Abstract
Background
A more extensive surgical resection of glioma contributes to improved overall survival (OS) and progression-free survival (PFS). However, some patients miss the chance of surgical resection when the tumor involves critical structures.
Purpose
The present study aimed to assess the feasibility of neoadjuvant 125I brachytherapy followed by total gross resection for initially inoperable glioma.
Methods
Six patients diagnosed with inoperable glioma due to invasion of eloquent areas, bihemispheric diffusion, or large tumor volume received 125I brachytherapy. Surgical resection was performed when the tumor shrank, allowing a safe resection, assessed by the neurosurgeons. Patients were followed up after surgery.
Results
Shrinkage of the tumor after adjuvant 125I brachytherapy enabled a total gross resection of all six patients. Four patients were still alive at the last follow-up, with the longest survival time of more than 50 months, two of which returned to everyday life with a KPS of 100. Another two patients had neurological injuries with KPSs of 80 and 50, respectively. One patient with grade II glioma died 34 months, and another with grade IV glioma died 40 months after the combined therapy.
Conclusions
In the present study, the results demonstrated that 125I brachytherapy enabled a complete resection of patients with initially unresectable gliomas. 125I brachytherapy may offer a proper neoadjuvant therapy method for glioma.
Similar content being viewed by others
Background
Gliomas originate from glial, stem, or neuronal precursor cells. According to histology, World Health Organization (WHO) classified glioma into four grades (grade I-IV). Gliomas are now better characterized by molecular changes. Usually, grade I to II gliomas are low-grade gliomas, and grade III and IV gliomas are called high-grade or malignant gliomas [1]. For glioma, surgical resection was the first choice [1, 2]. Numerous studies have suggested the therapeutic efficacy of maximal surgical resection while avoiding neurological damage to improve progression-free survival (PFS) and overall survival (OS) [3]. However, due to the situation of eloquent areas, bihemispheric diffusion, or considerable volume limitation, the extent of resection (EOR) is limited [1, 4]. Recent studies suggested that preoperative chemotherapy could decrease the tumor volume, thereby facilitating a safe and extensive resection [5, 6] and prolonging the PFS and OS of patients with gliomas.
Gliomas easily recur due to their characteristic diffuse infiltrative spread from the origin site [7, 8]. Based on these characteristics, local treatment with 125I is possible. Brachytherapy with iodine-125 seeds offered a safe and effective local treatment option for patients, including glioma in the eloquent brain [9,10,11,12]. Furthermore, 125I brachytherapy showed advantages in tumor shrinkage as the 125I seeds were inserted into the tumors, continuously releasing γ rays. However, the feasibility of the therapy strategy, combining neoadjuvant 125I brachytherapy with subsequent surgical resection, has never been thoroughly evaluated to the best of our knowledge. Thus, we undertook the present study to assess whether neoadjuvant 125I brachytherapy could facilitate a safe total gross resection for patients with initially unresectable gliomas and whether the combination therapy is effective.
Methods
All methods were performed in accordance with the relevant guidelines and regulations.
Patient criteria
Patient data were obtained from the hospital database, spanning from January 01, 2016, to December 31, 2017. The retrospective study was approved by the Institutional Review Board of the local hospital (Reference number: QYFY WZ LL 26,579), and the requirement for informed consent was waived. The inclusion criteria were as follows: (1) Age ≥ 18 years old; (2) Enhanced MRI or CT deemed unresectable gliomas upon neurosurgical evaluation; (3) Combined therapeutic approach, consisting of neoadjuvant 125I brachytherapy and subsequent surgical resection; (4) Availability of adequate laboratory examination information, including hematologic parameters, clotting, hepatic and renal function, etc. Patients with glioma involving the brain stem or ependymal surface were excluded.
