Abstract
Background
Little is known about whether age at initial diagnosis influences the prognosis of recurrent metastatic breast cancer (rMBC). Here, we analyzed the association between age at initial diagnosis and rMBC mortality in China.
Methods
A total of 1636 women diagnosed with rMBC between 1989 and 2020 at West China Hospital, Sichuan University were included in this study. The age at initial diagnosis was categorized as young (≤ 40 years), middle-aged (41–64 years) and elderly (≥ 65 years). Post-metastasis mortality was the primary outcome and its associated factors were analyzed by Cox proportional hazards models.
Results
During a median follow-up of 5.2 years after initial diagnosis of breast cancer, 620 deaths were identified. Compared with middle-aged patients, elderly patients had a 70% increased risk of post-metastasis mortality (95%CI, 1.24–2.33) after adjusting for demographics, tumor characteristics and treatment modes. Similarly, elderly patients were associated with a 75% increased risk of post-metastasis mortality (95%CI, 1.19–2.59) compared with young patients. Subgroup analyses also showed similar trends.
Conclusion
Our findings suggest that in breast cancer, elderly patients at initial diagnosis face a higher risk of post-metastasis mortality.
Similar content being viewed by others
Background
Recently, the International Agency for Research on Cancer releases the latest estimates on the global burden of cancer and provides estimates of incidence and mortality for 2020. One of the most shocking changes is the rapid growth in the newly diagnosed cases of breast cancer, which has replaced lung cancer as the world's largest cancer. According to the latest estimated data in 2020, there will be 2.26 million new breast cancers and 670,000 cancer deaths around the world [1].
Globally, due to the aging of the population, the World Report on Ageing and Health estimates that the number of people older than 60 years will double by 2050. More than 50% of breast cancers are diagnosed in patients older than 60 years in the USA. This proportion is still nearly 30% in China [2, 3]. The global cancer burden will increase by 50% by 2040 compared with 2020 [4]. Thus, cancer has become a major public health issue worldwide, especially for elderly patients.
The rate of metastasis for breast cancer is still increasing. Approximately 20%-30% of patients with early breast cancer will experience distant metastatic relapse and 90% of cancer-related deaths are attributed to metastasis [5, 6]. Distant metastasis can lead to a dramatic reduction of 5-year overall survival rate to only approximately 25%, compared with 80% for breast cancer patients without metastasis [6, 7]. Current treatments for metastatic breast cancer include palliation with chemotherapeutic, hormonal, and biologic agents, neither of which has an adequate effect on improving survival [8].
Age is one of the major risk factors for breast cancer. The World Health Organization and Medicare define the elderly as individuals older than 65 years [9]. Some studies have shown a striking relationship between increasing age at initial diagnosis and an increased risk of disease-specific mortality in breast cancer [10, 11]. However, an opposite association was observed in other studies [12, 13]. Although the overall prognosis of breast cancer has been substantiated in numerous studies, few studies focused on survival in patients with recurrent metastatic breast cancer (rMBC). Utilizing a large-scale cohort of patients in China diagnosed during 1989–2020, we investigated the association of age at initial diagnosis with the risk of post-metastasis mortality (PMM).
Methods
Study population
A retrospective cohort study was conducted using the Breast Cancer Information Management System (BCIMS). The BCIMS database covers virtually all patients diagnosed with invasive breast cancer at West China Hospital, Sichuan University from 1989 to 2020 and collects information on demographics, clinical characteristics, laboratory examinations, treatments, and follow-up visits [14].
A cohort of 15,660 breast cancer cases diagnosed between 1989 and 2020 was reviewed in this study. Of these, only 2,059 cases of metastatic breast cancer were included. Then 10 male patients, 18 patients with primary bilateral breast cancer and 395 patients with de novo metastatic disease were excluded, leaving 1,636 female patients with rMBC in the final analysis. Recurrent metastatic breast cancer was defined as distant recurrence or metastatic and identified by annual follow-up in patients with primary stage I-III breast cancer, while patients with locoregional recurrence were excluded from this analysis.
This study was approved by the Clinical Trial and Biomedical Ethics Committee at West China Hospital, Sichuan University (reference number: 2012–130). Informed consent forms were obtained from all patients.
Construction of variables
The primary independent variable of interest in this study was age at initial diagnosis of breast cancer, stratified as young (≤ 40 years old), middle-aged (41–64 years) and elderly (≥ 65 years), after reference to previous studies [15,16,17].
