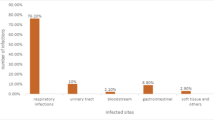
Abstract
Background
Patients with multiple myeloma (MM) remain at an increased risk of infection due to the disease process, as well as the ensuing treatments.
Methods
We performed a systematic review to evaluate the monthly risk of grade III/IV infection, pneumonia, and neutropenia in patients with myeloma enrolled in randomized clinical trials (RCTs).
Results
The risk of grade III or higher infection, pneumonia, and neutropenia persists among all phases of treatment. There was no statistical difference in grade III or higher infection, pneumonia, and neutropenia between frontline and relapsed/refractory setting. In the maintenance setting, the complications of infection, pneumonia, and neutropenia were low, but not negligible. Three-drug regimens were no more likely than two-drug regimens to have an increased risk of Grade III or higher infection.
Conclusions
This is the first study to quantify the monthly risk of grade III or higher infection, pneumonia, and neutropenia across different treatment regimens in the frontline, maintenance, and relapsed/refractory settings. The results of our systematic review demonstrate a significant risk for severe infection, pneumonia, and neutropenia in patients with MM. Further studies are needed to determine the value of antibiotic prophylaxis in a broader myeloma patient population, as well as other approaches that will further mitigate the morbidity and mortality related to infection in this vulnerable patient population.
Similar content being viewed by others
Introduction
Patients with multiple myeloma (MM) remain at an increased risk of infection due to the immunosuppressive nature of the underlying disease process, as well as the ensuing treatments [1,2,3]. Postulated risk factors for infection risk in myeloma include impaired host defenses with disease progression (leukopenia, T-cell immunodeficiency, hypogammaglobulinemia, poor performance status, increasing age, renal failure), multifactorial immunosuppression with prolonged steroid exposure and previous treatments (reduced CD4+, CD45+, CD19+, and NK cells), and disease evolution with mutational changes and clonal evolution and heterogeneity [4]. The risk of infection is the greatest within the first 3 months following diagnosis, and infections remain an important contributing factor to early morbidity and mortality for patients with MM [2, 5, 6].
Regimens known to increase the risk of severe infections include immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs). IMiDs like lenalidomide and pomalidomide, cause neutropenia, increasing risk of infection [7, 8]. The PI, bortezomib is associated with reactivation of varicella-zoster-virus due to impairment of T-cell function [9].
Given the increased risks of severe morbidity and mortality, it is imperative to assess the degree of immunosuppression and risk of infection with different treatment regimens, across all phases of treatment. Such information is useful for patients and providers in the risk assessment and mitigation decision-making process. No study has every reported the monthly-associated risk of infection with different treatment regimens in clinical trials across frontline, maintenance, and relapsed/refractory settings. We performed a systematic review and meta-analysis evaluating the monthly risk of infection, pneumonia, and neutropenia in patients with myeloma on treatment enrolled in randomized clinical trials (RCTs).
Methods
Search strategy
Three databases were searched i.e., MEDLINE/PubMed, Embase, and Cochrane Registry of Controlled Trials. An example search strategy using Embase is highlighted in Supplementary Table 1. Two independent reviewers (GRM, NB) screened all studies, and any conflict was resolved through mutual discussion. Furthermore, for the purpose of our analysis, we strictly adhered to predefined reporting criteria. This systematic review and meta-analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations [10].
Inclusion and exclusion criteria
Our search strategy was performed to include RCTs from January 1, 2015 to December 30, 2019. The search was last updated on April 1, 2020. Studies were only included for quantitative analysis if authors clearly reported the median duration of treatment or median number of cycles corresponding to their reported toxicities. If a study only reported combined leukopenia/neutropenia as a composite outcome, that study was not included in our neutropenia category. The use of antimicrobial prophylaxis was also obtained. Studies evaluating different phases of treatment (induction/consolidation/maintenance) that did not clearly elucidate reported timeframe of toxicities were not included in our analysis. All other studies including editorials, case reports, case series, review articles, case control, retrospective/prospective cohort, and single arm studies were excluded. Studies of regimens that only reported the efficacy/safety of autologous transplant were also excluded as our main focus was to evaluate the toxicity of MM regimens, including those used prior to or after a transplant, rather than the toxicity of the transplant itself. The search strategy was not restricted to language. Abstracts from conference proceedings that were captured via our search strategy (such as those listed on Embase) were also included.
