Abstract
Background
The revised 8th Edition American Joint Committee on Cancer (AJCC) Head and Neck Staging Manual distinguishes HPV-mediated from non-HPV-mediated oropharyngeal cancer (OpSCC). The objective was to analyze OpSCC treatment modalities and outcomes.
Methods
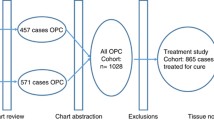
A retrospective study of OpSCC patients treated with radiotherapy or chemoradiotherapy between January 1st, 2000, and December 31st, 2008, as identified from the BC Cancer Registry. All patients received treatment at cancer clinics and had at least 5 years follow-up post-treatment. A total of 1259 OpSCC patients were identified. After initial chart reviews, 288 patients were excluded from further analysis and the majority (n = 198) was due to not receiving curative treatment. Based on the availability of formalin-fixed, paraffin-embedded (FFPE) tissue, patients were divided into two cohorts: Study Cohort (FFPE available, n = 244) and General Cohort (FFPE unavailable, n = 727). The Study Cohort was restaged according to AJCC 8th Edition based on p16 immunohistochemistry status. Kaplan-Meier analysis was used to estimate the 5-year overall survival (OS), disease-specific survival (DSS), and locoregional recurrence-free survival (LFS).
Results
Among 971 patients, OpSCC age-adjusted incidence rate was observed to have increased from 2.1 to 3.5 per 100,000 between 2000 and 2008. The General Cohort was relatively older than the Study Cohort (60.1 ± 10.5 vs. 57.3 ± 9.4), but both cohorts were predominantly males (78.3% vs. 76.2%). Amongst the Study Cohort, 77.5% were p16-positive, of whom 98.4% were down staged in the 8th Edition. These early-stage patients showed OS improvement for those treated with chemoradiation, compared to radiation alone (85.8% vs. 73.1%, p = 0.05).
Conclusions
OpSCC incidence is increasing in BC. The addition of chemotherapy to radiotherapy may portend a benefit in OS even for early-stage p16-positive OpSCC. Additional research is necessary to assess the safety of treatment de-escalation even among early-stage disease.
Similar content being viewed by others
Background
Efforts to reduce tobacco use have resulted in a decline of the incidence of head and neck cancer. However, oropharyngeal squamous cell carcinoma (OpSCC) continues to increase [1]. Persistent high-risk human papillomavirus (HR-HPV) infections can lead to cancer development and HPV subtypes 16 and 18 has been recognized to cause OpSCC [2]. In conjunction with an increase in the prevalence of oral HPV in the population [3, 4], an accompanied increase in the incidence of HPV-mediated OpSCC is being observed [5].
HPV-positive OpSCC patients often present with small primary tumors, but high nodal burden, compared to their HPV-negative counterparts [6, 7]. The 7th Edition of the American Joint Committee on Cancer (AJCC) Head and Neck Staging Manual, which stresses the significance of nodal involvement [8], will stage most HPV-positive OpSCC to locally advanced disease. Treatment for OpSCC disease is either primary radiation therapy (RT), with or without chemotherapy, or primary surgery, with or without adjuvant RT and/or chemotherapy. Independent of treatment modality, HPV-positive OpSCC patients have significantly improved outcomes compared to HPV-negative OpSCC [9], which has stimulated trials to study the effect of treatment de-escalation, in the hopes of limiting treatment-induced side effects whilst maintaining locoregional control [10, 11]. In recognition of HPV-positive OpSCC being a distinct clinical entity, the 8th Edition of the AJCC Head and Neck Staging Manual has included HPV status, determined by p16 overexpression, as part of its OpSCC staging [12].
The main objectives of this study were to review the trends of OpSCC patients in British Columbia (BC) and to associate survival outcomes with treatment modality of patients with OpSCC.
