Abstract
Background
The aim of the present study was to assess the prevalence of deficient mismatch repair (MMR) in Chinese ovarian clear cell carcinoma (CCC) patients and its association with clinicopathologic features.
Methods
Immunohistochemistry with four antibodies against MLH1, PMS2, MSH2 and MSH6 was performed on whole section slides, and the results were correlated with clinicopathologic variables.
Results
A total of 108 cases were included in the present study with a median age of 52 years at first diagnosis. Early-stage disease and platinum-sensitive recurrence accounted for 62.3 and 69.6%, respectively, of the total cases. Overall, the estimated 5-year overall survival was 70.3 and 20.7% in patients with early- and late-stage tumors, respectively. Deficient MMR was identified in 5.6% (6/108) of the cohort and included MSH2/MSH6 (n = 4) and MLH1/PMS2 (n = 2). The average age of the six patients with deficient MMR was 45.6 years, and the rate of MMR-deficient tumors in women ≤50 years was relatively higher than that in women over 50 years (10.0% vs. 2.9%; P = 0.266). Half of the patients with deficient MMR were diagnosed with synchronous (endometrial or colorectal) and metachronous (endometrial) cancer, which was significantly more than their intact counterparts (P = 0.002). All six patients with deficient MMR had early-stage tumors, and the majority (83.3%) were platinum sensitive. The median progression-free survival was slightly higher in patients with defective MMR expression than in their intact counterparts (30 months vs. 27 months), but significance was not achieved (P = 0.471).
Conclusions
Young ovarian CCC patients with concurrent diagnosis of endometrial and colorectal cancer are more likely to have MMR-deficient tumors, thereby warranting additional studies to determine whether patients harboring MMR abnormalities have a favorable prognosis.
Similar content being viewed by others
Background
The histological subtypes of ovarian cancer are distinct diseases, each with different clinical and molecular characteristics [1]. Ovarian clear cell carcinoma (CCC) has unique epidemiological correlations with ethnicity, endometriosis, genetic/epigenetic alterations and specific immune-related molecular profiles [2]. In addition, ovarian CCC is challenging to treat due to its aggressiveness and chemoresistance [3]. The objective response rate of ovarian CCC to conventional chemotherapy is 9% in platinum-sensitive patients and 1% in platinum-resistant recurrence [4]. Recent clinical trials have shown that ovarian CCC patients show surprising sensitivity to immune checkpoint inhibitors, but ovarian cancer patients with high-grade serous carcinoma show modest responses [5, 6]. However, given the rarity of the disease, only small numbers of cases were included in the trials, and further verification is needed.
Mismatch repair (MMR) deficiency has been demonstrated to be a biomarker of sensitivity to immune checkpoint blockade with antibodies against programmed death receptor-1 across various types of tumors [7]. Defective MMR leads to the accumulation of mutations in the genome and microsatellite instability in tumors [8]. Lynch syndrome is characterized by loss of expression of MMR genes [9], and the most clinically significant genes are MLH1, MSH2, MSH6 and PMS2 [8, 10]. Women with Lynch syndrome are at increased risk of ovarian carcinoma, mostly clear cell and endometrioid histology [9]. In the past 5 years, several publications have focused on MMR deficiency in ovarian CCC [11,12,13,14,15,16,17,18]. The frequency of deficient MMR varies from study to study, and most studies include a small number of clear cell carcinoma patients [13,14,15,16, 18].
In the present study, we assessed the status of MMR proteins in a well-annotated unselected cohort of Chinese ovarian CCC patients. The frequency of MMR deficiency was evaluated by immunohistochemistry, and the association of MMR deficiency with clinicopathologic variables was also evaluated.