Data collection
Two authors (GRM and NB) performed and verified all data extraction. Extracted data was tabulated using Microsoft Excel (Microsoft, Redmond, Washington, United States). We identified number of participants in each study and characteristics of studies such as the nature of MM patient population (“transplant-eligible” versus “non-transplant-eligible”) and regimens used as “first-line” or “relapsed/refractory.” We also identified what class of drug was used in each regimen based on whether a PI/IMiD/Anti-CD38 agent was included or not. When pooled analysis was presented for a class of drugs, maintenance studies were excluded as the toxicities would be expected to be much lower. We also collected the publication year of data related to each study. We systematically screened each of the trials for outcomes pertaining to the incidence of infection, the grade/type of such infection, and neutropenia. Other infection-specific variables were captured including if antibiotic prophylaxis was permitted, number of participants who used prophylactic antibiotics, and death from infection. Immunomodulatory drugs were classified as thalidomide and its analogs (pomalidomide, lenalidomide). Bortezomib, carfilzomib, and ixazomib were identified as proteasome inhibitors. The median number of cycles received for each treatment regimen, in order to standardize outcome reporting was also collected.
Primary and secondary outcomes
Our study had three primary outcomes which we assessed across each treatment phase of myeloma (frontline treatment, relapsed/refractory setting, and maintenance). The primary outcomes of the studies were the incidence of Grade III or higher infections per month on treatment amongst patients with MM enrolled in RCTs, the incidence of Grade III or higher pneumonia per month on treatment amongst patients with MM enrolled in RCTs, and the incidence of Grade III or higher neutropenia per month in treatment amongst patients with MM enrolled on RCTs.
Heterogeneity and Bias assessment
We assessed heterogeneity in studies using the I2 statistic as defined by Cochrane Handbook for Systematic Reviews. I2 < 30%, 30–60%, 61–75, and > 75% were suggestive of low, moderate, substantial, and considerable heterogeneity, respectively [11, 12]. Study quality using Cochrane risk-of-bias tools for RCTs was assessed [11, 13]. The influence of individual studies was examined by leaving out one study and recalculating the meta-analysis.
Statistical analysis
Pooled proportion rates for all outcomes were compared using risk ratio (RR) and 95% confidence intervals (CI) with p-values generated. A p-value of < 0.05 was considered statistically significant. We calculated outcomes using the DerSimonian-Laird method along with random effects. Due to software limitations, we multiplied the actual incidence/prevalence of outcomes by 100, to generate infection risk per 100 months. Results were displayed as per monthly risk of grade III or higher infection/pneumonia/neutropenia. Open meta-analyst (CEBM, Brown University, Rhode Island, USA) and Comprehensive Meta-analysis (Biostat, Englewood, New Jersey, US) were used as the computing software.
Results
After excluding trials not meeting defined time-period, duplicates, trials in progress with no results, and non-randomized studies, we included 31 RCTs for analysis in our study (Fig. 1).
Thirty-one studies clearly reported either incidence of Grade III or higher infection, pneumonia, or neutropenia, and provided a clear duration of treatment/number of cycles. Supplementary Table 2 lists characteristics of the studies included in our analysis. Supplementary Table 3 highlights the risk of bias in each of the included studies. The treatment regimens incorporated in these RCTs were analysed according to the phase of treatment (frontline, relapsed/refractory, and maintenance).
Incidence of grade III or higher infection, pneumonia, neutropenia in RCTs evaluating patients in the frontline setting
Nine RCTs using 11 unique treatment regimens (n = 2656 patients) reported on the incidence of infection per cycle/month of therapy in the frontline setting (Fig. 2) [14,15,16,17,18,19,20,21,22].
In the frontline setting, bortezomib/lenalidomide/dexamethasone (VRD) was associated with a monthly incidence of grade III or higher infection of 2.6% (2.4–2.8%, I2 = 98.1%) [21] in non-transplant patients and 1.5% (1.4–1.6%, I2 = 98.1%) in transplant eligible patients [17]. Daratumumab/lenalidomide/dexamethasone (DRd) was associated with a monthly incidence of grade III or higher infection of 1.3% (1.2–1.4%, I2 = 98.1%) [20]. Bortezomib/dexamethasone (Vd) was associated with a monthly incidence of grade III or higher infection of 2.2% (2.0–2.4%, I2 = 98.1%) [14].
A total of 6 RCTs, 8 regimens, (n = 2231 patients) reported on the incidence of Grade III or higher pneumonia (Supplementary Figure 1) [14, 15, 17, 19, 20, 23]. Amongst these regimens, daratumumab/bortezomib/melphalan/prednisone was associated with the highest risk of monthly grade III or higher pneumonia in newly diagnosed MM (3.7% (3.5–3.9%, I2 = 99.4%) [15].
Ten RCTs with 12 regimens (n = 3459 patients) in newly diagnosed MM reported incidence of grade III or higher neutropenia per cycle/month of treatment (Supplementary Figure 2) [14,15,16,17,18,19,20, 23,24,25]. Bortezomib/melphalan/prednisone was associated with the highest risk of monthly grade III or higher neutropenia in newly diagnosed MM (4.0% (3.7–4.3%, I2 = 99.6%) [14]. Bortezomib/melphalan/prednisone had a significantly higher risk of monthly neutropenia compared to the other regimens in the UPFRONT study – bortezomib/dexamethasone [0.3% (0.2–0.4%, I2 = 99.6%)] and bortezomib/thalidomide/dexamethasone [0.5% (0.4–0.7%, I2 = 99.6%) [14]. VRD was associated with a monthly incidence of grade III or higher neutropenia of 2.1% (2.0–2.3%, I2 = 99.6%) [17].