Methods
Study population – general and study cohorts
Patients with OpSCC referred to the BC Cancer Agency (BCCA) for treatment between January 1st, 2000, and December 31st, 2008, were retrospectively identified from the BC Cancer Registry. OpSCC patients included anatomical site codes of C01.9 (base of tongue), C02.4 (lingual tonsil), C05.1 (soft palate), C05.2 (uvula), C09 (tonsil), and C10 (oropharynx), following the 3rd Edition of the International Classification of Diseases for Oncology (ICD-O-3) [13]. Additional ICD-O-3 morphological codes of 80,103, 80,203, 80,213, 80,523, 80,703, 80,713, 80,723, 80,733, 80,743, and 80,763 further defined patients for pathological diagnoses of carcinomas and squamous cell carcinomas. Incidence rates of newly diagnosed OpSCC patients were age-adjusted to standardize against the population of BC in 2000. Patient charts were electronically reviewed through the Cancer Agency Information System (CAIS) to collect information on age at diagnosis, sex, primary anatomical site, smoking history, intent and type of primary treatment received, and patient survival 5 years post-diagnosis. Smoking history was categorized as ever-smokers, defined as patients that were former or current smokers, or never-smokers, patients that have never smoked. Patients referred to the BCCA were mainly treated using RT alone or RT with concurrent chemotherapy (CRT). Due to its small number, curative surgical patients referred to the BCCA for adjuvant RT/CRT were excluded from this study. For patient survival, the last follow-up appointment date or the death date, was recorded as the last contact date for alive or deceased patients, respectively. Among deceased patients, the cause of death was categorized as death due to disease or death due to other causes.
A subset of the Study Population with available formalin-fixed, paraffin-embedded (FFPE) tumor biopsy tissues was furthered analyzed as the Study Cohort. The remaining Study Population without FFPE tissues were considered as the General Cohort. The electronic charts of the Study Cohort were further reviewed for TNM staging, adjuvant treatment (if any), and recurrences. The dates for recurrences were collected from reviewing, in descending order of availability, pathology reports, imaging results, or clinical assessments. This study was approved by the University of British Columbia Research Ethics Board (REB #H10–03153).
Restaging of patients in the study cohort
The Study Cohort consisted of patients that were diagnosed between 2000 and 2008, which used the 7th Edition of the AJCC Head and Neck Cancer Staging Manual for staging. The 8th Edition AJCC Head and Neck Cancer Staging Manual was published in October 2016, and went into effect for cancer cases diagnosed on or after January 1st, 2018. Patients in the Study Cohort were restaged using the AJCC 8th Edition criteria prior to survival analysis. HPV status was first determined by p16 immunohistochemistry (IHC) staining, according to the manufacturer’s protocol, using the E6H4 clone (mouse monoclonal primary antibody, ready-to-use; Ventana Medical Systems, Inc., Tucson, AZ, USA) [12]. Tissue sections, consisted of either cases from whole tissue sections (n = 40 cases, 5-μm thick FFPE) or tissue microarray cores (n = 204 cases, two 0.6-mm cores per case), were scored at 200-400x magnification by a certified oral pathologist. Positive p16 staining was defined as strong diffuse nuclear and cytoplasmic staining in ≥70% of tumor areas [14].
Following p16 IHC staining, all p16-positive patients in the Study Cohort, were restaged according to the 8th Edition AJCC Head and Neck Staging Manual [12]. Due to limited pre-treatment MRI investigations, the presence of extranodal extension (ENE) could not be reliably attained and therefore, p16-negative patients in the Study Cohort were not restaged.
