Abstract
Background
Cervical cancer is a common malignancy of the female genital tract. Treatment options for cervical cancer patients diagnosed at FIGO (2009) stage IB2 and IIA2 remains controversial.
Methods
We perform a Bayesian network meta-analysis to directly or indirectly compare various interventions for FIGO (2009) IB2 and IIA2 disease, in order to improve our understand of the optimal treatment strategy for these women. Three databases were searched for articles published between 1971 and 2020. Data on included study characteristics, outcomes, and risk of bias were abstracted by two reviewers.
Results
Seven thousand four hundred eighty-six articles were identified. Thirteen randomized controlled trials of FIGO (2009) IB2 and IIA2 cervical cancer patients were included in the final analysis. These trials used six different interventions: concomitant chemoradiotherapy (CCRT), radical surgery (RS), radical surgery following chemoradiotherapy (CCRT+RS), neoadjuvant chemotherapy followed by radical surgery (NACT+RS), adjuvant radiotherapy followed by Radical surgery (RT + RS), radiotherapy alone (RT).SUCRA ranking of OS and Relapse identified CCRT+RS and CCRT as the best interventions, respectively. Systematic clustering analysis identified the CCRT group as a unique cluster.
Conclusion
These data suggest that CCRT may be the best approach for improving the clinical outcome of cervical cancer patients diagnosed at FIGO (2009) stage IB2/IIA2. Phase III randomized trials should be performed in order to robustly assess the relative efficacy of available treatment strategies in this disease context.
Similar content being viewed by others
Introduction
Cervical cancer is a major cause of morbidity and mortality, and remains one of the four most common malignant tumors in women. Globally, more than 560,000 new cases of cervical cancer are diagnosed each year, of which 80% occur in developing countries [1, 2].
Treatments of stage IB2/IIA2 cervical cancer revolves around chemoradiotherapy (CCRT), radical Surgery (RS), radical surgery following chemoradiotherapy (CCRT+RS), neoadjuvant chemotherapy followed by radical surgery (NACT+RS), adjuvant radiotherapy followed by Radical surgery (RT + RS), radiotherapy alone (RT). Previous studies have suggested that CCRT is the most appropriate treatment strategy [3,4,5,6]. However, other investigators have reported that NACT + RS improves the long-term DFS and OS of patients with locally advanced disease [7,8,9]. Other treatment regimens, such as CCRT+RS [10, 11], RT + RS [11, 12], RT [13, 14] and RS [14, 15], remain controversial. We therefore sough to perform a network meta-analysis of currently available findings in order to determine the most effective treatment for patients with stage IB2/IIA2 cervical cancer.
Systematic reviews and meta-analyses are widely considered to represent the pinnacle of the medical evidence pyramid [16]. However, traditional meta-analysis typically compare only two intervention types. In contrast, network meta-analyses can process all possible comparison indicators in the same model multiple times or in combination, and collect direct and indirect evidence at the same time [17, 18]. Moreover, network meta-analyses are thought to produce more accurate and reliable models compared to traditional meta-analysis, representing the premier guideline evidence for clinical practice [19, 20]. A network meta-analysis compares multiple treatment options for the same disease, which may be useful for developing clinical practice guidelines [21].
Here, we present a Bayesian network meta-analysis to address the currently conflicting data surrounding optimal treatment strategies for FIGO IB2/IIA2 cervical cancer patients. We aim to summarize and analyze the existing evidence to explore the clinical outcome of patients treatment with various regimens, using overall survival (OS) and disease recurrence as primary endpoints, in order to identify the optimal approach for management of locally advanced disease.
Material and methods
Search strategy and study selection
Two authors performed independent searches using PubMed, the Cochrane Central Register of Controlled Trials and Embase to identify Randomized Controlled Trials (RCTs) for the treatment of cervical cancer from 1971 to 2020, according to the Cochrane System Intervention Review Manual [22]. A comprehensive search was carried out through Boolean logic operators with Medical Subject Headings (MeSH) combined with entry words, using “Uterine Cervical Neoplasms”, “Chemoradiotherapy”, “General Surgery”, “Surgical Procedures, Operative”, “Gynecologic Surgical Procedures”, “Hysterectomy”, “Chemotherapy, Adjuvant”, “Drug Therapy”, “Radiotherapy” and “Randomized controlled trials”. This study was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for systematic reviews and meta-analysis [23] (Material S2). The specific search strategy is detailed in Material S1.