Risk of infection, pneumonia, neutropenia in RCTs evaluating patients in relapsed/refractory setting
In the relapsed/refractory MM (RRMM) setting, we identified 9 RCTs with 10 treatment regimens (n = 1980 patients) reporting the incidence of Grade III or higher infection per cycle/month of treatment (Fig. 3, 26–34].
The highest monthly risk of infection was in patients treated with pomalidomide/dexamethasone-containing regimens: pembrolizumab/pomalidomide/dexamethasone, with a monthly risk for grade III or higher infection of 3.9% (3.5–4.2%, I2 = 99.5%) [26] followed by bortezomib/pomalidomide/dexamethasone at 3.5% (3.3–3.7%, I2 = 99.5%) [27].
Seven RCTs with 8 regimens (n = 1614 patients) reported the incidence of Grade III or higher pneumonia per cycle/month of treatment (Supplementary Figure 3) [26,27,28,29,30,31,32]. In the RRMM setting, the highest monthly risk of grade III or higher pneumonia was pembrolizumab/pomalidomide/dexamethasone [3.2% (2.9–3.6%, I2 = 97.5%)], whereas commonly used daratumumab-based regimens like DRd and Daratumumab/bortezomib/dexamethasone (DVd) were associated with monthly risks of Grade III or higher pneumonia of 0.8% (0.7–0.9%, I2 = 97.5%) [30] and 0.7% (0.6–0.8%, I2 = 97.5%) [31], respectively.
Fifteen RCTs, 17 regimens (n = 3691 patients) with RRMM reported the incidence of Grade III or higher neutropenia per cycle/month of treatment (Supplementary Figure 4) [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. The monthly incidence of grade III or higher neutropenia with a contemporary pomalidomide-based triplet such as isatuximab/pomalidomide/dexamethasone was 8.3% (7.9–8.8%, I2 = 99.6%)] [35]. Commonly used daratumumab regimens such as DRd and DVd were associated with monthly risks of Grade III or higher neutropenia of 2.2% (2.0–2.4%, I2 = 99.6%) [30] and 1.1% (0.9–1.1%, I2 = 99.8%) [31], respectively.
Incidence of infection, pneumonia, neutropenia in RCTs evaluating patients in maintenance setting
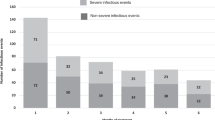
Four RCTs involving 7 regimens with 1265 patients on maintenance therapy reported incidence of Grade III or higher infection risk per month of treatment [41,42,43,44]. Across these 7 regimens the incidence of Grade III or higher infection risk per month of treatment was 0.4, 95% CI = 0.2–0.6% (Fig. 4).
Only one trial reported on incidence of grade III or higher pneumonia [42]. Four RCTs, with 7 treatment regimens involving 1265 patients with RRMM reported incidence of Grade III or higher neutropenia risk per month/cycle of treatment (Supplementary Figure 5) [41,42,43,44]. Across these 7 regimens, the incidence of Grade III or higher neutropenia per month was 0.5% (95% CI 0.4–0.7%).
Comparison of risks for frontline versus relapsed-refractory patients
In the frontline setting, risk of grade III or higher infection, pneumonia, and neutropenia per month of treatment were 1.7 (95% CI = 1.3–2.0%), 1.3 (95% CI = 0.8–1.8%), and 1.9% (95% CI = 1.3–2.6%), respectively. In the RRMM groups, rates of Grade III or higher infection, pneumonia, and neutropenia per month were 1.5 (95% CI = 1.0–2.0%), 1.2 (95% CI = 0.9–1.6%), and 2.7% (95% CI = 2.1–3.3%), respectively.
Comparison of risks for 2-drug versus 3-drug regimens
There was no statistical difference in 2-drug versus 3-drug regimens for grade III or higher infection, pneumonia, and neutropenia across all phases of treatment. The risk of Grade III or higher infection in 2-drug regimens were 1.5% (95% CI = 0.1–2.9%) (2 RCTs, 2 regimens, n = 332) [14, 34], while 3-drug regimens were 1.7% (95% CI = 1.3–2.2%) (10 RCTs, 11 regimens, n = 2049) [14, 17, 19,20,21,22, 26, 33, 35, 36] (Supplementary Figures 6 and 7). For grade III or higher pneumonia, the risk of 2-drug regimens and 3-drug regimens were 1.3 (95% CI = 0.4–2.2%) (2 RCTs, 2 regimens, n = 332) [14, 34] and 1.0 (95% CI = 0.8–1.3%), respectively (10 RCTs, 11 regimens, n = 2548) [14, 17, 19, 20, 23, 26, 30, 31, 33, 35] (Supplementary Figures 8 and 9). The risk of Grade III or higher neutropenia in 2-drug regimens was 2.6 (95% CI = -1.9–7.2%) (2 RCTs, 2 regimens, n = 332) [14, 34] and 2.5 (95% CI = 2.0–3.1%) in 3-drug regimens (15 RCTs, 16 regimens, n = 3888) [14, 17, 19, 20, 23,24,25,26, 30, 31, 33, 35, 36, 38, 39] (Supplementary Figures 10 and 11).