Statistical analysis
The Study Cohort was compared to the General Cohort for age at diagnosis, sex, primary anatomical site, smoking history, type of primary treatment received, and 5-year overall survival (OS). Sub-anatomical sites other than the tonsils or base of tongue were grouped together as “other oropharynx”. The continuous variable of age was analyzed using Student’s t-test. Fisher’s exact test was used to analyze the remaining categorical variables. Kaplan-Meier (KM) survival analysis was used to estimate the 5-year OS, disease-specific survival (DSS), and locoregional recurrence-free survival (LFS). Date of diagnosis, as based on pathology report, was used as the initial timepoint for time-to-outcome events. For 5-year OS, any cause of death was considered an event. For DSS, death due to the disease, including regional and distant metastasis, was considered an event. Local and regional recurrences were grouped into LFS and the earlier date was used as the endpoint for patients that developed both recurrences at separate instances. Log-rank tests were used to determine the statistical significance between groups. Statistical analysis was conducted using the R software (ver. 3.3.3, R Core Team, Vienna, Austria) and KM survival analysis was conducted using the survival package (ver. 2.40–1). Results were considered statistically significant at p ≤ 0.05.
Results
Burden of OpSCC in British Columbia
Based on the inclusion criteria, 1259 patients were identified from the BC Cancer Registry. After initial chart reviews, 288 patients were excluded from further analysis due to not receiving curative treatment (n = 198), the treatment prescribed was not primarily RT (n = 65) or incomplete data (n = 25). A total of 971 patients were included for analysis, representing 77.1% of the total OpSCC cases diagnosed in BC. During this time period, the age-adjusted incidence rate (AAIR) of OpSCC treated with curative intent radiotherapy with or without chemotherapy increased from 2.1 to 3.5 per 100,000 population, with the male AAIR increasing from 3.2 to 6.5 per 100,000, and the female AAIR decreasing from 1.1 to 0.7 per 100,000 (Fig. 1).
Patient demographics and clinical characteristics between study and general cohorts
Among 971 analyzed patients, 25.1% of the patients (n = 244) had available FFPE tissues for p16 IHC staining and referred as the Study Cohort, with the remaining patients (n = 727) referred as the General Cohort. Comparison of the two cohorts assessed the representativeness of the Study Cohort (Table 1). Compared to the General Cohort, the Study Cohort had a younger mean age at diagnosis and higher representation of tonsil primary. Radiation was administered at a median dosage of 66 Gy. Among CRT treated patients, Cisplatin was the most commonly administered chemotherapy agent in both General (66.7%, n = 198) and Study Cohorts (78.7%, n = 85). In the Study Cohort, 21.3% of patients (n = 52) developed locoregional recurrence.
Restaging the study cohort to 8th edition
The FFPE was stained for p16 IHC and revealed p16-positivity rate of 77.5% (Table 1). Compared to p16-negative patients, p16-positive patients were relatively younger, more likely to have tonsillar primaries, more likely to be never-smokers, and had better 5-year OS. There were no statistically significant differences in terms of sex (p = 0.21), and primary treatment (CRT vs. RT alone, p = 0.22) between p16-positive and p16-negative tumors.
With available p16 status, p16-positive (n = 189) patients were restaged according to the 8th Edition of the AJCC Head and Neck Staging Manual (Additional File 1) [12]. In our Study Cohort, 98.4% of p16-positive patients were down staged, such that 88.9% of p16-positive patients that were either stage III or IV (7th Edition), have been reduced to 16.9% as stage III (8th Edition). Overall Kaplan-Meier survival analysis for p16-positive patients, based on stage according to the 7th and 8th Edition, is presented in Fig. 2A and B, respectively. The 8th Edition was superior to the 7th Edition in stratifying 5-year OS. There was no statistically significant difference in 5-year OS between stages II, III, and IV (72.8, 65.0 and 66.8%, respectively, p = 0.91) based on the 7th Edition. In contrast, the 8th Edition stage III patients had significantly poorer 5-year OS (51.0%) when compared to both stage I (79.8%, p < 0.01) and stage II (74.9%, p = 0.04). The 55 p16-negative patients could not be restaged based on the 8th Edition because of the inconsistent use of MRI to determine ENE status.