As specified in the predetermined inclusion criteria, all searched articles were individually evaluated by the two authors. We first screened the initial inclusion of studies based on the title and abstract, and deleted duplicate studies. Remaining articles were subject to full text screening by the two authors to evaluate study relevance. All citations were managed in Endnote X9. In order to ensure that further analysis can proceed smoothly, it is necessary to check the veracity and completeness of the data. Discrepancies between the two authors were resolved by a third empirical observer through discussion.
Inclusion and exclusion criteria, data extraction
The two authors independently extracted relevant data for each included trial. Discrepancies were addressed via discussion and consensus, with external arbitration where required.
Detailed inclusion and exclusion criteria are shown in Table S1. In our inclusion and exclusion criteria, treatment is defined as a preference. Treatments were defined as an intervention following discussion of the physician and patient, including surgery, radiotherapy, chemotherapy, or a combination of these regimens. All included randomized controlled trials were coded according to treatment type and are divided into 6 treatment groups. Differences in coding between the two authors were resolved by discussion and consensus, with external arbitration where required.
Quality appraisal, evaluation of endpoints
We used Cochrane tools to assess the risk of bias (ROB) of the included studies [22]. The two authors separately assessed seven areas of ROB. ROB evaluation is conducted in Review Manager (version 5.1).
The primary endpoints were overall survival and disease relapse; comparisons of all interventions were performed. All surviving patients contribute to OS, regardless of their disease status. Where exact case numbers of deceased and surviving patients were not available, these were estimated from Kaplan-Meir survival curves; corresponding authors of included studied were contacted where necessary. Both local recurrence and distant metastasis were included as disease relapse.
Statistical analysis
Compared with traditional meta-analysis, Bayesian network meta-analysis has greater analytical power, in that it summarizes all possible intervention comparisons simultaneously [20]. Using the minimum information prior distribution based on the random effect Bayesian statistical model, a connection network is formed combining direct and indirect evidence. Six intervention therapies were compared simultaneously; first, we performed regular pairwise meta-analysis. The I2 index was used to determine heterogeneity; indices of 25, 50, and 75% represent mild heterogeneity, moderate heterogeneity, and high heterogeneity, respectively [24]. A funnel chart was produced to detect publication bias. In order to reveal all available treatment evidence, a simple summary description network diagram was generated. The above analysis was performed in STATA, version 14.2. Endpoint analysis effect sizes were summarized as odds ratios (OR) with corresponding confidence intervals (CrI). Bayesian stratified random effects were used to directly and indirectly compare multiple interventions. The Bayesian method is used to calculate endpoint results; first, three parallel Markov chains with randomly selected states are established to simulate accurate estimation of statistical models [25]. Each chain generates 50,000 iterations, and because of the aging cycle, the first 20,000 iterations will be abandoned to ensure minimization of deviation of the initial value [26]. Convergence of the model was judged through the diagnostic curve [27]. The surface under the Cumulative Ranking Curve (SUCRA) is regarded as the ranking probability map for each intervention. The higher the SUCRA value, the more likely it is that an intervention is at the highest level or very effective, while a value of 0 means that the treatment is least effective [28]. Consistency between the two comparisons was evaluated by comparing the DIC values between the consistency and inconsistency models (a difference greater than 5 is considered as inconsistency between models) [29]. Node splitting was used to further assess for local inconsistencies in our network [30]. These analyses were performed using R (X64 version 3.5.3) with the “Gemtc” package (0.8–4 version), “JAGS” (version 4.3.0) and OpenBUGS (version 3.2.3).
Cluster analysis of the treatments
After Bayesian network analysis, by sorting out the SUCRA data of OS and relapse, a systematic cluster analysis of various treatment options was performed. Two to five cluster types were chosen and a vertical icicle diagram was used to visualize different clustering forms. After the systematic clustering analysis, the results were further analyzed through Online Analytical Processing (OLAP). The above analysis uses IBM SPSS version 26.0 for analysis.
