Abstract
Background
The oncogenic drivers of triple-negative breast cancer (TNBC), which is characterized by worst prognosis compared with other subtypes, are poorly understood. Although next-generation sequencing technology has facilitated identifying potential targets, few of the findings have been translated into daily clinical practice. The present study is aimed to explore ZNF703 (Zinc finger 703) function and its underlying mechanism in TNBC.
Methods
ZNF703 expressions in tissue microarray were retrospectively examined by immunohistochemistry. The cell proliferation by SRB assay and colony formation assay, as well as cell cycle distribution by flow cytometry were assessed. The protein levels associated with possible underlying molecular mechanisms were evaluated by western blotting. Kaplan-Meier analysis was used to plot survival analysis.
Results
Our data suggest that ZNF703 expressed in 34.2% of triple-negative human breast tumors by immunohistochemistry. In vitro, ZNF703 knockdown had potent inhibitory effects on TNBC cell proliferation and cell cycle, with cyclin D1, CDK4, CDK6, and E2F1 downregulated, while Rb1 upregulated. Moreover, Kaplan-Meier analysis showed that high mRNA expression of ZNF703 was correlated to worse overall survival (HR for high expression was 3.04; 95% CI, 1.22 to 7.57, P = 0.017).
Conclusions
Taken together, the results identified that targeting ZNF703 contributed to the anti-proliferative effects in TNBC cells, due to induced G1-phase arrest. This study is the first to identify ZNF703 as a potentially important protein that is involved in TNBC progression.
Similar content being viewed by others
Introduction
Triple-negative breast cancer (TNBC), defined as lack of expression of estrogen receptor α (ERα), progesterone receptor (PR) and human epidermal growth receptor 2 (HER2) / erb-b2 receptor tyrosine kinase 2 (ERBB2), which does not benefit from routine targeted therapies and is associated with poor outcome [1, 2], is the most aggressive subtype of breast cancer. Although patients with early stages of TNBC may be cured with chemotherapy, median overall survival is rather limited in those who suffer from recurrent or metastatic diseases [3, 4]. The inner mechanisms that drive the abnormal proliferation of TNBC are still poorly understood; targeted agents are still to be developed and could result in improved overall survival for TNBC patients [5,6,7]. Most early TNBC patients are treated with chemotherapy, including anthracyclines, paclitaxel, or platinum. Metastatic TNBC patients are likely to be resistant to chemotherapy and have little choices to be treated with specific targeted therapies to prolong survival [8, 9]. Clinical trials have demonstrated few effective targeted drugs, including PARP inhibitors [10], PD-1 or PD-L1 inhibitors [11,12,13]. TNBC encompasses molecularly different subgroups [14]; however, molecular-subgroup-based therapies have not been established.
Scientists have explored about ZNF703 (Zinc finger 703) in cancer fields. It is a transcriptional factor, which is also an oncogene in luminal B breast cancer, identified by genome-wide measurements of DNA copy number using comparative genomic hybridization [15, 16]. Some studies [17] have used integrated analysis of copy number and gene expression in a discovery and validation set of almost 2000 primary breast tumors, in which copy number changes of ZNF703 are very obvious and common in breast tumors, secondary to ERBB2 and CCND1. Therefore, ZNF703 is a new and very important oncogene in breast cancer, and it should be considered as a therapeutic target in ~15% of breast tumors [18]. The rearrangements of individual tumors in a cohort of 560 breast cancers were systematically investigated, and it reveals that simultaneous amplification of chromosome 8—ZNF703/FGFR1—and chromosome 11—CCND1—where there is a chromosome 8–chromosome 11 translocation, is likely to be an early, critical, initiating event in breast cancer [19]. However, it seems that those amplified genes are not always overexpressed [20].
In the present study, for the first time, we discovered that ZNF703 was also expressed in part of triple-negative breast cancer, whether in the human tumor specimens or cancer cell lines. Here we assessed, for the first time to our knowledge, the activity of ZNF703 inhibition and the underlying mechanisms in TNBC cell lines: MDA-MB-468 and BT549, as well as analyzed the relationship between overall survival and ZNF703 expression in TNBC.