Use of prophylactic antibiotics in RCTs
Only three RCTs noted whether use of prophylactic antibiotics was mandated (Supplementary Table 4) [24, 27, 43]. Among these studies, name of antibiotics given and number of patients receiving antibiotics were not reported. Only 10 RCTs reported on death from infection [17, 20, 21, 25,26,27,28, 35, 37, 43].
Sensitivity analysis
Omitting single studies successively showed no study had a significant influence on the overall results (Supplementary Figure 12).
Discussion
Our study is the first study to quantify monthly risk for infection, pneumonia, and neutropenia in various regimens across all phases of treatment in patients with multiple myeloma on clinical trials. We demonstrate that rates of grade III or higher infection, pneumonia, and neutropenia are clinically significant across frontline and RRMM setting. Compared to those in the frontline and RRMM, the complications of infection, pneumonia, and neutropenia in the maintenance setting were low, but not negligible. Our study also indicates that three-drug regimens are no more likely than two-drug regimens to have an increased risk of Grade III or higher infection, implying that multiple patient/host factors (and not just the drugs) play a major part in the causation of infection. Our study thus questions whether there is indeed cumulative toxicity as pertains to infection when additional classes of drugs are added. This merits further study with patient-level information in future work. The importance of host factors is also evident in the frontline setting when using the same drug, VRD, in two different patient populations. VRD was associated with a monthly incidence of grade III or higher infection of 2.6% (2.4–2.8%, I2 = 98.1%) [21] in non-transplant patients compared to 1.5% (1.4–1.6%, I2 = 98.1%) in transplant eligible patients [17]. The difference in infection risks highlights the importance of host factors when assessing infection risks in these patients.
The safety profiles for MM regimens have improved over time, as evidenced by pomalidomide/dexamethasone containing regimens in which the highest risk of Grade III or higher infection per month was seen in the earlier studies (Richardson et al. [45] and MM-003 [46]), with the risk subsequently decreased in later studies (3.5% with bortezomib/pomalidomide/dexamethasone [27], 2.4% for isatuximab, pomalidomide/dexamethasone [35], and 1.6% with elotuzumab/pomalidomide/dexamethasone [29]). As pomalidomide moved from a heavily pre-treated patient population in the earliest aforementioned studies, to earlier in the disease course in more contemporary studies, the risk of Grade III or higher infection decreased significantly. It should be noted that the decreased dose of dexamethasone in contemporary regimens is also likely a contributing factor in the decrease in infections in more modern regimens, as low-dose dexamethasone was associated with better short-term overall survival and lower toxicity when compared to high-dose dexamethasone [47].
Our work provides numerical data to clinicians and patients of the risks of infections, pneumonia, and neutropenia associated with treatment regimens in clinical trials. While extrapolating these results to patients in clinic, it is important to consider differences in baseline characteristics between clinical trial patients and those seen in routine clinical practice, who are often likely to be older, have more comorbidities and potentially be at a higher risk of complications of treatment. Furthermore, the monitoring of infection for routine patients outside of clinical trials may not be as vigorous. Given the risk of infection in trials in not only the newly diagnosed setting, but also the relapsed/refractory setting, consideration must be made for antibiotic prophylaxis strategies in these patients, and trials designing such strategies are needed.
Unfortunately, uniform use of prophylactic antibiotics in clinical trials with MM has historically been lacking [48]. In our analysis, the reporting of whether or not prophylactic antibiotic use was done was inconsistent and sparse and hence could not be ascertained. The use of prophylactic antibiotics has proven to be beneficial in a multicenter randomized trial of levofloxacin prophylaxis for 12 weeks at the start of therapy for newly diagnosed myeloma compared to placebo, owing to reduced febrile episodes and deaths without increasing health-care associated infections [49]. In our study, we did see that because the infection risk did not considerably change between frontline and RRMM, the use of prophylactic antibiotics should be considered in both the frontline and RRMM setting, and this should be an area of future investigation. Subsequent trials should look at the value of antibiotic prophylaxis, as well as the downstream implications such as changes in microbial resistance patterns in the community. As we found that reporting of use antibiotic prophylaxis has historically been poor, ongoing and future trials should clearly report this information.
