Abstract
Background
Soft-tissue sarcomas (STS) are rare tumors of the soft tissue. Recent diagnostic studies on STS mainly dealt with only few cases of STS and did not investigate the post-therapeutic performance of MRI in a routine clinical setting. Therefore, we assessed the long-term diagnostic accuracy of MRI for detecting recurrent STS at a multidisciplinary sarcoma center.
Methods
In all, 1055 postoperative follow-up MRIs of 204 patients were included in the study. MRI follow-up scans were systematically reviewed for diagnostic values (true-positive/−negative and false-positive/−negative results) in detecting recurrences. Pathological reports and follow-up MRIs were set as baseline references.
Results
The median age of the patients was 55.3 ± 18.2 years. Of the patients, 34.8% presented with recurrences. Here, 65 follow-up scans were true positive, 23 false positive, 6 false negative, and 961 true negative. The overall sensitivity and specificity of MRI for detecting recurrences were 92 and 98%, respectively, with an accuracy of 97%. For intramuscular lesions and after surgery alone the sensitivity was higher (95 and 97%, respectively) than for subcutaneous lesions and surgery with additional radiation therapy (83 and 86%, respectively), at similarly high specificities (96–98%). The 6 false-negative results were found in streaky (n = 2) and small ovoid/nodular (n = 4) recurring lesions. The false-positive lesions imitated streaky (n = 14), ovoid/nodular (n = 8), and polycyclic/multilobulated recurring tumors (n = 1). All false-positive results were found in patients in whom the primary tumors were polycyclic/multilobulated in appearance.
Conclusion
MRI shows a high diagnostic accuracy for detecting recurrent STS, with a high sensitivity and specificity. The diagnostic accuracy decreases in subcutaneous lesions and after surgery with radiation therapy, compared to intramuscular lesions and surgery alone. Radiologists should pay particular attention to streaky and small ovoid/nodular recurring lesions and patients with polycyclic/multilobulated primary tumors.
Similar content being viewed by others
Background
Soft-tissue sarcomas (STS) constitute a rare and heterogeneous group of tumors, accounting for only 1% of adult malignancies [1]. Due to the rarity of these malignancies, there is often only little experience in dealing with STS and in postoperative surveillance outside of specialty centers [2]. More than 50 different subtypes have been described, and the extremities are the most common sites of STS [1, 3]. Surgery is the most common treatment option for STS, with additional radiotherapy in selected cases [4]. In the literature, different strategies for postoperative surveillance of STS patients have been reported. Nevertheless, a unified strategy is still lacking [1, 5,6,7]. The most commonly used imaging modalities for the post-therapeutic follow up of STS patients are ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI). While CT is usually used for the screening of distant metastasis, ultrasound and MRI are used for detecting local recurrences [8, 9]. Studies on the diagnostic accuracy of MRI in detecting STS already exist. Nevertheless, recent studies on this topic mostly included only few cases of STS and did not examine the performance of post-therapeutic MRI in a routine clinical setting [10,11,12]. Therefore, we analyzed the diagnostic value of MRI for detecting recurrent STS in the long-term, postoperative follow-up at a multidisciplinary sarcoma center. Furthermore, we analyzed whether the localization of STS in the soft tissue or the type of therapy has an impact on the diagnostic value of MRI.