Materials and methods
Cell culture, reagents and antibodies
All breast cancer cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). MDA-MB-468 and BT-549 were cultured in RPMI 1640 medium (Gibco) with 10% Fetal bovine serum (FBS, Gibco) supplemented with 2 mM L-glutamine. Other cell lines were cultured followed by instructions from ATCC guideline. Among them, cell lines were classified into four distinguished subtypes, including normal breast epithelial cell line, luminal-type breast cancer cell line, HER2-positive breast cancer cell line, and triple-negative breast cancer cell line (Fig. 1A).
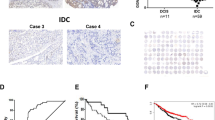
ZNF703 expression in breast cancer. A Immunoblotting (IB) for ZNF703 in total cell lysates from five triple-negative breast cancer (TNBC) cell lines (red circles), two normal breast epithelial cell lines MCF-10 A, HBL-100 (green circles) and representative examples of other breast cancer subtypes (yellow circles for luminal-type, and blue circles for HER2-positive subtype). A GAPDH antibody was used as a loading control. B Representative image of IHC staining for ZNF703 in TNBC specimens. Left: low ZNF703 expression; Right: high ZNF703 expression. The bar represents 50 μm. C Immunostaining scores of ZNF703 in 76 TNBC patients. The vertical axis indicates the differences between the score of each patient and the median score. High expression group was indicated as positive numbers, and low/no expression group was indicated as zero or negative numbers
The antibodies used in this study were as follows: ZNF703 for Western blot (1:1000 dilution, Abcam, No.ab137054), ZNF703 for immunohistochemistry (1:50 dilution, Sigma-Aldrich, St. Louis, MO, USA, No.HPA023930), HSP90α (all at a 1:1000 dilution, Abcam); cyclin D1 (No. 55,506), CDK4 (No.12,790), CDK6 (No.13,331), Rb1 (No.9313), E2F1 (No.3742), GAPDH (No.5174) and HSP90α (No.4877) [all at a 1:1000 dilution purchased from Cell Signaling Technology, Boston, MA, USA].
Immunoblot analysis
Cells were treated and harvested as described. The assay was performed as previously described [21]. Immunolabeling was visualized by an ECL (electrochemiluminescence) detection kit from Ammersham Biosciences according to the manufacturer’s instructions. The blots were from original gels which had to be cropped before hybridizing with secondary antibodies. GAPDH or HSP90α was used as a loading control.
RNA interference and proliferation assays
Cell lines were transfected with short-interfering RNA (siRNAs, 30 nM final concentration) in 6-well plates with RNAiMAX (Invitrogen) according to the manufacturer’s instructions and harvested 48 hours after transfection, which could be cultured to enter following experiments. Target sequences for the siRNA of ZNF703: sense strand-5’ CCACACACUUUGGGCCUAA dTdT 3’; antisense-strand-3’ dTdT GGUGUGUGAAACCCGGAUU 5’. Non-targeting control siRNA was designed and synthesized by Guangzhou RuiBoBio (Guangzhou, China). Proliferation assay and colony-forming assay were performed as previously described [22]. Cell proliferation was measured by sulforhodamine B (SRB) (Sigma) assay. Relative growth was calculated as the value relative to controlled cells. In colony-forming assay, cells were seeded into 6-well plates (1000 cells per well). After several proper days, colonies were fixed in 10% acetic acid, 10% methanol and 80% ddH2O, and then stained with crystal violet (0.5% w/v).
Cell cycle analysis
TNBC cells treated with non-targeting control siRNA or the siRNA of ZNF703 were seeded in 6-well plates at a 60–70% confluence for 24 h. After that, TNBC cells were washed twice with PBS and fixed in 75% ethanol for 2 h at 4 ℃. Then, the TNBC cells were trypsinized and then suspended in fresh medium and centrifuged at 1,000 rpm for 5 min. Cell cycle analysis was performed as previously described [23]. The cells were washed with PBS and then stained with 0.05 µg/mL PI (Sigma-Aldrich), 1 µg/mL DNase-free RNase (Sigma-Aldrich) for 30 min. FACSCalibur analyzer (Becton-Dickinson, San Jose, CA, USA) was used to acquire events and Modfit software (Verity Software House, Topsham, ME, USA) was used to collect and analyze cell-cycle data.