There are limitations to our study. As most trials use regimens in combination, the exact contribution of each treatment class to the risk of infection, pneumonia, and neutropenia is unknown, and an analysis of the risk of infection per treatment class was not performed. The methodology of our study cannot fully account for other factors on an individual patient level such as prior lines of treatment and other differences in patient characteristics among these clinical trials. Indeed the risk of infection reflects patient specific factors in addition to the toxicity of the drug, and thus direct comparisons must be taken with this caveat. Our search was limited to randomized trials, hence non-randomized study data on regimens currently used such as selinexor-dexamethasone [50] were not included here. Furthermore, unpublished regimens could also not be captured through our search strategy. Studies not clearly mentioning the duration of treatment or our primary outcomes were not included for analysis, decreasing the number of studies evaluated. The marked heterogeneity amongst the patients enrolled on these trials also raises the need for caution before using information for clinical application. Furthermore, our study did not analyze individual types/areas of infection other than pneumonia; Although it is a well-known fact that the patterns of infection vary with class of drugs, such as respiratory infections with the use of anti-CD38 therapy, herpes zoster/herpes simplex infections with PIs, and Pneumocystis jirovecii with steroids [1]. We also could not account for when in the course of treatment these infections occurred, or the exact organism that was causing these infections, due to lack of reporting of these variables in the studies analyzed. As our study includes only trials before the onset of COVID-19, our data cannot be used to ascertain the risk of COVID-19 with these regimens, although the data is still of relevance in the COVID era given the well-known complications of neutropenia as they pertain to secondary bacterial infections [51]. There was high statistical heterogeneity in our study as evidenced by the high I2 values, owing to the inclusion of various studies with varying sample sizes and patient populations. This persisted despite sensitivity testing (leave one out analysis).
Prior to our study, there were meta-analyses that studied the risk of infection in MM in specific subsets of patients, however these looked specifically at certain classes of drugs, and not comprehensively at all classes and all phases of treatment [8, 52]. To the best of our knowledge, our study is the first to provide comprehensive quantitative estimates of monthly incidence of events by accounting for duration of treatment, although future work is needed looking at patient level data to account comprehensively for all risk factors.
In summary, our study demonstrates a significant risk of infection in patients treated with various regimens for MM, even in the era of contemporary novel treatments. A transition from chemotherapeutic agents to novel agents has resulted in a decrease in incidence of severe neutropenia, but the incidence of severe infection and pneumonia persists. Infection, pneumonia, and neutropenia remain a risk in frontline, maintenance, and relapsed/refractory setting. Further studies are needed to determine the value of antimicrobial prophylaxis – not only antibacterial, but also antiviral and antifungal medications- in a broader myeloma patient population, as well as other approaches that will further mitigate the morbidity and mortality related to infection in this vulnerable patient population.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- MM:
-
Multiple myeloma
- IMiDs:
-
Immunomodulators
- PI:
-
Protease inhibitors
- RCTs:
-
Randomized clinical trials
- PRISMA:
-
Preferred Reporting Items for Systemic Reviews and Meta-Analyses
- CI:
-
Confidence intervals
- VRD:
-
Bortezomib/lenalidomide/dexamethasone
- DRd:
-
Daratumumab/lenalidomide/dexamethasone
- Vd:
-
Bortezomib/dexamethasone
- RRMM:
-
Relapsed/refractory MM
- DVd:
-
Daratumumab/bortezomib/dexamethasone
References
Blimark C, Holmberg E, Mellqvist UH, Landgren O, Bjorkholm M, Hultcrantz M, et al. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica. 2015;100(1):107–13. https://doi.org/10.3324/haematol.2014.107714.
Augustson BM, Begum G, Dunn JA, Barth NJ, Davies F, Morgan G, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002--Medical Research Council adult Leukaemia working party. J Clin Oncol. 2005;23(36):9219–26. https://doi.org/10.1200/JCO.2005.03.2086.
Nucci M, Anaissie E. Infections in patients with multiple myeloma in the era of high-dose therapy and novel agents. Clin Infect Dis. 2009;49(8):1211–25. https://doi.org/10.1086/605664.
Schütt P, Brandhorst D, Stellberg W, Poser M, Ebeling P, Müller S, et al. Immune parameters in multiple myeloma patients: influence of treatment and correlation with opportunistic infections. Leuk Lymphoma. 2006;47(8):1570–82. https://doi.org/10.1080/10428190500472503.
Holmstrom MO, et al. Causes of early death in multiple myeloma patients who are ineligible for high-dose therapy with hematopoietic stem cell support: a study based on the nationwide Danish myeloma database. Am J Hematol. 2015;90(4):E73–4. https://doi.org/10.1002/ajh.23932.
Hsu P, Lin TW, Gau JP, Yu YB, Hsiao LT, Tzeng CH, et al. Risk of early mortality in patients with newly diagnosed multiple myeloma. Medicine (Baltimore). 2015;94(50):e2305. https://doi.org/10.1097/MD.0000000000002305.