Results
Study characteristics and ROB quality assessment
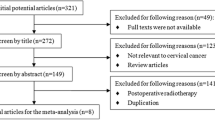
Among the 7486 citations, 4500 records were retained after deletion of duplicates. Four thousand two hundred thirty-two citations were removed after evaluation of title and abstract. Two hundred fifty-five records were excluded during full-text screening: 75 studies did not include stage IB2 and IIA2 cervical cancer, 65 studies were not randomized controlled trials, 12 studies had no relevant results, 15 studies did not determine the control group, 15 studies were supplements and 73 were excluded for other reasons such as foreign language, abstract etc.13 articles were included in the final study (Fig. 1).
These studies included 2733 participants undergoing 6 different interventions and provided sufficient data published from 1987 to 2020. Table 1 summarizes the main characteristics of the participants and interventions in the 13 included studies. Overall, 1399 patients were randomly assigned to the intervention group, while the remaining 1334 patients were assigned to the control group. In different studies, age is reported as the mean or median, ranging from 18 to 70 years. Across the 13 randomized controlled trials, most (more than half) of the participants were from Asia, followed by North America and Europe.
The 13 included studies included 6 interventions. The number of events and the The quality of individual and overall research levels are plotted in Figure S1 and Figure S2, respectively. In all 13 trials, all sequences were randomly generated, nine randomized controlled trials described their allocation concealment method, one trial design was not double-blind, and four randomized controlled trials had incomplete data on outcome indicators. There is 1 randomized controlled trial with higher risk, which originated from allocation concealment and double-blind design.
I2 analysis indicated no statistically significant heterogeneity in our preliminary meta-analysis (I2 = 0 for OS, P > 0.05, I2 = 8% for relapse, P > 0.05). The funnel chart indicated no obvious publication bias for OS (Figure S3) or relapse (Figure S4).
Visual network geometry was performed to show each arm. Each intervention has its own unique nodes, whose size depends on their number in the entire network. The two interventions are connected by straight lines, and the thickness of each straight line represents the number of comparisons (Fig. 2a, Fig. 3a).
a Network diagram for OS. Total studies = 9, Total patients in network = 2342. b The odds ratio (OR) table for each pair of intervention measures, with a confidence interval (CRI) of 95%. Odds ratio for OS: Treatment in top left is better. Abbreviations:CCRT = concomitant chemotherapy and radiotherapy,RS = Radical Surgery,NACT = neoadjuvant chemotherapy,RT = radiotherapy
a Network diagram for relapse. Total studies = 10, Total patients in network = 1950. b The odds ratio (OR) table for each pair of intervention measures, with a confidence interval (CRI) of 95%. Odds ratio for relapse: The treatment in the upper left corner is better. Abbreviations:CCRT = concomitant chemotherapy and radiotherapy,RS = Radical Surgery,NACT = neoadjuvant chemotherapy,RT = radiotherapy
Among the 2733 patients, the final number of OS and relapses were 1692 in 2342 and 470 in 1950, respectively (Table 2). A SUCRA line was drawn to rank the hierarchy of each interventions (shown in Fig. 2b and Fig. S5 for OS), which indicated that CCRT+RS got the highest probability (SUCRA = 0.7986) in IB2/IIA2 patients compared with the other 5 active interventions, Following by NACT+RS (SUCRA = 0.5214), RT (SUCRA = 0.5070), CCRT (SUCRA = 0.4832), RT + RS (SUCRA = 0.4462), RS (SUCRA = 0.2436) got an inferior ranking. Another SUCRA line was drawn to rank the hierarchy of each interventions (shown in Fig. 3b and Figure S6 for relapse), which indicated that CCRT got the highest probability (SUCRA = 0.8389) in IB2/IIA2 patients compared with the other 5 active interventions, Following by RT + RS (SUCRA = 0.6504), NACT+RS (SUCRA = 0.6295), RT (SUCRA = 0.4897), CCRT+RS (SUCRA = 0.2427), RS (SUCRA = 0.1488) got an inferior ranking.