Materials and methods
Study population
All methods were carried out in accordance with relevant guidelines and regulations. After obtaining approval from the Institutional Review Board (050432–4-1212B), we identified all the patients by searching the surgical pathology archives for “ovarian clear cell carcinoma” from 2008 to 2018. In our institution, one surgical specimen is generally reviewed by two pathologists (one young and one senior doctor) as a routine. A third experienced pathologist will review the slides in some difficult cases or resolve discrepancies. In the present study, we included all patients with archived tissue blocks, and all available hematoxylin & eosin-stained slides were reviewed to confirm the diagnosis. Cases were excluded if they were focal carcinomas or if the clear cell component was less than 50% in mixed tumors. The requirement for written informed consent was waived considering the retrospective design of the study.
Clinicopathological information and survival outcomes were obtained from medical records. The following data were collected: the age at diagnosis of ovarian CCC; personal and family history of cancer; date and type of primary surgery; International Federation of Gynecology and Obstetrics (FIGO) stage at initial diagnosis [19]; consistent endometriosis (presence of endometriosis in the same specimen [20]); residual disease; platinum-free interval (the time interval from completion of the last platinum-based chemotherapy to disease recurrence); time of disease progression or recurrence; and tumor status at last contact. Patients were considered platinum sensitive if the platinum-free interval was more than 6 months. Progression-free survival (PFS) and overall survival (OS) were defined as the time interval from the date of the primary surgery to the date of first recurrence and death or last contact, respectively. Due to the retrospective design, some clinicopathological information was missing.
Immunohistochemistry
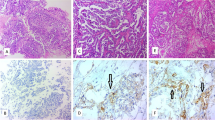
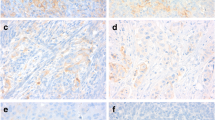
Four-micrometer-thick, formalin-fixed, paraffin-embedded whole-block sections were used for immunohistochemistry. All staining was performed using an automated slide stainer (Ventana BenchMark ULTRA) according to our protocols as MMR protein immunohistochemistry is routinely conducted at our institution [21, 22]. The primary antibodies included anti-MLH1 (Clone G168–728), anti-MSH2 (Clone G219–1129), anti-MSH6 (Clone 44) and anti-PMS2 (Clone EPR3947) (Roche, Basel, Switzerland).
All the slides were reviewed independently by two pathologists who were blinded to the clinical information. MMR stains were interpreted as abnormal (loss of nuclear staining in all tumor cells) and normal (retained nuclear staining) [21, 22]. Lymphocytes and stromal cells served as positive internal controls.
Statistical analyses
Continuous data are presented as the median/mean (range), and categorical data are presented as proportions. Parametric Student’s t tests were utilized to evaluate continuous variables, while chi-square tests (continuity correction chi-square) were used to evaluate categorical variables. Survival time was evaluated using the Kaplan–Meier model. All reported P values were two-sided, and P < 0.05 was considered statistically significant. Statistical Package for Social Science (SPSS) (Version 17.0, SPSS, Inc., Chicago, IL, USA) was used for the statistical analyses, and GraphPad Prism (Version 5.0, GraphPad Software, Inc., La Jolla, CA, USA) was used to generate the figures.
Results
Clinical features of the study patients
In total, 108 patients were included in the present study after excluding 8 cases with immunohistochemistry technical failure. For the entire cohort, the median age was 52 years (mean of 51.8 years and range of 26–79 years), and 37.0% of the patients were 50 years or younger. Nine patients had a personal history of cancer and/or synchronous cancer. Of these patients, four were diagnosed synchronously with a malignancy of the endometrium (n = 3) or colon (n = 1). The patient with synchronous colon and ovarian cancer developed endometrial cancer 2 years later. A previous history of breast cancer and thyroid cancer was noted in two patients and one patient, respectively. The remaining two patients had metachronous urothelial cell cancer and lung cancer after the diagnosis of ovarian CCC. In addition, 23 patients reported a family history of cancer, mostly colorectal cancer (n = 4), pancreatic cancer (n = 3) and urothelial cell cancer (n = 3).