Association of Survival with treatment modality
Outcomes for 5-year OS, DSS, and LFS were compared based on 8th Edition staging and treatment modality of either CRT or RT alone (Fig. 3). Interestingly, a comparison of early-stage patients (n = 157) found that those who received CRT (n = 69) than RT (n = 88) had improved 5-year OS (85.8% vs. 73.1%, p = 0.05), DSS (90.3% vs. 80.4%, p = 0.07), and LFS (93.6% vs. 76.9%, p < 0.01) (Fig. 3A-C). Stage III patients (n = 32) who received CRT (n = 19) than RT (n = 13) also had improved 5-year OS (56.7% vs. 43.3%, p = 0.27), DSS (60.0% vs. 47.2%, p = 0.31) and LFS (72.7% vs. 68.4%, p = 0.53), but none reaching statistical significance (Additional File 2).
Kaplan-Meier survival and log-rank analysis of treatment for early-stage, p16-positive oropharyngeal squamous cell carcinoma patients for 5-year overall survival (A), disease-specific survival (B), and locoregional recurrence-free survival (C). Patients were assessed using the 8th Edition for early-stages of the American Joint Committee on Cancer Head and Neck Staging Manual. Abbreviations: RT: Radiotherapy; CRT: Concurrent chemoradiotherapy
Discussion
The current study retrospectively reviewed the population based incidence and management of OpSCC treated with curative intent radiotherapy with or without chemotherapy, in BC between 2000 and 2008. BC is a province of Canada with a population of ~ 5 million, where all newly diagnosed OpSCC are registered at a central intake and treated at one of six cancer agencies. While tobacco consumption continues to decline in BC [15], the AAIR of OpSCC over the study period has increased from 2.1 to 3.5 per 100,000, mostly comprised of males patients. This trend is witnessed worldwide and is attributed to HPV-induced oncogenesis [9]. Consistent with previous literature, the p16-positive patients, compared to p16-negative, had a higher proportion of males, younger age, decreased tobacco use, and a drastically improved 5-year OS [9, 16,17,18].
Since the introduction of the 8th Edition AJCC Head and Neck Staging Manual, studies have evaluated the improved prognostic predictability of this new staging criteria [19,20,21]. Indeed, the provincial-wide data presented here, reveals no significant difference in OS for stage I to IV patients, based on the AJCC 7th Edition. However, p16-positive patients restaged using the 8th Edition revealed a significant difference of 5-year OS when comparing Stage III (51.0%) to either Stage I (79.8%) or Stage II (74.9%). In this study, patients were not routinely stained for p16 upon initial diagnosis and therefore, management was not influenced by HPV status.
Restaging the present cohort according to the 8th Edition found a significant stage redistribution. Notably, early-stage disease patients who received CRT than RT alone revealed an improved DSS, and significant improvement in OS and LFS. The addition of chemotherapy to RT appears play some role to improve OS even for early-stage disease. A study from the National Cancer Database (NCDB) restaged patients from the 7th to 8th Edition, reported a similar 3-year OS of stage I patients compared to our stage I patient population of 90.3% vs. 91.3%, respectively. The NCDB study also revealed a statistically improved OS of stage I OpSCC treated with CRT vs. RT (91.3% vs. 80.6%), similar to our findings [21]. A retrospective review of ~ 280 node positive AJCC 8th stage I p16-positive OpSCC was designed to identify adverse features affecting prognosis. Using multivariate analysis, the addition of chemotherapy, which was received by 70% of stage I patients, was one of the four independent variables in predicting disease-free survival [22]. Importantly, treatment in the aforementioned studies, including the presented case series, was nonrandomly assigned, and therefore the perceived improvement in DFS and OS by the addition of systemic therapy could be biased.