Inconsistency detection
The posterior values of the random effects inconsistency and consistency model were estimated; for OS and relapse, the difference in DIC values between the consistency and inconsistency model was 2.6 and 2.0, respectively. These indicated no substantial inconsistency between models.
Overall ranking of SUCRA for each endpoint and cluster analysis
Intervention ranking were distinct for the two endpoints measures (OS and relapse). Clinically, high OS is highly desirable; however, high recurrence rate also represent a substantial burden on patients. In order to make an overall assessment of the best treatment plan, the SUCRA value of each endpoint of all 13 interventions was added to obtain a cumulative SUCRA score. This analysis determined CCRT as the optimal treatment strategy (Fig. 4). Subsequently, based on the sum of SUCRA of OS and relapse, systematic cluster analysis divides the CCRT into a cluster, further supporting this strategy as the best option (Fig. 5).
Further OLAP cube analysis demonstrated that when using a three-category approach, CCRT and RS were divided into a single group, indicating CCRT to be the optimal intervention and RS to be the worst (Table 3, Fig. 5).
Discussion
We performed an NMA study of treatments related to locally advanced cervical cancer in women to assess the relative effectiveness of various treatments in trials to date. Among all interventions evaluated, CCRT demonstrated the highest comprehensive efficacy, as evidenced by the sum of SUCRA value. After Bayesian analysis, a systematic cluster analysis was performed to determine the treatment interventions that can be evenly grouped according to the sum of SUCRA values of the two endpoints obtained by NMA, setting the cluster numbers to 2–5 categories to facilitate observation. At 3 clusters, CCRT and RS are classified into different groups. From the SUCRA value, it is apparent that the top-ranked treatments vary depending on the endpoint of the assessment. The sum of the SUCRA value of each of the two endpoints implies that CCRT is the optimal intervention for FIGO stage IB2/IIA2 cervical tumor. Hierarchical cluster analysis further verified that the CCRT separated into an independent group. Therefore, in FIGO stage IB2/IIA2 cervical cancer, CCRT appears the optimal management strategy for cases.
Cervical cancer is a serious women’s health issue worldwide; most cervical tumors are caused by high-risk human papillomavirus (HR-HPV) infection [41]. An appreciable proportion of cervical cancer is diagnosed at FIGO stage IB2/IIA2. Previous reports have compared these cases against stage IB1 disease, reporting an increased risk of death from FIGO stage IB2 cervical cancer disease representing a close-to-doubling of risk (HR 1.98, 95% CI 1.62–2.41, P < 0.001) [42]. Optimal management of these cases is therefore crucial.
The efficacy of CCRT in the treatment of locally advanced cervical cancer has been compared in previous randomized controlled trials or meta-analysis; these studies have suggested the superiority of CCRT versus other regimens [43,44,45]. Gupta et al. [31] suggested that in locally advanced cervical cancer, cisplatin-based concurrent radiotherapy and chemotherapy can achieve better disease-free survival compared with radical surgery after neoadjuvant chemotherapy.
Other studies suggest that - although only significant for patients with stage IB2-IIB - NACT plus RS seems to confer survival benefit compared to RT [34]. Compared with RS alone, especially compared with CCRT, NACT + RS may improve the long-term disease-free survival rate and overall survival rate of patients with locally advanced cervical cancer stage IB2-IIB [7]. Moreover, total hysterectomy after NACT may be an option for patients with stage Ib2-IIb cervical adenocarcinoma [46]. However, this study found that NACT did not improve overall survival, but reduced the number of patients receiving postoperative radiotherapy [47]. Lee et al. [48] described no therapeutic advantage of NACT + RS compared to CCRT. Some scholars believe that preoperative brachytherapy in the vaginal cavity can be used as an effective treatment method for comprehensive treatment of stage Ib2 and IIa cervical cancer, with a satisfactory local control rate for stage Ib2 and IIa cervical cancer [32]. The findings of Landoni et al. [13] indicate that, in terms of survival, there is no alternative treatment for early cervical cancer. Long-term follow-up confirmed that the best treatment for individual patients should take into account clinical factors, such as menopausal status, comorbidities, histological type, and tumor diameter. In light of our findings in the context of this controversy, CCRT appears to be the most appropriate therapeutic option.