Table 1 shows that 62.3% of the patients presented with early-stage disease (FIGO I + II), and most of these patients (45.3%) presented with FIGO stage I. Moreover, 25.9% of the patients had concurrent endometriosis, and 91.2% of patients had residual disease ≤1 cm. For the patients with advanced disease, the debulking results indicated that 41.7% of the patients (15/36) had no gross residual disease and that 66.7% of the patients (24/36) had residual disease ≤1 cm. Concerning the chemotherapy response, platinum-sensitive recurrence accounted for 69.6% of the patients.
Clinicopathological features of patients with defective MMR
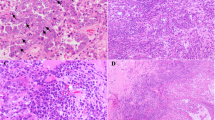
A total of six (5.6%) patients harbored abnormal MMR expression, including MSH2/MSH6 (n = 4) and MLH1/PMS2 (n = 2) (Fig. 1). Table 2 shows the clinical and pathological characteristics of ovarian CCC patients with deficient MMR. The average age of the six MMR-deficient patients was 45.6 years, which was younger than patients with MMR-intact tumors (average age of 52.1 years), but statistical significance was not achieved (P = 0.153). In addition, the rate of MMR-deficient tumors in women ≤50 years was relatively higher than that in those over 50 years (10.0% vs. 2.9%; P = 0.266, continuity correction chi-square) (Supplementary Table 1). In terms of personal history of cancer, half of the patients with deficient MMR were diagnosed with synchronous or metachronous cancer, which was significantly higher than their counterparts with intact expression (P = 0.002, continuity correction chi-square) (Supplementary Table 1). Two patients had synchronous endometrioid endometrial cancer (No. 3 and No. 5). One patient (No. 2) had an accidental diagnosis of ovarian CCC during the scheduled colorectal cancer surgery and was diagnosed with metachronous endometrioid endometrial cancer 2 years later. A family history of cancer was reported in two patients (No. 3 and No. 6). All six patients had early-stage (FIGO I + II) tumors at first diagnosis. Concerning platinum response, the majority (5/6, 83.3%) of the patients were platinum sensitive.
The two patients with a family history underwent subsequent genetic testing. Patient No. 6 was demonstrated to carry the following MHL1 germline mutation: c.1756G > C (p. Ala586Pro) (Class 5, pathogenic [23]). Patient No. 3 had synchronous endometrial cancer and ovarian CCC at diagnosis. In addition, patient No. 3 had a significant family history as follows: her mother had endometrial cancer, and two brothers of her mother had colorectal and pancreatic cancer. She was found to have a complex gene rearrangement in MSH2, which has never been reported (Class 3, uncertain significance [23]). Further multiplex ligation-dependent probe amplification tests were negative. Based on the gene mutation test, the diagnosis of underlying Lynch syndrome for patient No. 3 should be made with caution despite the clinical history.
Survival analysis
Follow-up information was available in the majority of the patients (96.3%, 104/108). After a mean follow-up time of 46 months (range, 1–178 months), 46.1% of patients (48/104) died of disease, 20.2% of patients (21/104) were still alive with disease, and 33.7% of patients (35/104) had no evidence of disease. Of note, 26 patients had a follow-up time less than 24 months. Of these, six patients were alive without disease, and the remaining 20 patients all died. Figure 2 shows the survival curves for the entire cohort stratified by stage. The median PFS of patients with early and late disease was 35 and 12 months (P = 0.002), respectively (Fig. 2A). Similarly, the median OS of patients with early-stage tumors was significantly better than that of patients with advanced disease (109 vs. 31 months, P < 0.001) (Fig. 2B). The estimated 5-year overall survival was 70.3% in patients with early-stage tumors and 20.7% in those with advanced disease.