Iodine-125 implantation
Iodine-125 seeds were implanted, as we previously reported [12]. Briefly, patients were safely fixed on the CT bed with a negative pressure vacuum pad. Then, 125I seeds (diameter of 0.8 mm, length of 4.5 mm, seeds radioactivity: 0.7 mCi; half-life of 59.4 days; Model 7711, Beijing Atom and High Technique Industries, Inc., Beijing, China) were implanted according to the preimplantation plan made with the computerized treatment planning system (TPS; Beijing Astro Technology Ltd. Co., Beijing, China). Briefly, Urethral catheterization was performed after general anesthesia. A homemade locator was placed on the head to confirm the puncture point. Holes were made with an electric cranial drill, flat needles were inserted into the tumors, and 125I seeds were implanted with the needles. Dynamic CT scans were performed during the surgery to confirm the distribution of the seeds. The average number of seeds implanted into the six patients was 55 (range, 21–100). Patients were required to stay in bed for 24 h, and dehydration medications were given for 7–14 days. After the implantation of the 125I seeds, patients received outpatient examinations every two months. The neurosurgeons evaluated the examinations. Surgical resection was conducted when the glioma shrank to a suitable size.
Results
As Tables 1 and 2 show, two females and four males were included in this study. The median age was 43.5 years old (range 22–65). The median follow-up time from diagnosis was 32.5 months (range 21–47). The average number of seeds implanted into the six patients was 55 (range, 21–100). The median prescribed dose was 110 Gy (range, 90–140 Gy). The presenting symptoms were headache in three patients, nausea in one patient, vomiting in one patient, dizziness in one patient, myodynamia decline in one patient, vision decline in one patient, language disorders in two patients, and seizures in five patients. The median KPS was 85 (range 70–90). The anatomical location of the tumor was frontal in four patients, parietal in one patient, and left hippocampus in one patient. Three patients had low-grade gliomas, and the other three had high-grade gliomas. One patient had her first neuropathological diagnosis by biopsy; the other five were diagnosed with craniotomy. One patient with recurrent glioma had previously received surgical resection accompanied by external beam radiotherapy (total dose 50 Gy, 2 Gy/time) and chemotherapy with temozolomide (TMZ, 75 mg/m2). One patient had previously received bevacizumab treatment (400 mg/time, 4 times total). The other four patients received 125I brachytherapy as initial therapy. 125I seeds were implanted with TPS guidance, and verification of the dosage distribution was conducted immediately after implantation (Figs. 1 and 2). All patients received total surgical resection after 125I brachytherapy with a median therapy interval of 6 months (range 2–23 months).
Pre-implantation plan of 125I brachytherapy for a patient (Figs. 1, 2, 3 and 4 are from the same patient) with grade IV glioma. a The gross target volume (GTV) was outlined with a red line, the clinical target volume (CTV) was outlined with a blue line. Different colored lines showed the dose distribution. Additionally, the needle paths were designed with TPS. b Pre-implantation dose-volume histogram (DVH) of GTV and CTV. c Pre-implantation D100, D90, D80, V150, V100, V90 of the GTV and CTV
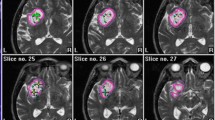
Post-implantation verification of 125I brachytherapy for a patient with grade IV glioma. a-d The gross target volume (GTV) was outlined with a red line, the clinical target volume (CTV) was outlined with a blue line. Different colored lines showed the dose distribution after 125I implantation. e-f 3D-reconstruction of the CT-MRI infusion images after 125I implantation. g Post-implantation DVH of GTV and CTV. h Post-implantation D100, D90, D80, V150, V100, V90 of the GTV and CTV
One patient with glioblastoma returned to everyday life with a KPS of 100 and was still alive at the last follow-up (survival time, 51 months) (Figs. 3, 4 and 5). Another patient with grade II oligodendroglioma had already survived for 52 months at the last follow-up, with a KPS of 100, and was still alive when we followed up. No neurological symptoms were found in these two patients after the combination therapy. They were free from seizures after the combined therapy. Another two patients with grade III and II gliomas were alive when we followed up (survival time: 33 months and 31 months, respectively), with KPSs of 50 and 80, respectively. One patient (grade II glioma) died with a survival time of 34 months. Furthermore, another patient with glioblastoma died with a survival time of 40 months.