NMA comes with conceptual and technical considerations [49], including the need to meet transitivity and consistency assumptions. The transitivity hypothesis means that the diverse treatments in all studies are comparable in terms of the characteristics that may affect the results. In order to ensure transmissibility, except for treatment interventions, other aspects of the included study should be relatively similar [49, 50]. In order to meet this transitivity assumption, we limited the study to locally advanced cervical cancer.
Consistency described the statistical consistency between the direct comparison and the indirect comparison of each paired comparison in NMA. Differences indicate inconsistency [19, 29, 49]. We use the confidence interval in the network Meta-analysis to test the heterogeneity and consistency of the two endpoints and use the node splitting method to detect local inconsistencies [30]. No major heterogeneity or consistency issues were identified in the OS or relapse analysis.
The advantage of this study is that our NMA compares each intervention for locally advanced cervical cancer. At present, the treatment of stage IB2/IIA2 cervical cancer is still controversial; our findings are therefore of clear clinical interest.
We acknowledge several limitations of our study. We acknowledge the subjectivity of the risk bias assessment. Some of the include studies lacked blinding of participating subjects, personnel or external reviewers. Moreover, some studies had incomplete outcome data. One randomized control trial demonstrated higher risk, which originated from allocation concealment and double-blind design. The quality of several studies may have affected our analysis. In addition, due to incomplete data, very few data were available, so the endpoint of complication rate and type of different treatments were lacking. Another limitation of the study is that all 13 studies included cervical cancer stage IB2/IIA2, but a few studies not only included cervical cancer stage IB2 /IIA2. This may have some impact on our research.
Conclusions
We report an analysis of all RCTs using different interventions in FIGO IB2/IIA2 cervical cancer; NMA identified that, in terms of effectiveness and safety, overall survival and relapse, CCRT may be the optimal treatment strategy in locally advanced cervical cancer. RS alone may be the least effective strategy. However, since these interventions have not yet been directly compared face-to-face, additional verification is necessary for the Phase 3 multicenter randomized controlled trial.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Vu M, Yu J, Awolude OA, Chuang L. Cervical cancer worldwide. Curr Probl Cancer. 2018;42(5):457–65. https://doi.org/10.1016/j.currproblcancer.2018.06.003.
Hsieh HY, Huang JW, Lu CH, Lin JC, Wang L. Definite chemoradiotherapy is a competent treatment option in FIGO stage IB2 cervical cancer compared with radical surgery +/− neoadjuvant chemotherapy. J Formos Med Assoc. 2019;118(1 Pt 1):99–108. https://doi.org/10.1016/j.jfma.2018.01.015.
Thakur P. Prospective randomized study comparing concomitant chemoradiotherapy using weekly cisplatin and paclitaxel vs concomitant chemoradiotherapy using weekly cisplatin in locally advanced carcinoma cervix. J Cancer Res Ther. 2014;10:S45.
Li Z, Yang S, Liu L, Han S. A comparison of concurrent chemoradiotherapy and radiotherapy in Chinese patients with locally advanced cervical carcinoma: a multi-center study. Radiat Oncol. 2014;9:212.
Huang P, Zhou L, Wang LH, Li Y, Peng X, Zhu AN, et al. Clinical effect of preoperative chemoradiotherapy and radiotherapy for the patients with I B2 and II a cervical cancer. Chin J Cancer Prevent Treat. 2011;18(16):1290–2.
Yin M, Zhao F, Lou G, Zhang H, Sun M, Li C, et al. The long-term efficacy of neoadjuvant chemotherapy followed by radical hysterectomy compared with radical surgery alone or concurrent chemoradiotherapy on locally advanced-stage cervical cancer. Int J Gynecol Cancer. 2011;21(1):92–9. https://doi.org/10.1111/IGC.0b013e3181fe8b6e.
Qin T, Zhen J, Zhou M, Wu H, Ren R, Qu B, et al. Efficacy of neoadjuvant chemotherapy plus radical surgery in patients with bulky stage II cervical squamous cell carcinoma: a retrospective cohort study. Int J Surg. 2016;30:121–5. https://doi.org/10.1016/j.ijsu.2016.04.038.