Davies F, Baz R. Lenalidomide mode of action: linking bench and clinical findings. Blood Rev. 2010;24(Suppl 1):S13–9. https://doi.org/10.1016/S0268-960X(10)70004-7.
Ying L, YinHui T, Yunliang Z, Sun H. Lenalidomide and the risk of serious infection in patients with multiple myeloma: a systematic review and meta-analysis. Oncotarget. 2017;8(28):46593–600. https://doi.org/10.18632/oncotarget.16235.
Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–98. https://doi.org/10.1056/NEJMoa043445.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. https://doi.org/10.1371/journal.pmed.1000100.
Higgins JP, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928. https://doi.org/10.1136/bmj.d5928.
Shuster JJ. Review: Cochrane handbook for systematic reviews for interventions, version 5.1.0, published 3/2011. Res Synth Methods. 2011;2(2):126–30. https://doi.org/10.1002/jrsm.38 Julian P.T. Higgins and Sally green, editors.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. https://doi.org/10.1136/bmj.327.7414.557.
Niesvizky R, Flinn IW, Rifkin R, Gabrail N, Charu V, Clowney B, et al. Community-based phase IIIB trial of three UPFRONT Bortezomib-based myeloma regimens. J Clin Oncol. 2015;33(33):3921–9. https://doi.org/10.1200/JCO.2014.58.7618.
Mateos MV, Cavo M, Blade J, Dimopoulos MA, Suzuki K, Jakubowiak A, et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial. Lancet. 2020;395(10218):132–41. https://doi.org/10.1016/S0140-6736(19)32956-3.
Voorhees P, Kaufman JL, Laubach J, Sborov D, Reeves B, Rodriguez C, et al. Daratumumab + Lenalidomide, Bortezomib & Dexamethasone Improves Depth of response in transplant-eligible newly diagnosed multiple myeloma: GRIFFIN. Clin Lymphoma Myeloma Leukemia. 2019;19(10):e353–4. https://doi.org/10.1016/j.clml.2019.09.583.
Rosiñol L, Oriol A, Rios R, Sureda A, Blanchard MJ, Hernández MT, et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood. 2019;134(16):1337–45. https://doi.org/10.1182/blood.2019000241.
Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394(10192):29–38. https://doi.org/10.1016/S0140-6736(19)31240-1.
Horvath N, Spencer A, Kenealy M, Joshua D, Campbell PJ, Lee JJ, et al. Phase 3 study of subcutaneous bortezomib, thalidomide, and prednisolone consolidation after subcutaneous bortezomib-based induction and autologous stem cell transplantation in patients with previously untreated multiple myeloma: the VCAT study. Leuk Lymphoma. 2019;60(9):2122–33. https://doi.org/10.1080/10428194.2019.1579322.
Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Daratumumab plus Lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. 2019;380(22):2104–15. https://doi.org/10.1056/NEJMoa1817249.
Durie BGM, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389(10068):519–27. https://doi.org/10.1016/S0140-6736(16)31594-X.
Jacobus SJ, Rajkumar SV, Weiss M, Stewart AK, Stadtmauer EA, Callander NS, et al. Randomized phase III trial of consolidation therapy with bortezomib-lenalidomide-dexamethasone (VRd) vs bortezomib-dexamethasone (Vd) for patients with multiple myeloma who have completed a dexamethasone based induction regimen. Blood Cancer J. 2016;6(7):e448. https://doi.org/10.1038/bcj.2016.55.
Facon T, Lee JH, Moreau P, Niesvizky R, Dimopoulos M, Hajek R, et al. Carfilzomib or bortezomib with melphalan-prednisone for transplant-ineligible patients with newly diagnosed multiple myeloma. Blood. 2019;133(18):1953–63. https://doi.org/10.1182/blood-2018-09-874396.
Jackson GH, Davies FE, Pawlyn C, Cairns DA, Striha A, Collett C, et al. Response-adapted intensification with cyclophosphamide, bortezomib, and dexamethasone versus no intensification in patients with newly diagnosed multiple myeloma (myeloma XI): a multicentre, open-label, randomised, phase 3 trial. Lancet Haematol. 2019;6(12):e616–29. https://doi.org/10.1016/S2352-3026(19)30167-X.
Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373(7):621–31. https://doi.org/10.1056/NEJMoa1505654.
Mateos MV, Blacklock H, Schjesvold F, Oriol A, Simpson D, George A, et al. Pembrolizumab plus pomalidomide and dexamethasone for patients with relapsed or refractory multiple myeloma (KEYNOTE-183): a randomised, open-label, phase 3 trial. Lancet Haematol. 2019;6(9):e459–69. https://doi.org/10.1016/S2352-3026(19)30110-3.