The demographics included calendar year at diagnosis, ethnic group, insurance type and educational level (as proxies for socioeconomic status), marital status, and body mass index (BMI). Insurance type was classified as urban (i.e. Urban Resident-Based Basic Medical Insurance Scheme [URBMI], Urban Employee-Based Basic Medical Insurance Scheme [UEBMI], and/or commercial insurances) and rural (i.e. New Rural Cooperative Medical Scheme) schemes [14]. According to the recommendation to Asian populations [18], BMI was classified into < 23 kg/m2 (non-overweight) and ≥ 23 kg/m2 (overweight). Clinical characteristics included menopausal status, comorbidity, histological type, histological grade, tumor stage, hormone receptor status including both estrogen receptors (ER) and progesterone receptors (PR), human epidermal growth factor receptor 2 (HER2) status, Ki-67 level, molecular subtype and metastatic sites. Molecular subtypes of breast cancer are divided into four categories by joint HR/HER2/Ki-67 status: Luminal A (HR + /HER2-/Ki-67 < 14%), Luminal B (HR + /HER2 + or ER + /HER2-/Ki-67 ≥ 14%), HER2-positive (HR-/HER2 +), and triple-negative (HR-/HER2-) [19]. Metastatic sites were classified as bone, brain, visceral (lung, liver, and intracavity lymph nodes, etc.), contralateral breast, and others (skin, soft tissue, distant lymph nodes, etc.). Local recurrence was defined as the recurrence in any part of the ipsilateral breast, chest wall, and regional lymph nodes. Treatment modes were categorized as surgery, any chemotherapy, any radiotherapy, any hormonal therapy, and any targeted therapy.
Post-metastasis mortality, overall mortality and breast cancer-specific survival
All patients were actively followed up through telephone and medical visits until death or June 22st, 2020, whichever came first. The underlying cause of death was ascertained from the medical records, whenever possible, or informed by the immediate family members.
The post-metastasis (PMS) or overall survival (OS) was defined as the time elapsed between the first onset of metastasis or primary breast cancer diagnosis and the date of death due to any cause, respectively. And the breast cancer-specific survival (BCSS) was calculated as the time from the date of diagnosis to the date of death attributed to breast cancer. All patients still alive were censored at the date of last follow-up. Metastatic-free interval was defined as time between date of diagnosis of primary breast cancer and date of diagnosis of first distant recurrence metastatic.
Statistical analysis
The demographics, clinical characteristics and treatment modes of patients in different age groups were described. Pearson's chi-square test was used to compare the differences in proportions among the age groups.
The differences in survival of patients among age groups were assessed. Survival curves were obtained using the Kaplan–Meier method and the curves were compared with log rank test.
The cumulative rates and 95% confidence intervals (CIs) of post-metastasis (PMM), overall (OM) and breast cancer-specific (BCSM) mortality by age was measured and plotted using a competing risk model. Hazard ratios (HRs) and 95% CIs were then estimated from Cox regression by contrasting age groups. In Cox regression analysis, the demographics (calendar year at diagnosis, ethnic group, insurance type and educational level and marital status), clinical characteristics (comorbidity, histological type, tumor stage, hormone receptor status, HER2 status, Ki-67 level and recurrent/metastatic sites), and treatment modes (surgery, chemotherapy, radiotherapy, hormonal therapy and targeted therapy) were adjusted.
All analyses were performed in STATA statistical software (version 14; STATA, College Station, TX). P value < 0.05 indicated statistical significance.
Results
Patients’ characteristics
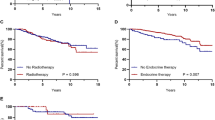
Overall, 1636 patients (median age, 47 years; range, 22–89 years) were included in the study, of which 363 were younger than 40 years at diagnosis (22.19%; median age, 35 years), 1,173 were aged 40 to 64 years (71.70%; median age, 49 years), and 100 were aged 65 years or older (6.11%; median age, 69 years). A significant age-associated increase was observed in patients with urban schemes, married and postmenopausal status, higher BMI and comorbidities at diagnosis as well as less well-educated level and lower proliferative disease (Ki-67 < 14%). In addition, tumors were more likely to be HER2-negative in young and elderly patients. The administration of chemotherapy and radiotherapy significantly decreased with age, whereas the proportion of hormonal therapy increased significantly in young and elderly patients (both P< 0.05; Table 1). Further, we analyzed the palliative treatments which the women received after metastasis. The results showed that elderly patients generally use less systematic treatments, compared to young and middle-aged ones (Table 2).