We next evaluated the prognostic implication of MMR deficiency in the study population. The median PFS was higher in patients with abnormal MMR expression than in those with intact expression (54 months vs. 27 months), but statistical significance was not achieved (P = 0.471) (Fig. 2C). Considering that all patients with MMR deficiency had early-stage tumors, we further evaluated the prognostic impact of MMR deficiency in patients with early-stage tumors (Fig. 2D), but statistical significance was not achieved in terms of PFS. Overall survival comparison was not made given that all the patients with MMR deficient tumors were still alive (data censored) at last contact.
Discussion
Several recent publications focusing on MMR evaluation in ovarian CCC are summarized in Table 3. Five of the eight studies included relatively small sample sizes due to disease rarity [13,14,15,16, 18]. The prevalence of MMR deficiency ranges from 0 to 13% [11,12,13,14,15,16,17,18]. Bennett et al. conducted the largest series on whole section slides and correlated MMR expression to histological features [11]; they reported that diffuse intratumoral stromal inflammation and the presence of peritumoral lymphocytes may be associated with MMR loss in ovarian CCC [11]. Another study with a large sample size that assessed PROMISE algorithm-related markers, including MMR, in ovarian CCC by tissue microarray [17] has demonstrated a low frequency of abnormal MMR (2%) and no pathogenic DNA polymerase ε (POLE) mutation [17].
Universal testing of MMR in ovarian cancer is not routine in most hospitals, and the National Comprehensive Cancer Network (NCCN) guidelines recommend it as clinically indicated. Not surprisingly, higher frequencies of defective MMR have been reported in younger patients in several studies [9, 11, 12, 24]. Rambau et al. tested MMR proteins in 612 ovarian cancer patients by tissue microarray and found that deficient MMR is related to age < 50 years, synchronous endometrial endometrioid cancer and absence of ARID1A [12]. A previous study with a relatively large sample size focusing on ovarian CCC alone (n = 109) has shown that patients with abnormal MMR expression are significantly younger with a mean age of 40 years compared to 53.2 years for the overall cohort [11]. In the present cohort, the mean age of the six patients with loss of MMR expression was 45.6 years compared to 52.1 years for patients with MMR-intact tumors, but this difference was not statistically significant. Nevertheless, we noted a rate of 10.0% MMR deficiency in patients aged 50 years and younger.
MMR-deficient tumors have peculiar clinical behaviors, including early-onset metastatic potential, but generally favorable prognosis and remarkable responses to immune therapy [25]. The possible clinical implications of MMR deficiency in ovarian CCC have been evaluated in the literature [11, 12, 17]. However, no consensus has been reached, mainly due to disease rarity and the low frequency of MMR abnormalities. In the present study, six MMR-deficient patients had early-stage disease, which was consistent with the finding that MMR-deficient colorectal cancers are strongly enriched in the early stages of diagnosis [26]. Moreover, patients with loss of MMR expression tended to have longer progression-free survival than those patients with preserved expression, but the difference was not significant. Although the present study was based on a small number of cases, our findings suggested that MMR-deficient tumors may confer a good prognosis in ovarian CCC. Similarly, the prognostic implication of MMR deficiency in ovarian CCC has been evaluated in two studies [11, 17], but no conclusion has been reached. Stewart et al. reported that two patients with advanced tumors harboring MMR abnormalities were alive at 160 months and 124 months following surgery [14].
To the best of our knowledge, the present study represents one of the largest series measuring MMR proteins in ovarian clear cell carcinoma patients. However, the present study had several limitations. First, considering disease rarity, we collected the cases over a long period of time, which led to missing data. Second, the cohort may be limited by the selection and surveillance biases often associated with studies from a single institution. Last, the present study did not evaluate the specific regimen of treatment, which may be a confounding factor for survival outcome.
Conclusions
The present study showed MMR loss in 5.6% of unselected tumors of ovarian clear cell carcinoma, but this rate increased to 10% when selecting for age (50 years and below). All patients presented with early-stage disease, and half of the patients had synchronous/metachronous endometrial/colorectal cancer. Patients with MMR deficiency seemed to have better progression-free survival when all patients were analyzed, but the difference was not as significance when only the early-stage group was investigated. Thus, additional studies are required to determine whether patients harboring MMR abnormalities have a favorable prognosis.