Immunohistochemistry Staining
Immunohistochemical analysis of tissue microarray sections were performed as previously described [22]. Tissue specimens were obtained from seventy-six patients who undergone surgical treatment at Ruijin Hospital (China) between January 2001 and December 2003 and were diagnosed of stage I-III primary breast cancer without history of other malignant tumors. Patients receiving chemotherapy or radiotherapy prior to surgery were excluded. Two pathologists were blinded to the clinicopathologic data and independently evaluated ZNF703 expression as well as breast cancer subtype. As for ZNF703, they assessed the intensity of nuclear staining (0 score: no staining; 1 score: weak, 2 scores: moderate, 3 scores: strong) as well as the percentage of stained cells (0 score: 0%, 1 score: 1–20%, 2 scores: 21–40%, 3 scores: 41–60%, 4 scores: 61–80%, 5 scores: 81–100%). The final immunoreactive score ranged from 0 to 15, which equaled to the number of multiplying the intensity score by the percentage score. The median value was 5, by which it could divide patients into high expression group (above score 5), and low/no expression group (equal or below score 5). The study protocol was designed according to the principles of the Helsinki guidelines and approved by the institutional ethical board of Ruijin hospital affiliated to Shanghai Jiaotong university school of medicine. Cases were classified into two groups: low/no expression or high expression, according to median score of nucleic staining. The antibody was titrated with negative and positive controls. Evaluation of hormone receptor (HR) status accords with the Allred scoring method [24].
Microarray data information from TCGA dataset and analysis
ZNF703 mRNA expression data and corresponding clinical information of 136 basal-like invasive breast cancer samples, including basal-like 1 (BL1) and basal-like 2 (BL2) were obtained from The Cancer Genome Atlas (TCGA) dataset (https://portal.gdc.cancer.gov/) in January 2020, in which the method of acquisition and application complied with the guidelines and policies. Patients were divided into two groups according to the median value (Table 2), including ZNF703-low expression (seventy patients) and ZNF703-high expression (sixty-six patients) subgroups. Median follow-up was 9.5 years. The Kaplan-Meier survival analysis with log-rank test was used to compare the difference of overall survival between two groups [25, 26].
Statistics
Data analysis was performed using the statistical package SPSS 26.0. Each experiment was repeated at least three times. Student’s t-test was used to evaluate numeric data. Chi-square test was used for comparisons of categorical data. For Kaplan–Meier curves, p-values, and hazard ratio (HR) with 95% confidence interval (CI) were generated by log-rank tests using GraphPad Prism (version 8.4.0). Statistical tests were two-sided, and P-values less than 0.05 were considered statistically significant.
Results
ZNF703 expression in TNBC
We detected the expression of ZNF703 in thirteen breast cancer cell lines and two normal breast epithelial cell lines by western blot (Fig. 1A, Fig. S1). We found that normal breast epithelial cell line MCF-7-10 A did not express ZNF703. HBL-100 and most of the HER2-positve breast cancer cell lines such as BT-474, SK-BR-3 and ZR-7530 [27], expressed little ZNF703 proteins. TNBC cell lines MDA-MB-435, MDA-MB-468, MDA-MB-231 and BT-549 expressed more amount of ZNF703 proteins, although not at high levels. Luminal cell line MCF-7 and one HER2-positve cell line MDA-MB-453 also expressed a certain level of ZNF703 proteins. We next selected BT-549 and MDA-MB-468 cell lines as the model to explore the role of ZNF703 in vitro. We also examined ZNF703 expression in the tumor tissue block of 76 TNBC patients by immunohistochemistry (Fig. 1C, Table 1). Median age was 53 years old. Twenty-six cases (34.2%) with high expression of ZNF703 were identified (Fig. 1B, C). ZNF703 was not associated with age, grade, tumor size, lymph node metastases, stage and pathological type in those patients (P > 0.05). These findings mean that ZNF703 expressed and could be detected in TNBC samples, whether in cell lines or in tumor specimen.
ZNF703 inhibition attenuates TNBC cell proliferation and colony formation
We established TNBC cell lines BT-549, MDA-MB-468 with non-targeting control siRNA (NC) or the siRNA of ZNF703, respectively (Fig. 2A). Next, we performed experiments to determine whether ZNF703 could increase cell proliferation. The results showed that ZNF703 inhibition could statistically significantly depress cell growth in a time-dependent model (Fig. 2B, C). We also performed a colony formation assay to verify the inhibitory effects of treatment with ZNF703-siRNA, as compared to control cells (Fig. 2D and E), with a statistically significant result.