Age at initial diagnosis and survival of breast cancer
During follow-up after breast cancer diagnosis (median 5.2 years, interquartile range, 2.8–8.8 years), 620 rMBC patients (37.89%) died and 548 of them were attribute to breast cancer. The median follow-up after metastatic recurrence was 2.4 years (interquartile range, 1.0–3.9 years). Median PMS for young patients was 8.69 years, while for middle-aged and elderly patients was 7.24 and 3.49 years, respectively. Ten-year PMS rates were 46.0%, 47.6% and 27.5% in groups of young, middle-aged and elderly age, respectively. Results showed that elderly patients had a worse PMS than young or middle-aged ones. Similar patterns were noticed for overall and breast cancer-specific survival (Fig. 1).
Age at initial diagnosis and mortality of breast cancer
The cumulative rates of PMM were higher among elderly patients, compared with young and middle-aged patients (Fig. 2). Similar patterns were noticed for overall mortality. When adjusting for demographics, clinical characteristics and treatment modes, elderly patients had a 70% increased risk of PMM (95%CI: 14% to 87%) compared with middle-aged patients (Fig. 3). Similarly, when compared with young patients, patients with elderly age had a 75% increased risk of PMM (95%CI: 11% to 82%) (Fig. 4). Similar trends were found for OM (Supplementary Figures S1 and S2) and BCSM (Supplementary Figures S3 and S4). Additionally, considering age is a continuous variable, we analyzed and confirmed the significantly prognostic role of age as a continuous variable in breast cancer (Supplementary Table 1).
Age at initial diagnosis and mortality of breast cancer by subtype stratification
In subtype analysis, with control for demographics, clinical characteristics and treatment modes, the association between age and prognosis was much more significant (Figs. 3 and 4). After stratification by molecular subtypes, elderly patients had a higher death risk in rMBC with luminal A subtype (HR = 5.32; 95%CI: 1.68–16.85) and triple-negative subtype (HR = 2.07; 95%CI: 1.06–4.04), compared with middle-aged patients. However, elderly patients showed a higher death risk (HR = 2.70; 95%CI: 1.14–6.39) only in triple-negative subtype, compared with young patients. It was noteworthy that, after stratification by brain metastatic site, elderly patients presented with a better prognosis than young and middle-aged patients, although there was no statistical difference due to the small sample size.
For treatment mode subgroups, administration of hormonal therapy for patients, elderly patients had a 72% increased risk of mortality (95%CI: 1.14–2.60) than middle-aged ones. In radiotherapy subgroup, elderly patients showed a higher death risk (HR = 1.83; 95%CI: 1.04–3.24) compared with young ones.
In addition, patients in subgroup like diagnosis year in 2008–2020, less well-education, urban schemes, breast cancer with hormone receptor-positive, HER2-negative, stage T1 or N3, the increased risk for the elderly patients still be significant. Similar patterns were found for OM (Supplementary Figures S1 and S2).
However, there was no subgroup difference between young and middle-aged patients in PMM, OM and BCSM (Supplementary Figures S5, S6 and S7, respectively).
Discussion
To the best of our knowledge, this is the first study to demonstrate that elderly patients with recurrent and distant metastatic breast cancer are at increased risk of PMM, compared with the young and middle-aged groups in China. Importantly, this association is partly but not entirely explained by known prognostic indicators, including demographic factors, tumor characteristics, and cancer treatment. In addition, the correlation was not significant between the young and middle-aged groups.
The relationship between age and prognosis of breast cancer has always been controversial. Findings from several studies have shown that the percentage of deaths attributed to breast cancer decreased with increasing age [12, 13]. However, an opposite association was observed in other studies [10, 11]. Additionally, some results reveal that young and elderly breast cancer patients are more likely to have a poorer outcome than middle-aged patients [20, 21]. Here, our data further illustrated that the increasing age is associated with PMM and overall mortality in rMBC patients independent of clinical factors.