Availability of data and materials
The dataset supporting the conclusions of this article is available upon request. Please contact Prof. Huijuan Yang (huijuanyang@hotmail.com).
Abbreviations
- MMR:
-
Mismatch repair
- CCC:
-
Clear cell carcinoma
References
Kobel M, Kalloger SE, Boyd N, McKinney S, Mehl E, Palmer C, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med. 2008;5:e232.
Oda K, Hamanishi J, Matsuo K, Hasegawa K. Genomics to immunotherapy of ovarian clear cell carcinoma: unique opportunities for management. Gynecol Oncol. 2018;151:381–9.
del Carmen MG, Birrer M, Schorge JO. Clear cell carcinoma of the ovary: a review of the literature. Gynecol Oncol. 2012;126:481–90.
Crotzer DR, Sun CC, Coleman RL, Wolf JK, Levenback CF, Gershenson DM. Lack of effective systemic therapy for recurrent clear cell carcinoma of the ovary. Gynecol Oncol. 2007;105:404–8.
Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and antitumor activity of anti-PD-1 antibody, Nivolumab, in patients with platinum-resistant ovarian Cancer. J Clin Oncol. 2015;33:4015–22.
Matulonis Ursula A, Ronnie S-F, Alessandro S, Sergeevua LA, Sandro P, Ignace V, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: Interim results from the phase 2 KEYNOTE-100 study. J Clin Oncol. 2018;36:5511.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science (New York, NY). 2017;357:409–13.
Li GM. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008;18:85–98.
Vierkoetter KR, Ayabe AR, VanDrunen M, Ahn HJ, Shimizu DM, Terada KY. Lynch syndrome in patients with clear cell and endometrioid cancers of the ovary. Gynecol Oncol. 2014;135:81–4.
Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76:1–18.
Bennett JA, Morales-Oyarvide V, Campbell S, Longacre TA, Oliva E. Mismatch repair protein expression in clear cell carcinoma of the ovary: incidence and morphologic associations in 109 cases. Am J Surg Pathol. 2016;40:656–63.
Rambau PF, Duggan MA, Ghatage P, Warfa K, Steed H, Perrier R, et al. Significant frequency of MSH2/MSH6 abnormality in ovarian endometrioid carcinoma supports histotype-specific Lynch syndrome screening in ovarian carcinomas. Histopathology. 2016;69:288–97.
Howitt BE, Strickland KC, Sholl LM, Rodig S, Ritterhouse LL, Chowdhury D, et al. Clear cell ovarian cancers with microsatellite instability: a unique subset of ovarian cancers with increased tumor-infiltrating lymphocytes and PD-1/PD-L1 expression. Oncoimmunology. 2017;6:e1277308.
Stewart CJ, Bowtell DD, Doherty DA, Leung YC. Long-term survival of patients with mismatch repair protein-deficient, high-stage ovarian clear cell carcinoma. Histopathology. 2017;70:309–13.
Willis BC, Sloan EA, Atkins KA, Stoler MH, Mills AM. Mismatch repair status and PD-L1 expression in clear cell carcinomas of the ovary and endometrium. Mod Pathol. 2017;30:1622–32.
Xiao X, Dong D, He W, Song L, Wang Q, Yue J, et al. Mismatch repair deficiency is associated with MSI phenotype, increased tumor-infiltrating lymphocytes and PD-L1 expression in immune cells in ovarian cancer. Gynecol Oncol. 2018;149:146–54.
Parra-Herran C, Bassiouny D, Lerner-Ellis J, Olkhov-Mitsel E, Ismiil N, Hogen L, et al. p53, mismatch repair protein, and POLE abnormalities in ovarian clear cell carcinoma: an outcome-based Clinicopathologic analysis. Am J Surg Pathol. 2019;43(12):1591–9.