Methods
Patients
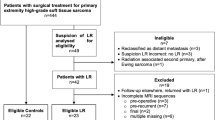
A total of 1707 postoperative follow-up MRI scans were performed in 242 patients with histologically proven STS between 2008 and 2020. Thirty-eight patients were excluded due to insufficient imaging and pathological data. Ultimately, 204 patients with a total of 1286 postoperative follow-up MRI scans were included in our study and presented complete data on imaging. Examinations in which predictive values could not be determined and the last examination of each patient were excluded (n = 231; Fig. 1). Either core needle or open biopsy was performed in all lesions suspected of being recurrences (n = 88). In these patients, the pathological reports were set as reference and the radiological findings were correlated to the pathological reports. All other MRIs were reviewed during the subsequent MRI follow-up examinations, showing whether lesions had been overlooked in the previous MRIs. These subsequent MRI follow-ups took place after 3 to 6 months. The follow-up MRIs were reviewed by two dedicated musculoskeletal radiologists with a minimum of 5 years of experience in sarcoma diagnostics, with findings reached by consensus. The reports were divided into two groups: presence of recurrence and absence of recurrence. From these findings we extracted true-positive/−negative and false-positive/−negative MRI findings in detecting recurrent STS. The false-negative results were derived retrospectively by reviewing the subsequent MRI follow-ups. Latest recurrences were clearly delimited after two such follow-ups.
The pre-established standard schedule for MRI examination of all patients was four times in the first year (every 3 months), twice in the second post-therapeutic year (every 6 months), and once a year for a minimum of three consecutive years thereafter. The first MRI examination routinely took place 3 months after primary tumor resection. Nevertheless, 173 patients of the 204 patients included in total strictly adhered to the MRI follow-up examinations that were provided. Thirty-one patients partially omitted MRI examinations. We excluded patients for whom intervals between the examinations were disproportionately long from the beginning.
The configurations of STS on MRI were used to describe recurrences or images mimicking a recurrence. The configuration corresponds to the morphological appearance of STS. Accordingly, recurrent STS mainly presented with the following main configurations: polycyclic/multilobulated, ovoid/nodular, and streaky. Polycyclic/multilobulated STS are mainly inhomogeneous tumors, which appear multilobulated and often polycystic. Ovoid/nodular configured STS are small and round/ovoid tumors. Streaky configured STS resemble elongated scars.
Magnetic resonance imaging
All patients were examined with a 1.5-Tesla MRI system (MAGNETOM Symphony, Siemens Healthineers, Erlangen, Germany). The MRI protocol included the following pulse sequences: T2-weighted (T2w) TSE (TE: 64–114 ms, TR: 3010–5840 ms, FOV: 22–44 cm2), T1-weighted (T1w) SE (TE: 10–14 ms, TR: 587–868 ms, FOV: 22–44 cm2), proton density-weighted (PDw) FS (TE: 26–36 ms, TR: 2740–4610 ms, FOV: 22–40 cm2), or Turbo-Inversion Recovery Magnitude (TIRM) (TE: 68–77 ms, TR: 4410–6980 ms, FOV: 37–45 cm2) and contrast-enhanced T1w SE FS (10–13 ms, TR: 533–1440 ms, FOV: 22–45 cm2). Slice thickness was 4–6 mm.
Statistical data
Diagnostic accuracy was determined by calculating predictive values (positive and negative), sensitivity, specificity, and accuracy using Fisher’s exact test and 2 × 2 tables. The 95% confidence interval was determined using the Wald test. A level of p < 0.05 was set as statistical significance for all tests. For statistical analysis, IBM-SPSS version 26.0 software package (IBM, Armonk, NY, USA) was used.
Results
The median age of the patients was 55.3 years (Min.: 10, Max.: 88, SD: 18.2). Of the patients, 52.9% were male (n = 108; Table 1). The overall median recurrence-free follow-up interval on MRI was 39 months (Min.: 3, Max.: 161). No significant difference was observed in the median recurrence-free follow-up intervals in a comparison of patients after surgery alone (37 months) and surgery with additional radiation therapy (35 months), or in subcutaneous (37 months) and intramuscular lesions (36 months). In all, 34.8% of the patients presented with recurrences. Sixty-five follow-up MRI scans were diagnosed as true positive, 23 as false positive, 6 as false negative, and 961 as true negative (Table 2 and Fig. 2). Overall, sensitivity and specificity of MRI for detecting recurrences were 92% and 98%, respectively, with an accuracy of 97%. For intramuscular lesions the sensitivity was higher than for subcutaneous lesions (95% and 83%, respectively), at similarly high specificities (97% and 98%, respectively; Tables 3 and 4). Furthermore, the sensitivity was higher in patients after surgery alone than after surgery with additional radiation therapy (97% and 86%, respectively), at similar specificities (96% and 98%, respectively; Tables 3 and 5). The 6 false-negative results were found in streaky (n = 2) and small ovoid/nodular (n = 4) recurrences. The false-positive lesions imitated streaky (n = 14), ovoid/nodular (n = 8; Fig. 3), and polycyclic/multilobulated recurring lesions (n = 1). All false-positive results were found in patients in whom the primary STS was polycyclic/multilobulated in appearance. Furthermore, 22 of the 23 false-positive results were derived from patients with R0 resection (95.7%). Altogether, 92.2% of the patients underwent R0 resection.