ZNF703 knockdown affects the tumorigenesis of BT-549 and MDA-MB-468 cells. A Immunoblotting (IB) of ZNF703 protein expression in BT-549 non-targeting siRNA control (NC), BT-549 siRNA, MDA-MB-468 NC, MDA-MB-468 siRNA cells. HSP90α was used as a loading control. B Growth curve of BT-549 NC and BT-549 siRNA cells. C Growth curve of MDA-MB-468 NC and MDA-MB-468 siRNA cells. Data are representative of three independent experiments and are presented as mean ± SD. D, E Cell growth was evaluated by the colony formation assay. Colony numbers were counted, and Fig. 2E represents an average of three independent experiments. (** P < 0.01, *** P < 0.001)
Anti-tumor effect of ZNF703 on TNBC through cell cycle signaling
To further evaluate the effect of ZNF703 on cell growth, we tested the effect of ZNF703-siRNA on the cell cycle distribution of TNBC cells. As it was shown, in one representative experiment (Fig. 3A, B), the analysis revealed cell cycle distribution of NC-siRNA treated cells showing 26.75%, 47.97% in G1, 44.58%, 39.97% in S-phase, 28.67%, 12.06% cells in G2/M for BT549 and MDA-MB-468, respectively; while 41.47%, 72.59% in G1, 43.40%, 13.53% in S-phase, 15.13%, 13.88% cells in G2/M for BT549 and MDA-MB-468 cells treated with ZNF703-siRNA, respectively. The G1 phase fraction increased in BT-549 cells and MDA-MB-468 cells, after treating with ZNF703-siRNA, implying that in comparison with NC-siRNA treated cells, ZNF703-siRNA induced an accumulation of cells in the G1 phase fraction. Besides, after knockdown of ZNF703, we found that cyclin D1, CDK4 and CDK6, as well as E2F1, which played a role in the G1 phase of cell cycle regulation [28,29,30], were downregulated by immunoblotting, while the tumor suppressor gene Rb1 was upregulated (Fig. 3C, Fig. S2).
ZNF703 regulates cell cycle of TNBC. A, B Inhibiting ZNF703 induced G1-phase arrest in BT-549 and MDA-MB-468 cell lines. Cells were treated with NC or ZNF703-siRNA for 72 h, and DNA contents were detected and analyzed by flow cytometry assay. The percentage of cells in G1, S and G2/M of cell cycle were calculated. These results were from one representative experiment of three independent experiments. C Immunoblotting (IB) of lysates of BT-549 NC, BT-549 siRNA, MDA-MB-468 NC and MDA-MB-468 siRNA cells using the indicated antibodies. A HSP90α antibody was used as a loading control. The experiment was repeated for three times and one representative result was shown
Prognosis of ZNF703 expression in basal-like invasive breast cancer patients from TCGA
TNBCs were classified into four transcriptomic subtypes, including basal-like 1 (BL1), basal-like 2 (BL2), mesenchymal (M) and luminal androgen receptor (LAR) [6, 31, 32]. Most of the TNBCs belong to basal-like subtypes. Here we collected and downloaded 136 basal-like invasive breast cancer samples from TCGA platform (Table 2). Median follow-up was 9.5 years. Kaplan-Meier survival analysis showed that (Fig. 4), high mRNA expression of ZNF703 were statistically significantly correlated to worse overall survival (HR for high expression was 3.04; 95% CI, 1.22 to 7.57, P = 0.017).
ZNF703 mRNA expression predicts overall survival by Kaplan-Meier survival analysis. Median follow-up was 9.5 years. One hundred and thirty-six basal-like invasive breast cancer samples from TCGA were analyzed by Kaplan-Meier to compare the difference of overall survival between two groups. (HR, hazard ratio, 95%CI, 95% Confidence Interval)
Discussion
TNBC accounts for 15–20% of newly diagnosed breast cancer cases [1] and lacks effective treatment options. The combination of a biomarker-based paradigm and a subtyping-based paradigm is recommended to prompt a suitable targeted treatment for individual TNBC [33]. Although next-generation sequencing technology has facilitated identifying potential targets, few of the findings have been translated into daily clinical practice for treating TNBC patients.