Fraune C, Rosebrock J, Simon R, Hube-Magg C, Makrypidi-Fraune G, Kluth M, et al. High homogeneity of MMR deficiency in ovarian cancer. Gynecol Oncol. 2020;156:669–75.
Chui MH, Ryan P, Radigan J, Ferguson SE, Pollett A, Aronson M, et al. The histomorphology of Lynch syndrome-associated ovarian carcinomas: toward a subtype-specific screening strategy. Am J Surg Pathol. 2014;38:1173–81.
Ye S, Yang J, You Y, Cao D, Bai H, Lang J, et al. Comparative study of ovarian clear cell carcinoma with and without endometriosis in People’s Republic of China. Fertil Steril. 2014;102:1656–62.
Bi R, Tu XY, Xiao YX, Shan BE, Wang HY, Cai X, et al. Expression of DNA mismatch repair protein in endometrial carcinomas and its correlation with clinicopathologic features. Zhonghua Bing Li Xue Za Zhi. 2016;45:302–7.
Zhang Q, Wang L, Ni S, Tan C, Cai X, Huang D, et al. Clinicopathological features and prognostic value of mismatch repair protein deficiency in gastric cancer. Int J Clin Exp Pathol. 2018;11:2579–87.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Jensen KC, Mariappan MR, Putcha GV, Husain A, Chun N, Ford JM, et al. Microsatellite instability and mismatch repair protein defects in ovarian epithelial neoplasms in patients 50 years of age and younger. Am J Surg Pathol. 2008;32:1029–37.
Germano G, Amirouchene-Angelozzi N, Rospo G, Bardelli A. The clinical impact of the genomic landscape of mismatch repair-deficient cancers. Cancer Discov. 2018;8:1518–28.
Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466–74.
Acknowledgments
None.
Funding
The present study was supported by grants from the National Natural Science Foundation of China (81702558) and Fudan University Shanghai Cancer Center (YJ201603). The funding bodies did not participate in the design of the study or the collection, analysis and interpretation of the data as well as the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the conception and design of the study. Data curation: Shuang Ye, Shuling Zhou, Siyuan Zhong, Boer Shan, Wenhua Jiang and Xu Cai. Formal analysis: Shuang Ye, Shuling Zhou, Siyuan Zhong, Wenhua Jiang, Xu Cai and Huijuan Yang. Funding acquisition: Shuang Ye. Investigation: Shuang Ye, Shuling Zhou, Siyuan Zhong, Wenhua Jiang, Xu Cai and Huijuan Yang. Methodology: Wentao Yang, Xu Cai and Huijuan Yang. Project administration: Shuang Ye, Boer Shan, Wentao Yang, Xu Cai and Huijuan Yang. Resources: Shuang Ye, Shuling Zhou, Siyuan Zhong, Boer Shan and Huijuan Yang. Software: Shuling Zhou, Siyuan Zhong, Wenhua Jiang, Wentao Yang and Xu Cai. Supervision: Wentao Yang, Xu Cai and Huijuan Yang. Visualization: Shuang Ye, Shuling Zhou and Siyuan Zhong. Writing - original draft: Shuang Ye, Shuling Zhou and Siyuan Zhong. Writing - review and editing: Boer Shan, Wentao Yang, Xu Cai and Huijuan Yang. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board at Fudan University Shanghai Cancer Center. The requirement for written informed consent was waived by the Institutional Review Board at Fudan University Shanghai Cancer Center due to the retrospective design.
Consent for publication
Not applicable.
Competing interests
The authors have nothing to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Clinical features of the study population (n = 108).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ye, S., Zhou, S., Zhong, S. et al. The frequency and clinical implication of mismatch repair protein deficiency in Chinese patients with ovarian clear cell carcinoma. BMC Cancer 22, 449 (2022). https://doi.org/10.1186/s12885-022-09588-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09588-z