1.5-T MRI (a: contrast-enhanced T1-weighted image with fat suppression in axial view of the thigh; b: Contrast-enhanced T1-weighted image with fat suppression in axial view of the groin; c: Proton density weighted image with fat suppression in coronal view of the thigh). a shows a small nodular recurrence with slight edema (white arrowhead). b presents an ovoid/nodular recurrence (white arrow), which can easily be misinterpreted as a lymph node. c shows a multilobulated recurrence (black arrow) with adjacent edema
Discussion
In our study we investigated the diagnostic accuracy of standard MRI for detecting recurring STS at a multidisciplinary sarcoma center.
STS include benign and malignant tumors and tumor-like lesions [13]. In our study we dealt with malignant lesions only. STS constitute a rare and heterogeneous group of tumors, which account for only about 1% of all malignancies [1]. The most recent histological classification of STS comes from the current WHO histological typing, in which more than 50 subtypes are described [1, 13, 14]. Previous studies on STS recurrence have reported a wide range of recurrence rates of up to 50% [15, 16]. Due to the rarity of STS, imaging studies on STS still remain scarce. Furthermore, most previous studies dealt with imaging features rather than with diagnostic accuracy. Additionally, previous diagnostic studies mostly included only small patient numbers or reviewed the available literature.
For postoperative surveillance, MRI is the imaging modality of choice and is widely used to assess STS recurrence [11, 17], as this technique has the advantage of a high soft-tissue contrast and no radiation. Nevertheless, distinguishing between post-treatment changes and recurrent STS on MRI is often reported to be challenging [18]. Previous publications have often shown that post-treatment changes and scar tissue can obscure recurring STS, which leads to unnecessary biopsies [19].
In patients with local recurrences, combined treatment with surgery and additional radiation therapy is usually chosen to improve local control [1, 20]. Nevertheless, a decision regarding the use of additional radiation therapy should be evaluated from case to case [1, 21]. Before starting the therapy, core needle biopsy is often performed to identify the pathology of the suspected lesions [22]. To start therapy quickly and to avoid unnecessary biopsy, precise postoperative MRI diagnostics are indispensable. Therefore, according to our study, it is of high clinical and diagnostic importance to determine the diagnostic reliability of MRI for postoperative surveillance of sarcoma patients in a routine clinical setting over a long time period.
In our study, both sensitivity (92%) and specificity (98%) were high overall, even after both surgery and radiation therapy and in both subcutaneous and intramuscular lesions. Indeed, sensitivity was lower after radiation therapy than after surgery alone and in subcutaneous lesions, compared to intramuscular lesions, but the sensitivity still remained at a high level. Reasons for the decreased sensitivities could lie in the increased rate of soft-tissue alterations after additional radiation therapy [19, 23] and the usually smaller sizes of the subcutaneous lesions. These two findings ultimately render it more difficult to distinguish between post-treatment changes and recurring tumor. The range of sensitivity and specificity that we found is high. However, some previous publications reported a lower specificity, ultimately leading to unneeded biopsies [11, 19, 24]. Afonso et al. described a sensitivity and specificity of only 58 and 73%, respectively, for conventional MRI [17], while for Del Grande F. et al. sensitivity and specificity of MRI were 100 and 52%, respectively, in detecting tumor recurrence in nonenhanced MRI, and 100 and 97%, respectively, in contrast-enhanced MRI [18]. In a recent review, Pennington A. et al. calculated a mean sensitivity and specificity of 88 and 86%, respectively, for local recurrences of primary vertebral tumors [25]. Other authors showed sensitivities and specificities of 64–88% and 85–96%, respectively, for MRI in detecting STS [17, 26,27,28,29]. Nevertheless, none of the previous studies investigated how MRI performed in a routine post-therapeutic clinical setting. Previous publications reported a lack of specificity of MRI in detecting recurring STS in nonenhanced T1- and T2-weighted images [19, 30, 31]. Therefore, contrast-enhanced MRI is reported to improve the diagnostic accuracy of MRI [32].