Hwang YY, Moon H, Cho SH, Kim KT, Moon YJ, Kim SR, et al. Ten-year survival of patients with locally advanced, stage ib-iib cervical cancer after neoadjuvant chemotherapy and radical hysterectomy. Gynecol Oncol. 2001;82(1):88–93. https://doi.org/10.1006/gyno.2001.6204.
Huguet F, Cojocariu OM, Levy P, Lefranc JP, Darai E, Jannet D, et al. Preoperative concurrent radiation therapy and chemotherapy for bulky stage IB2, IIA, and IIB carcinoma of the uterine cervix with proximal parametrial invasion. Int J Radiat Oncol Biol Phys. 2008;72(5):1508–15. https://doi.org/10.1016/j.ijrobp.2008.03.054.
Peters Iii WA, Liu PY, Barrett Ii RJ, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18(8):1606–13. https://doi.org/10.1200/JCO.2000.18.8.1606.
Li F, Wu Y, Kong W, Wang J, Hao X, Niu J, et al. Comparison of the effects of preoperative vaginal intracavitary irradiation plus surgery and surgery alone for stage I b2 and II a cervical cancer. Chin J Clin Oncol. 2008;35(14):797–800.
Landoni F, Colombo A, Milani R, Placa F, Zanagnolo V, Mangioni C. Randomized study between radical surgery and radiotherapy for the treatment of stage IB–IIA cervical cancer: 20-year update. J Gynecol Oncol. 2017;28(3):e34. https://doi.org/10.3802/jgo.2017.28.e34.
Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350(9077):535–40. https://doi.org/10.1016/S0140-6736(97)02250-2.
Frumovitz M, Obermair A, Coleman RL, Pareja R, Lopez A, Ribero R, et al. Quality of life in patients with cervical cancer after open versus minimally invasive radical hysterectomy (LACC): a secondary outcome of a multicentre, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2020;21(6):851–60. https://doi.org/10.1016/S1470-2045(20)30081-4.
Murad MH, Asi N, Alsawas M, Alahdab F. New evidence pyramid. Evid Based Med. 2016;21(4):125–7. https://doi.org/10.1136/ebmed-2016-110401.
Tonin FS, Rotta I, Mendes AM, Pontarolo R. Network meta-analysis: a technique to gather evidence from direct and indirect comparisons. Pharm Pract. 2017;15(1):943. https://doi.org/10.18549/PharmPract.2017.01.943.
Mills EJ, Bansback N, Ghement I, Thorlund K, Kelly S, Puhan MA, et al. Multiple treatment comparison meta-analyses: a step forward into complexity. Clin Epidemiol. 2011;3:193–202. https://doi.org/10.2147/CLEP.S16526.
Kiefer C, Sturtz S, Bender R. Indirect comparisons and network Meta-analyses. Dtsch Arztebl Int. 2015;112(47):803–8. https://doi.org/10.3238/arztebl.2015.0803.
Kruschke JK, Liddell TM. The Bayesian new statistics: hypothesis testing, estimation, meta-analysis, and power analysis from a Bayesian perspective. Psychon Bull Rev. 2018;25(1):178–206. https://doi.org/10.3758/s13423-016-1221-4.
Laws A, Tao R, Wang S, Padhiar A, Goring S. A comparison of National Guidelines for network Meta-analysis. Value Health. 2019;22(10):1178–86. https://doi.org/10.1016/j.jval.2019.05.013.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:Ed000142.
Welch V, Petticrew M, Petkovic J, Moher D, Waters E, White H, et al. Extending the PRISMA statement to equity-focused systematic reviews (PRISMA-E 2012): explanation and elaboration. J Clin Epidemiol. 2016;70:68–89. https://doi.org/10.1016/j.jclinepi.2015.09.001.
Turner RM, Jackson D, Wei Y, Thompson SG, Higgins JP. Predictive distributions for between-study heterogeneity and simple methods for their application in Bayesian meta-analysis. Stat Med. 2015;34(6):984–98. https://doi.org/10.1002/sim.6381.
van den Berg SM, Beem L, Boomsma DI. Fitting genetic models using Markov chain Monte Carlo algorithms with BUGS. Twin Res Hum Genet. 2006;9(3):334–42. https://doi.org/10.1375/twin.9.3.334.