Routine imaging examinations of the patients. A T2WI-Flair image of the patient on 2018.07.27. B T1WI-contrast image of the patient on Dec.16, 2018. C CT image of the patient on January 27, 2019 after the surgical resection. D T2WI-Flair image of the patient on Apr.22, 2019. E T2WI-Flair image of the patient on May 08, 2019. F T2WI-Flair image of the patient on June 23, 2019
Discussion
In the present study, we retrospectively analyzed patients with gliomas who did not qualify for surgical resection. 125I brachytherapy was used as adjuvant treatment for surgery. After significant tumor shrinkage due to 125I implantation, gross total resection was achieved in six cases, and the patients had a long survival time.
According to the European guidelines [2], surgical resection is the first-line treatment for gliomas, aiming to perform maximal tumor mass resection and avoid long-term neurological deficits. However, gliomas located in the eloquent cortex, deep structure, and corpus callosum or large volumes limit the extent of surgical resection, sometimes causing severe neurological deficits. Neoadjuvant therapy has been widely used in tumor downstaging, enabling secondary resectability [13]. Moreover, neoadjuvant therapy has been successfully used in glioma therapy [5, 6, 14]. 125I brachytherapy is emerging as a new therapeutic approach for tumors [15,16,17,18,19] and has also been used in glioma therapy, primarily as salvage therapy (Table 3). 125I brachytherapy showed effective local control of the gliomas. However, whether 125I brachytherapy can be used as a neoadjuvant therapy approach for patients with unresectable gliomas has not been explored.
In the present study, six patients received 125I brachytherapy. Surgical resection was not suitable for these patients due to the location and volume of the tumors. Two patients received other therapies before, and four received 125I brachytherapy as initial therapy. Most patients showed noticeable tumor shrinkage 2–8 months after 125I brachytherapy regardless of the tumor type. After a complete evaluation by the neurosurgeon, the six patients received total surgical resection of tumors at different time points after 125I brachytherapy. One patient with glioblastoma returned to everyday life after the combination therapy with a KPS of 100. No neurological system disorders were found after the therapy. Another patient with grade II oligodendroglioma also achieved a KPS of 100 after therapy and was free from seizures.
Furthermore, the patient was ready for marriage at the last follow-up. The two patients discussed above all faced a large tumor volume before the surgery. After therapy with 125I, the tumor volume decreased, which enabled total surgical resection. All six patients received the combination therapy of 125I brachytherapy followed by total surgical resection. Four of them were still alive when we last followed up, with a survival time of more than 31 months. Only one patient had language disorders after the therapy. Moreover, all the patients who survived were free from seizures. Two patients died with a survival time of more than 34 months.
The results showed that 125I brachytherapy showed practical efficacy in tumor shrinkage, enabling a subsequent total surgical resection. No severe neurological damage was found after the combined therapy. Furthermore, patients were free from seizures after the therapy. All patients survived a long time after therapy. For recurrent or primary gliomas, the combination therapy showed apparent efficacy. However, only six patients were included in this study, including low- and high-grade gliomas. A large number of cases should be included in further studies.
Conclusions
The results were not statistically significant. However, the results were positive and indicated a promising therapeutic approach for gliomas. Thus, we believe that neoadjuvant 125I brachytherapy followed by surgical resection might provide opportunities to patients with gliomas.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The data of the patients are available from the electronic medical record of the Affiliated Hospital of Qingdao University.
Abbreviations
- CT:
-
Computed Tomography
- EOR:
-
Extent of Resection
- HCC:
-
hepatocellular carcinoma
- KPS:
-
Karnofsky Performance Status
- MRI:
-
Magnetic Resonance Imaging
- OS:
-
Overall Survival
- PFS:
-
Progression-free survival
- TMZ:
-
Temozolomide
- TPS:
-
Treatment Planning System
- WHO:
-
World Health Organization
References
Eyüpoglu IY, Buchfelder M, Savaskan NE. Surgical resection of malignant gliomas—role in optimizing patient outcome. Nat Rev Neurol. 2013;9(3):141–51.