In our study, all of the patients were examined using contrast-enhanced MRI. Our data emphasize that MRI is a highly valuable imaging modality in the long-term postoperative surveillance of STS patients. Nevertheless, we found 23 false-positive and 6 false-negative results (8.5%). These 6 cases were all derived from streaky and small ovoid/nodular lesions, which were difficult to distinguish from the surrounding post-treatment tissue. A recent study showed that distinct post-therapeutic changes are the main reason for false-negative results on MRI [12]. All false-positive results were found in patients in whom the primary STS was polycyclic/multilobulated. This may well be due to the fact that polycyclic/multilobulated primary tumors are larger than other STS configurations in the mean and therefore perhaps lead to more heterogeneous post-treatment tissue changes. Furthermore, 22 of the 23 false-positive results were derived from patients with R0 resection. This finding is in contrast to a recent study showing that microscopic positive margins were the main reason for false-positive results [12]. Therefore, radiologists should pay particular attention to patients in whom the primary STS was polycyclic/multilobulated in shape and should carefully screen the soft tissue for streaky or small ovoid/nodular recurrences. However, this fact also demonstrates the limitations of MRI surveillance. In cases of recurrent lesions with a small and not clearly delimited appearance, it may become difficult to correctly detect a recurring tumor in the surrounding tissue. Therefore, for suspected recurrence or unclear cases, the subsequent MRI follow-up examinations should take place after 3 to 6 months. The justification for this time is also evident from our study as the two readers did find all false-negative recurrences retrospectively by reviewing the subsequent MRI follow-ups.
Our study has some limitations, as it is a single-center study with a retrospective design. Nevertheless, we could include 204 patients in a 12-year survey with a total of 1055 MRI follow-up scans. Another limitation is the verification of the true-positive/−negative and false-positive/−negative results. Biopsy was only performed in patients in whom recurrence was suspected. In the other cases, radiologists evaluated whether recurrences were overlooked or not. Therefore, we cannot completely rule out that individual findings might be false.
Conclusion
MRI shows a high diagnostic accuracy for detecting recurring STS in the long term, with a high sensitivity (92%) and specificity (98%). After resection of subcutaneous primary tumors and after radiation therapy, the sensitivity decreases to 83 and 86%, respectively, compared to intramuscular lesions and surgery alone (95 and 97%, respectively). Radiologists should pay particular attention to patients in whom the primary tumor was polycyclic/multilobulated in appearance and should carefully screen the post-treatment soft tissue for streaky and small ovoid/nodular recurrences, which are often difficult to distinguish from post-treatment changes.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MRI:
-
Magnetic resonance imaging
- STS:
-
Soft-tissue sarcoma
References
von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Ganjoo KN, et al. Soft tissue sarcoma, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2018;16(5):536–63. https://doi.org/10.6004/jnccn.2018.0025.
Ratan R, Patel SR. Chemotherapy for soft tissue sarcoma. Cancer. 2016;122(19):2952–60. https://doi.org/10.1002/cncr.30191.
Pazdur R, Coia LR, Hoskins WJ, Wagman LD. Cancer management: a multidisciplinary approach: F.A. Davis Company; 2003.
Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353(7):701–11. https://doi.org/10.1056/NEJMra041866.
Kane JM III. Surveillance strategies for patients following surgical resection of soft tissue sarcomas. Curr Opin Oncol. 2004;16(4):328–32. https://doi.org/10.1097/01.cco.0000127879.62254.d3.
Patel SA, Royce TJ, Barysauskas CM, Thornton KA, Raut CP, Baldini EH. Surveillance imaging patterns and outcomes following radiation therapy and radical resection for localized extremity and trunk soft tissue sarcoma. Ann Surg Oncol. 2017;24(6):1588–95. https://doi.org/10.1245/s10434-016-5755-5.
Whooley BP, Gibbs JF, Mooney MM, McGrath BE, Kraybill WG. Primary extremity sarcoma: what is the appropriate follow-up? Ann Surg Oncol. 2000;7(1):9–14. https://doi.org/10.1007/s10434-000-0009-x.
Briccoli A, Galletti S, Salone M, Morganti A, Pelotti P, Rocca M. Ultrasonography is superior to computed tomography and magnetic resonance imaging in determining superficial resection margins of malignant chest wall tumors. J Ultrasound Med. 2007;26(2):157–62. https://doi.org/10.7863/jum.2007.26.2.157.
Cho HS, Park I-H, Jeong WJ, Han I, Kim H-S. Prognostic value of computed tomography for monitoring pulmonary metastases in soft tissue sarcoma patients after surgical management: a retrospective cohort study. Ann Surg Oncol. 2011;18(12):3392–8. https://doi.org/10.1245/s10434-011-1705-4.
Kaste SC, Hill A, Conley L, Shidler TJ, Rao BN, Neel MM. Magnetic resonance imaging after incomplete resection of soft tissue sarcoma. Clin Orthop Relat Res. 2002;397:204–11.
Erfanian Y, Grueneisen J, Kirchner J, Wetter A, Podleska LE, Bauer S, et al. Integrated 18F–FDG PET/MRI compared to MRI alone for identification of local recurrences of soft tissue sarcomas: a comparison trial. Eur J Nucl Med Mol Imaging. 2017;44(11):1823–31. https://doi.org/10.1007/s00259-017-3736-y.
Hirschmann A, van Praag VM, Haas RL, van de Sande MAJ, Bloem JL. Can we use MRI to detect clinically silent recurrent soft-tissue sarcoma? Eur Radiol. 2020;30(9):4724.
Gielen JL, de Schepper AM, Vanhoenacker F, Parizel PM, Wang XL, Sciot R, et al. Accuracy of MRI in characterization of soft tissue tumors and tumor-like lesions. A prospective study in 548 patients. Eur Radiol. 2004;14(12):2320–30. https://doi.org/10.1007/s00330-004-2431-0.
Fletcher C. Pathology and genetics of tumors of soft tissue and bone, vol. 4: World Health Organization Classification of Tumors; 2002. p. 35–46.
James SLJ, Davies A. Post-operative imaging of soft tissue sarcomas. Cancer Imaging. 2008;8:8.
Kransdorf MJ, Murphey MD. Soft tissue tumors: post-treatment imaging. Radiol Clin. 2006;44(3):463–72. https://doi.org/10.1016/j.rcl.2006.01.006.
Afonso PD, Kosinski AS, Spritzer CE. Following unenhanced MRI assessment for local recurrence after surgical resection of mesenchymal soft tissue tumors, do additional gadolinium-enhanced images change reader confidence or diagnosis? Eur J Radiol. 2013;82(5):806–13. https://doi.org/10.1016/j.ejrad.2012.11.025.
Del Grande F, Subhawong T, Weber K, Aro M, Mugera C, Fayad LM. Detection of soft-tissue sarcoma recurrence: added value of functional MR imaging techniques at 3.0 T. Radiology. 2014;271:499–511.