Studies have demonstrated the inferiority of CRT with Cetuximab as compared to Cisplatin-based CRT for OS and locoregional control [23, 24]. Our study supported the use of Cisplatin-based concurrent CRT. In the RTOG 1016 study [23], 50.6% of stage I patients (n = 407/805) showed Cetuximab as inferior to Cisplatin. Similarly, the De-ESCALaTE HPV phase III trial [24] revealed a decrease in OS and an increase in recurrence rates in those randomized to Cetuximab vs. standard Cisplatin. In the NRG-HN002 phase II trial [25], patients receiving a reduced dose of curative RT without concurrent weekly Cisplatin experienced higher locoregional failure (9.5% vs. 3.3% respectively, p = 0.02) and worse progression free survival, compared to those receiving the same radiation therapy regimen with weekly Cisplatin. In our Study Cohort, early-stage patients who received CRT, rather than RT alone, had significantly improved 5-year LFS and OS. None of our Study Cohort patients received Cetuximab for comparison with contemporary studies. Whether Cisplatin is superior to other chemotherapy agents for improving patient outcome warrants further investigation.
Studies using de-escalation strategies are appealing for the p16-positive cohort. These strategies include concomitant CRT with possibility of less toxic chemotherapy regimens [23, 24], removal of chemotherapy (RT alone) [26], reducing the dose of RT [25, 27], and trans-oral surgery [28]. Our Study Cohort patients received the standard of care for RT, i.e., radiation dosage at 70 Gy, and the benefit of concurrent chemotherapy was still observed among our early-stage patients who had received CRT. Therefore, de-escalation studies that aim to reduce the RT dosage should be cautioned if concurrent chemotherapy is not administered, even for early-stage OpSCC.
One of the study limitation is that the study period predated the initiation of a Trans-Oral Robotics program (NCT01590355) [29] and therefore precluded surgery as a treatment modality for OpSCC. The application of trans-oral surgery in the context of treatment de-escalation has been studied prospectively in only one phase II randomized trial, but had its primary endpoint as the MD Anderson Dysphagia index and not oncologic outcomes [29]. Several prospective trials are currently underway with results pending [28, 30].
Other limitations of this study include the retrospective sample set with an inherent selection bias and an unequal stage distribution with limited numbers in some sub-groups precluding adequate power. Furthermore, the lack of controlling confounding clinical variables such as comorbidities and tobacco pack-years limits the findings and therefore the addition of chemotherapy to radiotherapy may not independently improve the outcome of early-stage p16-positive OpSCC patients. As well, it is possible that patients’ inherent health status precluded the addition of chemotherapy and therefore only suitable for RT alone and as a result may have correlated with their decreased survival. The Study Cohort was limited to the availability of FFPE on which p16 staining was performed, but nevertheless represented 25.1% of the total 971 analyzed patients. This resulted in a larger proportion of tonsillar subsite (vs. base of tongue) compared to that of the General Cohort, as this subsite is more amenable to biopsy with enough tissue for further HPV genotyping.
Conclusion
OpSCC has increased significantly in male patients in BC with a vast majority mediated by HPV, as determined by p16 IHC staining. Early-stage patients revealed a significant improvement in OS for those treated with CRT vs. RT alone. This study lends further support to the utility of the addition of chemotherapy to radiotherapy to improve OS, even for early-stage I/II p16-positive OpSCC. Further prospective study is required to investigate the oncologic safety of treatment de-escalation even for early-stage disease.
Availability of data and materials
The datasets used and/or analysed during the current study are not made publicly available due to ethics restrictions. However, the datasets are available from the corresponding author upon reasonable request.
Abbreviations
- OpSCC:
-
Oropharyngeal squamous cell carcinoma
- HR-HPV:
-
High-risk human papillomavirus
- AJCC:
-
American Joint Committee on Cancer
- BC:
-
British Columbia
- BCCA:
-
British Columbia Cancer Agency
- CAIS:
-
Cancer Agency Information System
- RT:
-
Radiation therapy
- CRT:
-
Concurrent chemoradiotherapy
- FFPE:
-
Formalin-fixed, paraffin-embedded
- IHC:
-
Immunohistochemistry
- ENE:
-
Extranodal extension
- KM survival analysis:
-
Kaplan-Meier survival analysis
- OS:
-
Overall survival
- DSS:
-
Disease-specific survival
- LFS:
-
Locoregional recurrence-free survival
- AAIR:
-
Age-adjusted incidence rate
- NCDB:
-
National Cancer Database
References
Pai SI, Westra WH. Molecular pathology of head and neck cancer: implications for diagnosis, prognosis, and treatment. Annu Rev Pathol. 2009;4:49–70.