Soffietti R, Baumert B, Bello L, Von Deimling A, Duffau H, Frénay M, Grisold W, Grant R, Graus F, Hoang-Xuan K. Guidelines on management of low‐grade gliomas: report of an EFNS–EANO* Task Force. Eur J Neurol. 2010;17(9):1124–33.
Jo J, Williams B, Smolkin M, Wintermark M, Shaffrey ME, Lopes MB, Schiff D. Effect of neoadjuvant temozolomide upon volume reduction and resection of diffuse low-grade glioma. J Neurooncol. 2014;120(1):155–61.
Duffau H, Capelle L. Preferential brain locations of low-grade gliomas: Comparison with glioblastomas and review of hypothesis. Cancer. 2004;100(12):2622–6.
Jo J, Williams B, Smolkin M, Wintermark M, Shaffrey ME, Lopes MB, Schiff D. Effect of neoadjuvant temozolomide upon volume reduction and resection of diffuse low-grade glioma. J Neuro-Oncol. 2014;120(1):155–61.
Blonski M, Taillandier L, Herbet G, Maldonado IL, Beauchesne P, Fabbro M, Campello C, Gozé C, Rigau V, Moritz-Gasser S. Combination of neoadjuvant chemotherapy followed by surgical resection as a new strategy for WHO grade II gliomas: a study of cognitive status and quality of life. J Neuro-Oncol. 2012;106(2):353–66.
Hu X, Qiu H, Zhang L, Zhang W, Ma Y, Qiao Z, Chen D, Han J, Duan G, Zhang F. Recurrent gliomas. Cancer Biol Ther. 2014;13(10):840–7.
Diamandis P, Aldape K. World Health Organization 2016 Classification of Central Nervous System Tumors. Neurol Clin. 2018;36(3):439–47.
Schwarz SB, Thon N, Nikolajek K, Niyazi M, Tonn J-C, Belka C, Kreth F-W. Iodine-125 brachytherapy for brain tumours-a review. Radiation Oncol. 2012;7(1):30.
Ruge MI, Kickingereder P, Simon T, Treuer H, Sturm V. Stereotactic iodine-125 brachytherapy for treatment of inoperable focal brainstem gliomas of WHO grades I and II: feasibility and long-term outcome. J Neuro-Oncol. 2012;109(2):273–83.
Ruge MI, Kickingereder P, Grau S, Dorn F, Galldiks N, Treuer H, Sturm V. Stereotactic iodine-125 brachytherapy for the treatment of WHO grades II and III gliomas located in the central sulcus region. Neuro Oncol. 2013;15(12):1721–31.
Wang C, Liu S, Peng L, Zhang K, Li W, Zhang H, Luan Y, Li P, Hu X. Permanent iodine-125 brachytherapy for patients with progressive or recurrent high-grade gliomas. BMC Cancer. 2020;20(1):591.
Burotto M, Wilkerson J, Stein WD, Bates SE, Fojo T: Adjuvant and neoadjuvant cancer therapies: a historical review and a rational approach to understand outcomes. In: Seminars in oncology: 2019: Elsevier; 2019: 83–99.
Blonski M, Pallud J, Gozé C, Mandonnet E, Rigau V, Bauchet L, Fabbro M, Beauchesne P, Baron M-H, Fontaine D. Neoadjuvant chemotherapy may optimize the extent of resection of World Health Organization grade II gliomas: a case series of 17 patients. J Neuro-Oncol. 2013;113(2):267–75.
Kickingereder P, Hamisch C, Suchorska B, Galldiks N, Visser-Vandewalle V, Goldbrunner R, Kocher M, Treuer H, Voges J, Ruge MI. Low-dose rate stereotactic iodine-125 brachytherapy for the treatment of inoperable primary and recurrent glioblastoma: single-center experience with 201 cases. J Neuro-Oncol. 2014;120(3):615–23.