Several possible underlying mechanisms may help to explain the results presented in this study. First, elderly patients may experience poor socioeconomic status, including poor educational level and health insurance, and have limited access to medical service, which may lead to delayed diagnosis and suboptimal treatment [22]. This is also supported by our data that in poor educational level subgroup, elderly patients suffer an increased risk of PMM compared with young and middle-age patients. However, compared with rural health insurance, we only provide arguments that PMM increases with age in urban health insurance subgroup. In China, rural health insurance includes the New Rural Cooperative Medical Scheme (covering the residents of rural households and launched in 2003), while urban health insurance includes URBMI (covering the unemployed, children, and elderly and launched in 2007), and UEBMI (covering employees and launched in 1998) [14, 22]. Apparently, age is associated with the type of urban health insurance. Compared with UEBMI, URBMI is limited to a lower reimbursement cap and covers a narrower spectrum of diseases [23]. Thus, elderly patients with inadequate insurance face a greater financial burden and are less likely to afford out-of-pocket medical expenses for early diagnosis and advanced therapy, especially for rMBC patients who have already invested large funds to earlier stage disease [.24]. In line with that, our data highlights the urgent need of promoting social support to significantly improve breast cancer prognosis in elderly patients.
Next, for analysis of molecular typing subgroup, our results showed that elderly patients were associated with increased risk of PMM in triple-negative breast cancer (TNBC) subgroup, compared with either young or middle-aged patients. There may be multi-factorial explanations. A lot of age-related factors may play important roles in metastasis and prognosis, including accumulation of immune dysfunction, DNA damage, chronic inflammation, drug resistance, etc. [25]. Immune checkpoint blockade has been of greatest interest in TNBC due to its immunogenicity, as evidenced by the presence of tumor-infiltrating lymphocytes and elevated programmed cell death-ligand 1 expression relative to other subtypes. However, Sceneay et al. implicated that the tumor microenvironment in aged patients with TNBC showed decreased interferon cell signaling and antigen presentation, suggesting failed innate immune activation with age and age-related immune dysfunction as a mechanism of immunotherapy resistance [25]. Besides, chemotherapy is the main available systemic therapy for TNBC patients. Elderly patients with TNBC obtain similar benefits from adjuvant chemotherapy compared with young patients, but are at greater risk of toxicity. Furthermore, elderly patients may suffer a greater incidence of comorbidity, use of multiple medications, and preexisting dysfunction, such that treatment-related toxicity may dramatically affect their prognosis. These call attention to us that elderly patients with TNBC face a special tumor heterogeneity and major treatment challenge [26, 27].
The proportion of hormone receptor-positive breast cancers increases with age and is considered as the more favorable tumor biology, since hormone receptor-positive tumors tend to grow more slowly and frequently respond to hormonal therapy. Significant treatment benefits with endocrine therapy have been shown in the elderly patient population [28]. However, for the administration of hormonal therapy subgroup, our results showed that elderly patients had a 72% increased rate of PMM risk (95%CI: 1.14–2.60) than middle-aged ones, after exhaustive adjustment for clinical factors. We found that nearly 30% elderly patients with postmenopausal status still chose a selective estrogen receptor modulator, tamoxifen and toremifene. In addition, the hormonal therapy time was less than five years in 60% patients. A previous study has demonstrated that aromatase inhibitors (AIs) have been shown to be more effective than tamoxifen in the metastatic setting [29]. The American Society of Clinical Oncology clinical practice guideline recommends that women with node-positive breast cancer receive extended therapy, including an AI, for up to a total of 10 years of endocrine treatment [30]. A growing body of evidence has shown significantly prolonged progression-free survival and a manageable toxicity profile for first-line CDK4/6 inhibitor plus AI in patients with hormone receptor-positive/HER2-negative advanced breast cancer [31, 32]. Even for elderly patients, CDK4/6 inhibitor plus endocrine therapy is an effective, well-tolerated treatment [33]. Obviously, due to the long treatment period and other reasons, endocrine therapy seems to be difficult to maximize its effect for elderly patients. These may cause cancer-related mortality increase with age.
The International Society of Geriatric Oncology has assembled a task force to make evidence-based recommendations for the treatment of breast cancer patients in the elderly [34]. Furthermore, new combination regimens that target multiple pathways have shown efficiency in elderly patients who were previously resistant to endocrine therapies. Future studies may need to focus on the combinations to improve outcomes of elderly patients with rMBC. With the rapid development of endocrine therapy, we believe that better results will be obtained in the future.
A major strength of our study is the large-scale cohort design with virtually complete follow-up, largely limiting the common sources of bias. The rich information on demographics and clinical characteristics helped to disentangle the direct influence of age on PMM, from the influence through demographics, tumor characteristics and treatment modes. Then we can further analyze the detailed correlation among subgroups. Our study also has several limitations. First, this cohort is based on a single regional medical center, the findings may not be generalized to the entire population. Furthermore, inadequate data of treatment limited analysis of its effect on the correlation between age and prognosis.