Shim SR, Kim SJ, Lee J, Rücker G. Network meta-analysis: application and practice using R software. Epidemiol Health. 2019;41:e2019013. https://doi.org/10.4178/epih.e2019013.
Toft N, Innocent GT, Gettinby G, Reid SW. Assessing the convergence of Markov chain Monte Carlo methods: an example from evaluation of diagnostic tests in absence of a gold standard. Prev Vet Med. 2007;79(2–4):244–56. https://doi.org/10.1016/j.prevetmed.2007.01.003.
Page MJ, Shamseer L, Altman DG, Tetzlaff J, Sampson M, Tricco AC, et al. Epidemiology and reporting characteristics of systematic reviews of biomedical research: a cross-sectional study. PLoS Med. 2016;13(5):e1002028. https://doi.org/10.1371/journal.pmed.1002028.
Neupane B, Richer D, Bonner AJ, Kibret T, Beyene J. Network meta-analysis using R: a review of currently available automated packages. PLoS One. 2014;9(12):e115065. https://doi.org/10.1371/journal.pone.0115065.
van Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods. 2016;7(1):80–93. https://doi.org/10.1002/jrsm.1167.
Gupta S, Maheshwari A, Parab P, Mahantshetty U, Hawaldar R, Sastri Chopra S, et al. Neoadjuvant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy in patients with stage IB2, IIA, or IIB squamous cervical Cancer: a randomized controlled trial. J Clin Oncol. 2018;36(16):1548–55. https://doi.org/10.1200/JCO.2017.75.9985.
Li C, Xu S, Zhou L, Zhu Y. Preoperative concurrent chemoradiotherapy for locally advanced cervical cancer. Chin J Clin Oncol. 2010;37(21):1242–1244+1248.
Curtin JP, Hoskins WJ, Venkatraman ES, Almadrones L, Podratz KC, Long H, et al. Adjuvant chemotherapy versus chemotherapy plus pelvic irradiation for high-risk cervical cancer patients after radical hysterectomy and pelvic lymphadenectomy (RH-PLND): a randomized phase III trial. Gynecol Oncol. 1996;61(1):3–10. https://doi.org/10.1006/gyno.1996.0087.
Benedetti-Panici P, Greggi S, Colombo A, Amoroso M, Smaniotto D, Giannarelli D, et al. Neoadjuvant chemotherapy and radical surgery versus exclusive radiotherapy in locally advanced squamous cell cervical cancer: results from the Italian multicenter randomized study. J Clin Oncol. 2002;20(1):179–88. https://doi.org/10.1200/JCO.2002.20.1.179.
Chang TC, Lai CH, Hong JH, Hsueh S, Huang KG, Chou HH, et al. Randomized trial of neoadjuvant cisplatin, vincristine, bleomycin, and radical hysterectomy versus radiation therapy for bulky stage IB and IIA cervical cancer. J Clin Oncol. 2000;18(8):1740–7. https://doi.org/10.1200/JCO.2000.18.8.1740.
Wang X, Chen J, Sun W, Zhu M, Li D, Chen G. Influences of neoadjuvant chemotherapy on clinical indicators, prognosis and neutrophil/lymphocyte ratio of stage IB2-IIB cervical cancer. J BUON. 2020;25(2):757–63.
Chen H, Liang C, Zhang L, Huang S, Wu X. Clinical efficacy of modified preoperative neoadjuvant chemotherapy in the treatment of locally advanced (stage IB2 to IIB) ervical ancer: randomized study. Gynecol Oncol. 2008;110(3):308–15. https://doi.org/10.1016/j.ygyno.2008.05.026.
Duan L, Zhang K, Wang Y, Jin J, Xie J, Luo X, et al. Effects of neoadjuvant chemotherapy combined with cervical cancer radical surgery for the treatment of cervical cancer. Biomed Res. 2017;28(22):9745–8.