Richardson PG, Oriol A, Beksac M, Liberati AM, Galli M, Schjesvold F, et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): a randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(6):781–94. https://doi.org/10.1016/S1470-2045(19)30152-4.
Moreau P, Mateos MV, Berenson JR, Weisel K, Lazzaro A, Song K, et al. Once weekly versus twice weekly carfilzomib dosing in patients with relapsed and refractory multiple myeloma (a.R.R.O.W.): interim analysis results of a randomised, phase 3 study. Lancet Oncol. 2018;19(7):953–64. https://doi.org/10.1016/S1470-2045(18)30354-1.
Dimopoulos MA, Dytfeld D, Grosicki S, Moreau P, Takezako N, Hori M, et al. Elotuzumab plus Pomalidomide and dexamethasone for multiple myeloma. N Engl J Med. 2018;379(19):1811–22. https://doi.org/10.1056/NEJMoa1805762.
Dimopoulos MA, San-Miguel J, Belch A, White D, Benboubker L, Cook G, et al. Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of POLLUX. Haematologica. 2018;103(12):2088–96. https://doi.org/10.3324/haematol.2018.194282.
Spencer A, Lentzsch S, Weisel K, Avet-Loiseau H, Mark TM, Spicka I, et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of CASTOR. Haematologica. 2018;103(12):2079–87. https://doi.org/10.3324/haematol.2018.194118.
Hájek R, Masszi T, Petrucci MT, Palumbo A, Rosiñol L, Nagler A, et al. A randomized phase III study of carfilzomib vs low-dose corticosteroids with optional cyclophosphamide in relapsed and refractory multiple myeloma (FOCUS). Leukemia. 2017;31(1):107–14. https://doi.org/10.1038/leu.2016.176.
Richardson PG, Jagannath S, Moreau P, Jakubowiak AJ, Raab MS, Facon T, et al. Elotuzumab in combination with lenalidomide and dexamethasone in patients with relapsed multiple myeloma: final phase 2 results from the randomised, open-label, phase 1b-2 dose-escalation study. Lancet Haematol. 2015;2(12):e516–27. https://doi.org/10.1016/S2352-3026(15)00197-0.
Spicka I, Ocio EM, Oakervee HE, Greil R, Banh RH, Huang SY, et al. Randomized phase III study (ADMYRE) of plitidepsin in combination with dexamethasone vs. dexamethasone alone in patients with relapsed/refractory multiple myeloma. Ann Hematol. 2019;98(9):2139–50. https://doi.org/10.1007/s00277-019-03739-2.
Attal M, Richardson PG, Rajkumar SV, San-Miguel J, Beksac M, Spicka I, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394(10214):2096–107. https://doi.org/10.1016/S0140-6736(19)32556-5.
Moreau P, et al. Updated Analysis of Bellini, a Phase 3 Study of Venetoclax or Placebo in Combination with Bortezomib and Dexamethasone in Patients with Relapsed/Refractory Multiple Myeloma. Blood. 2019;134(Supplement_1):1888.
Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hájek R, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27–38. https://doi.org/10.1016/S1470-2045(15)00464-7.
Usmani SZ, Schjesvold F, Oriol A, Karlin L, Cavo M, Rifkin RM, et al. Pembrolizumab plus lenalidomide and dexamethasone for patients with treatment-naive multiple myeloma (KEYNOTE-185): a randomised, open-label, phase 3 trial. Lancet Haematol. 2019;6(9):e448–58. https://doi.org/10.1016/S2352-3026(19)30109-7.
Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, et al. Oral Ixazomib, Lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374(17):1621–34. https://doi.org/10.1056/NEJMoa1516282.
Mateos MV, Nahi H, Legiec W, Grosicki S, Vorobyev V, Spicka I, et al. Subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma (COLUMBA): a multicentre, open-label, non-inferiority, randomised, phase 3 trial. Lancet Haematol. 2020;7(5):e370–80. https://doi.org/10.1016/S2352-3026(20)30070-3.
Morgan G, et al. Maintenance Therapy with the Oral Proteasome Inhibitor (PI) Ixazomib Significantly Prolongs Progression-Free Survival (PFS) Following Autologous Stem Cell Transplantation (ASCT) in Patients with Newly Diagnosed Multiple Myeloma (NDMM): Phase 3 Tourmaline-MM3 Trial. Biol Blood Marrow Transplant. 2019;25(3, Supplement):S19–20.
Bringhen S, D’Agostino M, Paris L, Ballanti S, Pescosta N, Spada S, et al. Lenalidomide-based induction and maintenance in elderly newly diagnosed multiple myeloma patients: updated results of the EMN01 randomized trial. Haematologica. 2020;105(7):1937–47. https://doi.org/10.3324/haematol.2019.226407.