Conclusions
In conclusion, our findings suggest that elderly patients face a higher risk of PMM in China, which may provide the knowledge of prognostic and predictive markers that will allow individualized therapy for rMBC.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files. The datasets generated and analyzed during the current study are available in the Breast Cancer Information Management System (BCIMS) database.
Abbreviations
- AI:
-
Aromatase inhibitor
- BCIMS:
-
Breast Cancer Information Management System
- BMI:
-
Body mass index
- BCSM:
-
Breast cancer-specific mortality
- BCSS:
-
Breast cancer-specific survival
- CI:
-
Confidence interval
- HER2:
-
Human epidermal growth factor receptor 2
- HR:
-
Hazard ratio
- OM:
-
Overall mortality
- OS:
-
Overall survival
- PMM:
-
Post-metastasis mortality
- PMS:
-
Post-metastasis survival
- rMBC:
-
Recurrent metastatic breast cancer
- TNBC:
-
Triple-negative breast cancer
- UEBMI:
-
Urban Employee-Based Basic Medical Insurance Scheme
- URBMI:
-
Urban Resident-Based Basic Medical Insurance Scheme
References
Goodman JE, Mayfield DB, Becker RA, Hartigan SB, Erraguntla NK. Recommendations for further revisions to improve the International Agency for Research on Cancer (IARC) Monograph program. Regul toxicol pharmacol. 2020;113:104639.
DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–51.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32.
Extermann M, Brain E, Canin B, Cherian MN, Cheung KL, de Glas N, Devi B, Hamaker M, Kanesvaran R, Karnakis T, et al. Priorities for the global advancement of care for older adults with cancer: an update of the International Society of Geriatric Oncology Priorities Initiative. Lancet Oncol. 2021;22(1):e29–36.
Lao C, Kuper-Hommel M, Elwood M, Campbell I, Edwards M, Lawrenson R. Characteristics and survival of de novo and recurrent metastatic breast cancer in New Zealand. Breast Cancer. 2021;28(2):387-97.
Liang Y, Zhang H, Song X, Yang Q. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Semin Cancer Biol. 2020;60:14–27.
Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–75.
Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, Barrios CH, Bergh J, Bhattacharyya GS, Biganzoli L, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020;31(12):1623–49.
Kocik J, Pajączek M, Kryczka T. Worse survival in breast cancer in elderly may not be due to underutilization of medical procedures as observed upon changing healthcare system in Poland. BMC Cancer. 2019;19:749.
Bastiaannet E, Liefers GJ, de Craen AJ, et al. Breast cancer in elderly compared to younger patients in the Netherlands: stage at diagnosis, treatment and survival in 127,805 unselected patients. Breast Cancer Res Treat. 2010;124:801–7.
van de Water W, Markopoulos C, van de Velde CJ, Seynaeve C, Hasenburg A, Rea D, et al. Association between age at diagnosis and disease-specific mortality among postmenopausal women with hormone receptor-positive breast cancer. Jama. 2012;307:590–7.
Beadle GF, McCarthy NJ, Baade PD. Effect of age at diagnosis of breast cancer on the patterns and risk of mortality from all causes: a population-based study in Australia. Asia Pac J Clin Oncol. 2013;9(2):129–38.
Du XL, Fox EE, Lai D. Competing causes of death for women with breast cancer and change over time from 1975 to 2003. Am J Clin Oncol. 2008;31(2):105–16.
Xie Y, Valdimarsdóttir UA, Wang C, Zhong X, Gou Q, Zheng H, Deng L, He P, Hu K, Fall K, et al. Public health insurance and cancer-specific mortality risk among patients with breast cancer: A prospective cohort study in China. Int J Cancer. 2021;148(1):28–37.
Maishman T. Cutress Ri, Hernandez A, Gerty S, Copson Er, Durcan L, Eccles DM: Local Recurrence and Breast Oncological Surgery in Young Women With Breast Cancer: The POSH Observational Cohort Study. Ann Surg. 2017;266(1):165–72.
Sopik V. International variation in breast cancer incidence and mortality in young women. Breast Cancer Res Treat. 2021;186(2):497–507.
Azim HA Jr, Partridge AH. Biology of breast cancer in young women. Breast Cancer Res. 2014;16(4):427.
Consultation WE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63.
Howlader N, Cronin KA, Kurian AW, Andridge R. Differences in Breast Cancer Survival by Molecular Subtypes in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27(6):619–26.