In a formalin-fixed paraffin-embedded (FFPE)-based next-generation sequencing (NGS) analysis in the Neoadjuvant GeparSepto Trial [34], high genetic heterogeneity was observed in different breast cancer types. In this most recent study, ZNF703 amplification occurred in 18.2% of triple-negative breast cancer patients, indicating the potential role played in TNBC development. In another study, which explored the associations between gene mutations and clinicopathologic characteristics by FoundationOne CDx assay in a cohort of 223 clinically advanced breast cancers, ZNF703 gene alterations were enriched in 7.2% of locally advanced TNBCs, but not in metaplastic TNBCs [35]. However, the inner mechanisms have not been investigated in these studies. In our study, 34.2% of TNBC patients with high expression of ZNF703 were identified by immunohistochemistry. There is a low correlation between amplification and overexpression in amplicon genes, and the amplicon does not influence tumor mutation burden in breast cancers [20]. Thus, intermediate, or even low expressions of genes are still likely to have an effect on tumor biology behaviors.
Besides luminal B breast tumors, ZNF703 was also reported to have been implicated in infiltrating lobular breast cancer or progression of lobular carcinoma in situ to invasive cancer [36]. One study showed that ZNF703 was a target of long noncoding RNA SPRY4-IT1 and played an oncogenic role in ER-negative breast cancer cells [37]. Furthermore, ZNF703 seemed to be associated with PR loss, exhibiting more ZNF703 amplification events in ER+PR-HER2- breast tumors than ER+PR+HER2- breast tumors [38]. These studies indicate that ZNF703 can influence the tumorigenesis of different kinds of breast cancer types, not only on the luminal B breast cancer. Levisticum officinale, an herbal plant, was proved to have anti-proliferative and apoptotic activities in a TNBC cell line, with higher expression of ZNF703 than in the less invasive MCF-7 cells [39]. In our study, we demonstrated that ZNF703 inhibition suppressed cell proliferation and cell cycle in two TNBC cell lines, indicating its expression regulates these processes. It is interesting that G1-phase arrest could be induced by inhibiting ZNF703 in TNBC cell lines, which is the new mechanism that was observed for ZNF703 in the context of TNBC. ZNF703 could have influences on several vital cell-cycle related proteins or kinases, such as cyclin D1, CDK4, CDK6 and Rb1, which triggered the changes of most important downstream transcriptional factor E2F1. However, there is a limitation that this result may need to be verified in vivo experiments in the future. In addition, inner mechanisms of how ZNF703 functions in cell cycle, for instance, through epigenetic molecules or protein-protein interactions, and the application of cell-cycle inhibitors like CDK4/6 inhibitor in combination with ZNF703 inhibitor, could be further explored.
Conclusions
Collectively, for the first time, our findings revealed that ZNF703 was a potentially vital protein for TNBC. Targeting ZNF703 contributed to the anti-tumor effects in TNBC cells through G1-phase arrest. ZNF703 can be explored as a novel therapeutic target for TNBC in further clinical trials.
Availability of data and materials
The data that support the findings of this study are available from The Cancer Genome Atlas (TCGA) dataset (https://portal.gdc.cancer.gov/) and the corresponding author [X. Z] upon reasonable request.
Abbreviations
- ZNF703:
-
Zinc finger 703
- TNBC:
-
Triple negative breast cancer
- ERα:
-
Estrogen receptorα
- PR:
-
Progesterone receptor
- HER2/ERBB2:
-
Human epidermal growth receptor 2/ erb-b2 receptor tyrosine kinase 2
- siRNA:
-
Short interfering RNA
- TCGA:
-
The Cancer Genome Atlas
- CDK4:
-
Cyclin-dependent kinase 4
- CDK6:
-
Cyclin-dependent kinase 6
- E2F1:
-
E2F transcription factor 1
- Rb1:
-
Human retinoblastoma susceptibility gene
References
Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721–8.
Criscitiello C, Azim HA, Jr., Schouten PC, Linn SC, Sotiriou C. Understanding the biology of triple-negative breast cancer. Ann Oncol. 2012;23 Suppl 6:vi13-8.