Yu Y-h, Wei C-y, Qin Q-h, Mo Q-g, Huang Z, Lian B. Efficacy of Iodine-125 Seed Implantation in Locoregionally Recurrent and Unresectable Breast Cancer: a Retrospective Study. Pathol Oncol Res. 2017;25(1):327–32.
He Y, Li L, Liu J, Zhang X. Iodine-125 seed brachytherapy inhibits non-small cell lung cancer by suppressing epithelial-mesenchymal transition. Brachyther. 2018;17(4):696–701.
Chen K, Chen G, Wang H, Li H, Xiao J, Duan X, He J, He K, Xiang G. Increased survival in hepatocellular carcinoma with iodine-125 implantation plus radiofrequency ablation: A prospective randomized controlled trial. J Hepatol. 2014;61(6):1304–11.
Zhang W, Li J, Li R, Zhang Y, Han M, Ma W: Efficacy and safety of iodine-125 radioactive seeds brachytherapy for advanced non–small cell lung cancer—A meta-analysis. Brachyther 2018, 17(2):439–448.
Suchorska B, Hamisch C, Treuer H, Mahnkopf K, Lehrke RE, Kocher M, Ruge MI, Voges J. Stereotactic brachytherapy using iodine 125 seeds for the treatment of primary and recurrent anaplastic glioma WHO degrees III. J Neurooncol. 2016;130(1):123–31.
David A, Larson JMS, Susan M, Chang Kathleen R, Lamborn Michael W, McDermott Penny K, Sneed Michael D, Prados William M, Wara WM, Nicholas MK, Berger MS. Permanent iodine 125 brachytherapy in patients with progressive or recurrent glioblastoma multiforme. Neuro Oncol. 2004;6(2):119–26.
Kickingereder P, Hamisch C, Suchorska B, Galldiks N, Visser-Vandewalle V, Goldbrunner R, Kocher M, Treuer H, Voges J, Ruge MI. Low-dose rate stereotactic iodine-125 brachytherapy for the treatment of inoperable primary and recurrent glioblastoma: single-center experience with 201 cases. J Neurooncol. 2014;120(3):615–23.
Wen EA PY, Black PM, Fine HA, Riese N, Levin JM, Coleman CN, Loeffler JS. Long Term Results of Stereotactic Brachytherapy Used in the Initial Treatment of Patients with Glioblastomas. Cancer. 1994;73(12):3029–36.
Bernstein M, Leung NL P, McKenzie S. Interstitial brachytherapy for malignant brain tumors: preliminary results. Neurosurg. 1990;26(3):371–9.
Acknowledgements
None.
Funding
This work was supported by the National Science Foundation for Youths of China (No. 81901800). China Postdoctoral Science Foundation (No. 2021M701812). Ministry of Science and Technology of the People’s Republic of China (No. 2019YFE0120100), Chinese Medical Education Association (No. 2020KTZ003), Science and Technology Plan of Shinan District, Qingdao (2022-2-001-YY).
Author information
Authors and Affiliations
Contributions
Congxiao Wang conducted all experiments, integrated data, edited figures, and wrote the manuscript; Chao Liu and Jun Chen collected and arranged the data. Han Jiang, Wei Zhang, Lili Yang, Xueda Li, Zixiang Li, and Lijing Peng provided essential assistance; Peng Sun and Xiaokun Hu directed this study, designed the research, and gave key advice. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study has obtained IRB approval from the institutional review board of the Affiliated Hospital of Qingdao University, and the need for informed consent was waived.
(Reference number: QYFY WZ LL 26579). The study was performed in accordance with the Declaration of Helsinki.
Fenyang hospital of Shanxi waived the need of informed consent, and Dr. Jun Chen got the approval and consent to participate section in the manuscript from Fenyang hospital of Shanxi .
Consent for publication
Not applicable.
Competing interests
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, C., Liu, C., Chen, J. et al. Effect of neoadjuvant iodine-125 brachytherapy upon resection of glioma. BMC Cancer 22, 397 (2022). https://doi.org/10.1186/s12885-022-09504-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09504-5