Iarc Working Group on the Evaluation of Carcinogenic Risks to Humans. A Review of Human Carcinogens. B. Biological Agents. Lyon: WHO Press; 2012. p. 475.
Chaturvedi AK, Graubard BI, Broutian T, Pickard RK, Tong ZY, Xiao W, et al. NHANES 2009-2012 findings: Association of Sexual Behaviors with higher prevalence of Oral oncogenic human papillomavirus infections in U.S. Men Cancer Res. 2015;75(12):2468–77.
Gillison ML, Broutian T, Pickard RK, Tong ZY, Xiao W, Kahle L, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307(7):693–703.
Chaturvedi AK, Engels EA, Pfeiffer RM. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–301.
Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma--an update. CA Cancer J Clin. 2015;65(5):401–21.
McIlwain WR, Sood AJ, Nguyen SA, Day TA. Initial symptoms in patients with HPV-positive and HPV-negative oropharyngeal cancer. JAMA Otolaryngol Head Neck Surg. 2014;140(5):441–7.
Edge SBD, Compton C, Fritz A, Greene F, Trotti A. AJCC Cancer staging manual. 7th ed. New York: Springer; 2011. p. 648.
Ang KK, Harris J, Wheeler R. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35.
Forastiere AA. Chemotherapy in the treatment of locally advanced head and neck cancer. J Surg Oncol. 2008;97(8):701–7.
Fung N, Faraji F, Kang H, Fakhry C. The role of human papillomavirus on the prognosis and treatment of oropharyngeal carcinoma. Cancer Metastasis Rev. 2017;36(3):449–61.
Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer staging manual, vol. XVII. 8th ed. New York: Springer International Publishing; 2016. p. 1032.
Fritz AG. International classification of diseases for oncology: ICD-O. 3rd edition, First revision, vol. viii. Geneva: World Health Organization; 2013. p. 242.
Westra WH. Detection of human papillomavirus (HPV) in clinical samples: evolving methods and strategies for the accurate determination of HPV status of head and neck carcinomas. Oral Oncol. 2014;50(9):771–9.
Reid JL, Hammond D, Rynard VL, Madill CL, Burkhalter R. Tobacco use in Canada: patterns and trends, 2017 Edition. Waterloo: Propel Centre for Population Health Impact, University of Waterloo; 2017.
Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36):4550–9.
Cramer JD, Hicks KE, Rademaker AW, Patel UA, Samant S. Validation of the eighth edition American joint committee on Cancer staging system for human papillomavirus-associated oropharyngeal cancer. Head Neck. 2018;40(3):457–66.
Nauta IH, Rietbergen MM, van Bokhoven A, Bloemena E, Lissenberg-Witte BI, Heideman DAM, et al. Evaluation of the eighth TNM classification on p16-positive oropharyngeal squamous cell carcinomas in the Netherlands and the importance of additional HPV DNA testing. Ann Oncol. 2018;29(5):1273–9.
Zhan KY, Eskander A, Kang SY, Old MO, Ozer E, Agrawal AA, et al. Appraisal of the AJCC 8th edition pathologic staging modifications for HPV-positive oropharyngeal cancer, a study of the National Cancer Data Base. Oral Oncol. 2017;73:152–9.
Geltzeiler M, Bertolet M, Albergotti W, Gleysteen J, Olson B, Persky M, et al. Staging HPV-related oropharyngeal cancer: validation of AJCC-8 in a surgical cohort. Oral Oncol. 2018;84:82–7.