Andre F, Zielinski CC. Optimal strategies for the treatment of metastatic triple-negative breast cancer with currently approved agents. Ann Oncol. 2012;23 Suppl 6:vi46-51.
Garrido-Castro AC, Lin NU, Polyak K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 2019;9(2):176–98.
Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–502.
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–67.
Mills MN, Yang GQ, Oliver DE, Liveringhouse CL, Ahmed KA, Orman AG, et al. Histologic heterogeneity of triple negative breast cancer: A National Cancer Centre Database analysis. Eur J Cancer. 2018;98:48–58.
Tutt A, Tovey H, Cheang MCU, Kernaghan S, Kilburn L, Gazinska P, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med. 2018;24(5):628–37.
Yu KD, Ye FG, He M, Fan L, Ma D, Mo M, et al. Effect of Adjuvant Paclitaxel and Carboplatin on Survival in Women With Triple-Negative Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6(9):1390–6.
Robson ME, Tung N, Conte P, Im SA, Senkus E, Xu B, et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30(4):558–66.
Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396(10257):1090–100.
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2018;379(22):2108–21.
Schmid P, Cortes J, Pusztai L, McArthur H, Kummel S, Bergh J, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med. 2020;382(9):810–21.
Zhao S, Ma D, Xiao Y, Li XM, Ma JL, Zhang H, et al. Molecular Subtyping of Triple-Negative Breast Cancers by Immunohistochemistry: Molecular Basis and Clinical Relevance. Oncologist. 2020;25(10):e1481-e91.
Sircoulomb F, Nicolas N, Ferrari A, Finetti P, Bekhouche I, Rousselet E, et al. ZNF703 gene amplification at 8p12 specifies luminal B breast cancer. EMBO Mol Med. 2011;3(3):153–66.
Holland DG, Burleigh A, Git A, Goldgraben MA, Perez-Mancera PA, Chin SF, et al. ZNF703 is a common Luminal B breast cancer oncogene that differentially regulates luminal and basal progenitors in human mammary epithelium. EMBO Mol Med. 2011;3(3):167–80.
Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486(7403):346–52.
Spellman P, Gray J. A new treasure in the breast cancer gene hunt. Nat Med. 2011;17(4):422–3.
Glodzik D, Purdie C, Rye IH, Simpson PT, Staaf J, Span PN, et al. Mutational mechanisms of amplifications revealed by analysis of clustered rearrangements in breast cancers. Ann Oncol. 2018;29(11):2223–31.
Voutsadakis IA. 8p11.23 Amplification in Breast Cancer: Molecular Characteristics, Prognosis and Targeted Therapy. J Clin Med. 2020;9(10). https://doi.org/10.3390/jcm9103079.
Zhang X, Emerald BS, Mukhina S, Mohankumar KM, Kraemer A, Yap AS, et al. HOXA1 is required for E-cadherin-dependent anchorage-independent survival of human mammary carcinoma cells. J Biol Chem. 2006;281(10):6471–81.
Zhang X, Mu X, Huang O, Xie Z, Jiang M, Geng M, et al. Luminal breast cancer cell lines overexpressing ZNF703 are resistant to tamoxifen through activation of Akt/mTOR signaling. PLoS One. 2013;8(8):e72053.
Huang O, Zhang W, Zhi Q, Xue X, Liu H, Shen D, et al. Teriflunomide, an immunomodulatory drug, exerts anticancer activity in triple negative breast cancer cells. Exp Biol Med (Maywood). 2015;240(4):426–37.
Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474–81.
Zhang Z, Lin E, Zhuang H, Xie L, Feng X, Liu J, et al. Construction of a novel gene-based model for prognosis prediction of clear cell renal cell carcinoma. Cancer Cell Int. 2020;20:27.
Lin W, Wu S, Chen X, Ye Y, Weng Y, Pan Y, et al. Characterization of Hypoxia Signature to Evaluate the Tumor Immune Microenvironment and Predict Prognosis in Glioma Groups. Front Oncol. 2020;10:796.
Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10(6):515–27.
Wang Z, Wang Y, Wang S, Meng X, Song F, Huo W, et al. Coxsackievirus A6 Induces Cell Cycle Arrest in G0/G1 Phase for Viral Production. Front Cell Infect Microbiol. 2018;8:279.
