HT use among poorly differentiated and locally advanced PCa cases in seven states which received RT between 2005 and 2014 (n = 2, 648).
Additional file 4
Factors associated with non-treatment among poorly differentiated (n = 3, 243) and locally advanced (n = 1, 690) PCa cases diagnosed between 2005 and 2014.
Additional file 5
Proportions of missing treatment data among non-metastatic PCa cases stratified by German federal states, 2005–2015 (n = 263,839)
Additional file 6
Multivariable binary logistic regression analysis showing predictors of missing hormonal treatment data in Schleswig-Holstein (n = 767)
Additional file 7
Proportions of missing TNM stage data in non-metastatic PCa cases stratified by German federal states, 2005–2015 (n = 263, 839)
Additional file 8
Multivariable binary logistic regression analyses showing predictors of missing stage data in five states, 2005–2015 (n = 74,098)
Additional file 9
Multivariable binary logistic regression analyses showing predictors of missing histopathological tumor grade data in five states, 2005–2015 (n = 74,098)
Additional file 10.
Mean GISD score of 16 German federal states based on 263,774 PCa cases diagnosed during 2005–2014 (1 = Schleswig-Holstein, 2 = Hamburg, 3 = Lower Saxony, 4 = Bremen, 5 = North Rhine-Westphalia, 6 = Hessen, 7 = Rhineland-Palatinate, 8 = Baden-Württemberg, 9 = Bavaria, 10 = Saarland, 11 = Berlin, 12 = Brandenburg, 13 = Mecklenburg-Vorpommern, 14 = Saxony, 15 = Saxony-Anhalt, and 16 = Thuringia)
Additional file 11.
Treatment recommendations of German S3-Guideline and EAU guidelines for localized high-risk prostate cancer patients, 2005 to 2021
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Abera, S.F., Bedir, A., Glowka, A. et al. Suboptimal use of hormonal therapy among German men with localized high-risk prostate Cancer during 2005 to 2015: analysis of registry data. BMC Cancer 22, 624 (2022). https://doi.org/10.1186/s12885-022-09677-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09677-z