Wang MX, Ren JT, Tang LY, Ren ZF. Molecular features in young vs elderly breast cancer patients and the impacts on survival disparities by age at diagnosis. Cancer Med. 2018;7(7):3269–77.
Johansson ALV, Trewin CB, Hjerkind KV, Ellingjord-Dale M, Johannesen TB, Ursin G. Breast cancer-specific survival by clinical subtype after 7 years follow-up of young and elderly women in a nationwide cohort. Int J Cancer. 2019;144(6):1251–61.
Meng Q, Fang H, Liu X, Yuan B, Xu J. Consolidating the social health insurance schemes in China: towards an equitable and efficient health system. Lancet. 2015;386(10002):1484–92.
Pan Y, Chen S, Chen M, Zhang P, Long Q, Xiang L, Lucas H. Disparity in reimbursement for tuberculosis care among different health insurance schemes: evidence from three counties in central China. Infect Dis Poverty. 2016;5:7.
Altice CK, Banegas MP, Tucker-Seeley RD, Yabroff KR. Financial Hardships Experienced by Cancer Survivors: A Systematic Review. J Natl Cancer Inst. 2017;109(2):djw205.
Sceneay J, Goreczny GJ, Wilson K, Morrow S, DeCristo MJ, Ubellacker JM, Qin Y, Laszewski T, Stover DG, Barrera V, et al. Interferon Signaling Is Diminished with Age and Is Associated with Immune Checkpoint Blockade Efficacy in Triple-Negative Breast Cancer. Cancer Discov. 2019;9(9):1208–27.
Shachar SS, Jolly TA, Jones E, Muss HB. Management of Triple-Negative Breast Cancer in Older Patients: How Is It Different? Oncology (Williston Park). 2018;32(2):58–63.
Tzikas AK, Nemes S, Linderholm BK. A comparison between young and old patients with triple-negative breast cancer: biology, survival and metastatic patterns. Breast Cancer Res Treat. 2020;182(3):643–54.
Jolly T, Williams GR, Jones E, Muss HB. Treatment of metastatic breast cancer in women aged 65 years and older. Womens Health (Lond). 2012;8(4):455–69 (quiz 470-451).
Mouridsen H, Chaudri-Ross HA. Efficacy of first-line letrozole versus tamoxifen as a function of age in postmenopausal women with advanced breast cancer. Oncologist. 2004;9(5):497–506.
Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KA, Giordano SH, Hudis CA, Solky AJ, Stearns V, et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J Clin Oncol. 2019;37(5):423–38.
Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375(20):1925–36.
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, Campone M, Petrakova K, Blackwell KL, Winer EP, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541–7.
Rugo HS, Turner NC, Finn RS, Joy AA, Verma S, Harbeck N, Masuda N, Im SA, Huang X, Kim S, et al. Palbociclib plus endocrine therapy in older women with HR+/HER2- advanced breast cancer: a pooled analysis of randomised PALOMA clinical studies. Eur J Cancer. 2018;101:123–33.
Biganzoli L, Wildiers H, Oakman C, Marotti L, Loibl S, Kunkler I, Reed M, Ciatto S, Voogd AC, Brain E, et al. Management of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol. 2012;13(4):e148-160.
Acknowledgements
We thank all staff members working on the BCIMS database for their contributions to data collection and management.
Funding
This work was supported by the Applied Basic Research Programs of Science and Technology Department of Sichuan Province of China (grant number: 2019YJ0070); the National Natural Science Foundation of China (grant number: 81902723). The funding source had no role in the study design, collection, analysis and interpretation of data, writing of the manuscript, or decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
YuxinXie, Qiheng Gou and Ting Luo conceived the study concept and design. YingjieZhang, Dan Zheng, Chuanxu Luo, Jiaojiao Suo and Xiaorong Zhong collected data.Yuxin Xie and Qiheng Gou performed statistical analysis. Yuxin Xie, Keqi Xieand Ting Luo drafted the manuscript, and all authors significantly contributedto the critical revision of the manuscript, data interpretation, and approvedthe submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental protocols and ethical consent of this study were approved by the Clinical Trial and Biomedical Ethics Committee at West China Hospital of Sichuan University, Chengdu, China (reference number: 2012–130). All methods were performed in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xie, Y., Gou, Q., Zhang, Y. et al. Association between age at initial diagnosis and post-metastasis mortality among women with recurrent metastatic breast cancer in China. BMC Cancer 22, 385 (2022). https://doi.org/10.1186/s12885-022-09454-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09454-y