Cheraghlou S, Yu PK, Otremba MD, Park HS, Bhatia A, Zogg CK, et al. Treatment deintensification in human papillomavirus-positive oropharynx cancer: outcomes from the National Cancer Data Base. Cancer. 2018;124(4):717–26.
Billfalk-Kelly A, Yu E, Su J, O'Sullivan B, Waldron J, Ringash J, et al. Radiologic Extranodal extension portends worse outcome in cN+ TNM-8 stage I human papillomavirus-mediated Oropharyngeal Cancer. Int J Radiat Oncol Biol Phys. 2019;104(5):1017–27.
Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393(10166):40–50.
Mehanna H, Robinson M, Hartley A, Kong A, Foran B, Fulton-Lieuw T, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. 2019;393(10166):51–60.
Yom SS, Torres-Saavedra P, Caudell JJ, Waldron JN, Gillison ML, Xia P, et al. Reduced-dose radiation therapy for HPV-associated Oropharyngeal carcinoma (NRG oncology HN002). J Clin Oncol. 2021;39(9):956–65.
O'Sullivan B, Huang SH, Siu LL, Waldron J, Zhao H, Perez-Ordonez B, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31(5):543–50.
Misiukiewicz K, Gupta V, Miles BA, Bakst R, Genden E, Selkridge I, et al. Standard of care vs reduced-dose chemoradiation after induction chemotherapy in HPV+ oropharyngeal carcinoma patients: the quarterback trial. Oral Oncol. 2019;95:170–7.
Howard J, Dwivedi RC, Masterson L, Kothari P, Quon H, Holsinger FC. De-intensified adjuvant (chemo)radiotherapy versus standard adjuvant chemoradiotherapy post transoral minimally invasive surgery for resectable HPV-positive oropharyngeal carcinoma. Cochrane Database Syst Rev. 2018;12:CD012939.
Palma D. A Phase II Randomized Trial for Early-stage Squamous Cell Carcinoma of the Oropharynx: Radiotherapy vs Trans-oral Robotic Surgery (ORATOR) (Clinicaltrials.gov Identifier NCT01590355) 2012. Available from: https://ClinicalTrials.gov/show/NCT01590355.
Nichols AC, Lang P, Prisman E, Berthelet E, Tran E, Hamilton S, et al. Treatment de-escalation for HPV-associated oropharyngeal squamous cell carcinoma with radiotherapy vs. trans-oral surgery (ORATOR2): study protocol for a randomized phase II trial. BMC Cancer. 2020;20(1):125.
Acknowledgements
Not applicable.
Funding
This work was supported by the Terry Fox Research Institute (2009–24), the Dr. Michele Williams Education and Research Fund, the BC Cancer Foundation, and University of British Columbia - Oral Cancer Research Fund.
Author information
Authors and Affiliations
Contributions
XJDL and EP designed the study, acquired data, analyzed the data, and prepared the first draft of the manuscript. EJ and JC acquired and analyzed data. All authors edited and critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the University of British Columbia Research Ethics Board (REB #H10–03153). This study was carried out in accordance with the principles of the Declaration of Helsinki. Due to the retrospective nature of this study, the need for a written, informed consent was waived by the University of British Columbia Research Ethics Board.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Restaging p16-positive OpSCC patients from 7th to 8th Edition of the AJCC Cancer Staging Manual.
Additional file 2.
Kaplan-Meier survival and log-rank analysis of treatment for stage III, p16-positive oropharyngeal squamous cell carcinoma patients for 5-year overall survival (A), disease-specific survival (B), and locoregional recurrence-free survival (C). Patients were assessed using the 8th Edition of the American Joint Committee on Cancer Head and Neck Staging Manual. Abbreviations: RT: Radiotherapy; CRT: Concurrent chemoradiotherapy
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lu, X.J.D., Jackson, E., Chew, J. et al. Combined chemoradiotherapy showed improved outcome with early-stage HPV-positive oropharyngeal cancers. BMC Cancer 22, 513 (2022). https://doi.org/10.1186/s12885-022-09515-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09515-2