Katsumata N, Yoshikawa H, Kobayashi H, Saito T, Kuzuya K, Nakanishi T, et al. Phase III randomised controlled trial of neoadjuvant chemotherapy plus radical surgery vs radical surgery alone for stages IB2, IIA2, and IIB cervical cancer: a Japan clinical oncology group trial (JCOG 0102). Br J Cancer. 2013;108(10):1957–63. https://doi.org/10.1038/bjc.2013.179.
Perez CA, Camel HM, Kao MS, Hederman MA. Randomized study of preoperative radiation and surgery or irradiation alone in the treatment of stage IB and IIA carcinoma of the uterine. Gynecol Oncol. 1987;27(2):129–40. https://doi.org/10.1016/0090-8258(87)90285-X.
Wardak S. Human papillomavirus (HPV) and cervical cancer. Med Dosw Mikrobiol. 2016;68(1):73–84.
Matsuo K, Machida H, Mandelbaum RS, Konishi I, Mikami M. Validation of the 2018 FIGO cervical cancer staging system. Gynecol Oncol. 2019;152(1):87–93. https://doi.org/10.1016/j.ygyno.2018.10.026.
Datta NR, Stutz E, Liu M, Rogers S, Klingbiel D, Siebenhüner A, et al. Concurrent chemoradiotherapy vs. radiotherapy alone in locally advanced cervix cancer: a systematic review and meta-analysis. Gynecol Oncol. 2017;145(2):374–85. https://doi.org/10.1016/j.ygyno.2017.01.033.
Rose PG. Chemoradiotherapy for cervical cancer. Eur J Cancer. 2002;38(2):270–8. https://doi.org/10.1016/S0959-8049(01)00352-5.
Green J, Kirwan J, Tierney J, Symonds P, Fresco L, Williams C, et al. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev. 2001. https://doi.org/10.1002/14651858.CD002225.pub2.
Gadducci A, Landoni F, Cosio S, Zizioli V, Zola P, Ferrero AM, et al. Neoadjuvant platinum-based chemotherapy followed by radical hysterectomy for stage Ib2-IIb adenocarcinoma of the uterine cervix - an Italian multicenter retrospective study. Anticancer Res. 2018;38(6):3627–34. https://doi.org/10.21873/anticanres.12637.
Yang Z, Chen D, Zhang J, Yao D, Gao K, Wang H, et al. The efficacy and safety of neoadjuvant chemotherapy in the treatment of locally advanced cervical cancer: a randomized multicenter study. Gynecol Oncol. 2016;141(2):231–9. https://doi.org/10.1016/j.ygyno.2015.06.027.
Lee J, Kim TH, Kim GE, Keum KC, Kim YB. Neoadjuvant chemotherapy followed by surgery has no therapeutic advantages over concurrent chemoradiotherapy in International Federation of Gynecology and Obstetrics stage IB-IIB cervical cancer. J Gynecol Oncol. 2016;27(5):e52. https://doi.org/10.3802/jgo.2016.27.e52.
Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3(2):80–97. https://doi.org/10.1002/jrsm.1037.
Jansen JP, Crawford B, Bergman G, Stam W. Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value Health. 2008;11(5):956–64. https://doi.org/10.1111/j.1524-4733.2008.00347.x.
Acknowledgements
The authors would like to express their gratitude to EditSprings for the expert linguistic services provided.
Funding
This work was supported by no funding.
Author information
Authors and Affiliations
Contributions
JC, BW, LX, BBL conceived and drafted experiments. BW and JC screened the literature and extracted the data, JC, BW, BBL, XCL, ZHL, RLC and RTW wrote manuscripts. LX supervised the entire process. All authors viewed and endorsed the final manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declared that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cheng, J., Liu, B., Wang, B. et al. Effectiveness comparisons of various therapies for FIGO stage IB2/IIA2 cervical cancer: a Bayesian network meta-analysis. BMC Cancer 21, 1078 (2021). https://doi.org/10.1186/s12885-021-08685-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-08685-9








 : When divided into 3 categories, CCRT and RS were divided into a single group respectively
: When divided into 3 categories, CCRT and RS were divided into a single group respectively