Zweegman S, van der Holt B, Mellqvist UH, Salomo M, Bos GMJ, Levin MD, et al. Melphalan, prednisone, and lenalidomide versus melphalan, prednisone, and thalidomide in untreated multiple myeloma. Blood. 2016;127(9):1109–16. https://doi.org/10.1182/blood-2015-11-679415.
Gay F, Oliva S, Petrucci MT, Conticello C, Catalano L, Corradini P, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015;16(16):1617–29. https://doi.org/10.1016/S1470-2045(15)00389-7.
Richardson PG, et al. Randomized, open label phase 1/2 study of Pomalidomide (POM) alone or in combination with low-dose dexamethasone (LoDex) in patients (pts) with relapsed and refractory multiple myeloma who have received prior treatment that includes Lenalidomide (LEN) and Bortezomib (BORT): phase 2 results. Blood. 2011;118(21):634.
San Miguel JF, et al. MM-003, a phase 3 study of pomalidomide+low-dose dexamethasone vs. High-dose dexamethasone in refractory or relapsed and refractory multiple myeloma: Outcomes by prior therapy and response depth. Haematologica. 2014;99:110.
Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11(1):29–37. https://doi.org/10.1016/S1470-2045(09)70284-0.
Jung SH, Kang SJ, Jang HC, Ahn JS, Yang DH, Lee SS, et al. Effect of levofloxacin prophylaxis for prevention of severe infections in multiple myeloma patients receiving bortezomib-containing regimens. Int J Hematol. 2014;100(5):473–7. https://doi.org/10.1007/s12185-014-1672-1.
Drayson MT, Bowcock S, Planche T, Iqbal G, Pratt G, Yong K, et al. Levofloxacin prophylaxis in patients with newly diagnosed myeloma (TEAMM): a multicentre, double-blind, placebo-controlled, randomised, phase 3 trial. Lancet Oncol. 2019;20(12):1760–72. https://doi.org/10.1016/S1470-2045(19)30506-6.
Chari A, Vogl DT, Gavriatopoulou M, Nooka AK, Yee AJ, Huff CA, et al. Oral Selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med. 2019;381(8):727–38. https://doi.org/10.1056/NEJMoa1903455.
Gibson C, Berliner N. How we evaluate and treat neutropenia in adults. Blood. 2014;124(8) quiz 1378:1251–8. https://doi.org/10.1182/blood-2014-02-482612.
Chen M, Zhao Y, Xu C, Wang X, Zhang X, Mao B. Immunomodulatory drugs and the risk of serious infection in multiple myeloma: systematic review and meta-analysis of randomized and observational studies. Ann Hematol. 2018;97(6):925–44. https://doi.org/10.1007/s00277-018-3284-y.
Acknowledgements
N/A
Funding
The article processing charges related to the publication of this article were supported by The University of Kansas (KU) One University Open Access Author Fund sponsored jointly by the KU Provost, KU Vice Chancellor for Research & Graduate Studies, and KUMC Vice Chancellor for Research, and managed jointly by the Libraries at the Medical Center and KU – Lawrence.
Author information
Authors and Affiliations
Contributions
Nicole Balmaceda performed the research, analyzed the data, and wrote the paper. Muhammad Aziz performed the research, contributed essential tools, and analyzed the data. Viveksandeep Thoguluva Chandrasekar performed the research, contributed essential tools, and analyzed the data. Brian McClune contributed essential tools and analyzed the data. Suman Kambhampati contributed essential tools and analyzed the data. Leyla Shune contributed essential tools and analyzed the data. Al-Ola Abdallah contributed essential tools and analyzed the data. Faiz Anwer contributed essential tools and analyzed the data. Aneela Majeed contributed essential tools and analyzed the data. Muzaffar Qazilbash contributed essential tools and analyzed the data. Siddhartha Ganguly contributed essential tools and analyzed the data. Joseph McGuirk contributed essential tools and analyzed the data. Ghulam Rehman Mohyuddin performed the research, designed the research study, contributed essential tools, analyzed the data, and wrote the paper. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Manuscript does not report or involve the use of animal or human data or tissue. Meta-analysis was performed. Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no conflict of interests to disclose, other than the following for Siddhartha Ganguly (SG) and Joseph McGuirk (JM).
SG: Daiichi Sankyo: Research Funding; Seattle Genetics: Speakers Bureau; Kite Pharma: Honoraria, Other: Advisory Board; Janssen: Honoraria.
JM: Kite Pharmaceuticals: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bellicum Pharmaceuticals: Research Funding; Astellas: Research Funding; Juno Therapeutics: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Fresenius Biotech: Research Funding; Gamida Cell: Research Funding; Pluristem Ltd.: Research Funding; Articulate Science.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Balmaceda, N., Aziz, M., Chandrasekar, V.T. et al. Infection risks in multiple myeloma: a systematic review and meta-analysis of randomized trials from 2015 to 2019. BMC Cancer 21, 730 (2021). https://doi.org/10.1186/s12885-021-08451-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-08451-x