Lessard F, Igelmann S, Trahan C, Huot G, Saint-Germain E, Mignacca L, et al. Senescence-associated ribosome biogenesis defects contributes to cell cycle arrest through the Rb pathway. Nat Cell Biol. 2018;20(7):789–99.
Bertero T, Gastaldi C, Bourget-Ponzio I, Mari B, Meneguzzi G, Barbry P, et al. CDC25A targeting by miR-483-3p decreases CCND-CDK4/6 assembly and contributes to cell cycle arrest. Cell Death Differ. 2013;20(6):800–11.
Jiang YZ, Ma D, Suo C, Shi J, Xue M, Hu X, et al. Genomic and Transcriptomic Landscape of Triple-Negative Breast Cancers: Subtypes and Treatment Strategies. Cancer Cell. 2019;35(3):428–40 e5.
Lehmann BD, Jovanovic B, Chen X, Estrada MV, Johnson KN, Shyr Y, et al. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS One. 2016;11(6):e0157368.
Zhao S, Zuo WJ, Shao ZM, Jiang YZ. Molecular subtypes and precision treatment of triple-negative breast cancer. Ann Transl Med. 2020;8(7):499.
Loibl S, Treue D, Budczies J, Weber K, Stenzinger A, Schmitt WD, et al. Mutational Diversity and Therapy Response in Breast Cancer: A Sequencing Analysis in the Neoadjuvant GeparSepto Trial. Clin Cancer Res. 2019;25(13):3986–95.
Freitag CE, Mei P, Wei L, Parwani AV, Li Z. Genetic alterations and their association with clinicopathologic characteristics in advanced breast carcinomas: focusing on clinically actionable genetic alterations. Hum Pathol. 2020;102:94–103.
Christgen M, Steinemann D, Kuhnle E, Langer F, Gluz O, Harbeck N, et al. Lobular breast cancer: Clinical, molecular and morphological characteristics. Pathol Res Pract. 2016;212(7):583–97.
Shi Y, Li J, Liu Y, Ding J, Fan Y, Tian Y, et al. The long noncoding RNA SPRY4-IT1 increases the proliferation of human breast cancer cells by upregulating ZNF703 expression. Mol Cancer. 2015;14:51.
Liu XY, Ma D, Xu XE, Jin X, Yu KD, Jiang YZ, et al. Genomic Landscape and Endocrine-Resistant Subgroup in Estrogen Receptor-Positive, Progesterone Receptor-Negative, and HER2-Negative Breast Cancer. Theranostics. 2018;8(22):6386–99.
Mollashahee-Kohkan F, Saravani R, Khalili T, Galavi H, Sargazi S. Levisticum Officinale Extract Triggers Apoptosis and Down-Regulates ZNF703 Gene Expression in Breast Cancer Cell Lines. Rep Biochem Mol Biol. 2019;8(2):119–25.
Acknowledgements
We thank Min Huang, Xun Huang, Jing Ai, Aijun Shen, Meiyu Geng, Kunwei Shen for suggestions of experiment designing. We also thank Xiaochun Fei, Hongchun Liu, Shuai Tang, Ying Wang, Xihua Yue, Danni Sun, Haotian Zhang, Chenchu Lin, Lu Wang, Liping Zhang, Hanlin Zeng, Dadong Zhang, Yinchun Ji and Wenyi Sun for technical helps.
Funding
This study was supported by [Quanzhou Science and Technology Project] (grant number 2019N017S), [Natural Science Foundation of Fujian Province] (grant number 2021J011395) and [High-level Talents Innovation and Entrepreneurship Project of Quanzhou Science and Technology Plan] (grant number 2019C073R). The funding body had no role in the design of the study, data collection, data analysis, interpretation of data, and in writing this manuscript.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: XZ and XM. Performed the experiments: XZ, XM, OH, ZTW and JLC. Analyzed the data: GW and DBC. Wrote and reviewed the paper: all authors. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethical Board of Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine, China. The requirement for obtaining informed consent was waived by the Ethical Board of Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine, China.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, X., Mu, X., Huang, O. et al. ZNF703 promotes triple-negative breast cancer cells through cell-cycle signaling and associated with poor prognosis. BMC Cancer 22, 226 (2022). https://doi.org/10.1186/s12885-022-09286-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09286-w