Garner HW, Kransdorf MJ, Bancroft LW, Peterson JJ, Berquist TH, Murphey MD. Benign and malignant soft-tissue tumors: posttreatment MR imaging. Radiographics. 2009;29(1):119–34. https://doi.org/10.1148/rg.291085131.
Catton C, Davis A, Bell R, O'Sullivan B, Fornasier V, Wunder J, et al. Soft tissue sarcoma of the extremity. Limb salvage after failure of combined conservative therapy. Radiother Oncol. 1996;41(3):209–14. https://doi.org/10.1016/S0167-8140(96)01856-7.
Torres MA, Ballo MT, Butler CE, Feig BW, Cormier JN, Lewis VO, et al. Management of locally recurrent soft-tissue sarcoma after prior surgery and radiation therapy. Int J Radiat Oncol Biol Phys. 2007;67:1124–9.
Kotilingam D, Lev DC, Lazar AJF, Pollock RE. Staging soft tissue sarcoma: evolution and change. CA Cancer J Clin. 2006;56:282–91.
Richardson ML, Zink-Brody GC, Patten RM, Koh W-J, Conrad EU. MR characterization of post-irradiation soft tissue edema. Skelet Radiol. 1996;25(6):537–43. https://doi.org/10.1007/s002560050131.
Bacci G, Lari S. Current treatment of high grade osteosarcoma of the extremity. J Chemother. 2001;13(3):235–43. https://doi.org/10.1179/joc.2001.13.3.235.
Pennington Z, Ahmed AK, Cottrill E, Westbroek EM, Goodwin ML, Sciubba DM. Systematic review on the utility of magnetic resonance imaging for operative management and follow-up for primary sarcoma—lessons from extremity sarcomas. Ann Transl Med. 2019;7(10):225. https://doi.org/10.21037/atm.2019.01.59.
Moulton JS, Blebea JS, Dunco DM, Braley SE, Bisset GS 3rd, Emery KH. MR imaging of soft-tissue masses: diagnostic efficacy and value of distinguishing between benign and malignant lesions. AJR Am J Roentgenol. 1995;164(5):1191–9. https://doi.org/10.2214/ajr.164.5.7717231.
McKeon KE, Wright BT, Lee DH. Accuracy of MRI-based diagnoses for distal upper extremity soft tissue masses. J Hand Microsurg. 2015;7(1):61–6. https://doi.org/10.1007/s12593-015-0174-6.
Chung WJ, Chung HW, Shin MJ, Lee SH, Lee MH, Lee JS, et al. MRI to differentiate benign from malignant soft-tissue tumours of the extremities: a simplified systematic imaging approach using depth, size and heterogeneity of signal intensity. Br J Radiol. 2012;85(1018):e831–6. https://doi.org/10.1259/bjr/27487871.
Lin G, Yang L-Y, Huang Y-T, Ng K-K, Ng S-H, Ueng S-H, et al. Comparison of the diagnostic accuracy of contrast-enhanced MRI and diffusion-weighted MRI in the differentiation between uterine leiomyosarcoma/smooth muscle tumor with uncertain malignant potential and benign leiomyoma. J Magn Reson Imaging. 2016;43(2):333–42. https://doi.org/10.1002/jmri.24998.
Fayad LM, Jacobs MA, Wang X, Carrino JA, Bluemke DA. Musculoskeletal tumors: how to use anatomic, functional, and metabolic MR techniques. Radiology. 2012;265(2):340–56. https://doi.org/10.1148/radiol.12111740.
Griffiths HJ, Thompson RC, Nitke SJ, Olson PN, Thielen KR, Amundson P. Use of MRI in evaluating postoperative changes in patients with bone and soft tissue tumors. Orthopedics. 1997;20(3):215–20.
Chou S-HS, Hippe DS, Lee AY, Scherer K, Porrino JA, Davidson DJ, et al. Gadolinium contrast enhancement improves confidence in diagnosing recurrent soft tissue sarcoma by MRI. Acad Radiol. 2017;24(5):615–22. https://doi.org/10.1016/j.acra.2016.12.010.
Acknowledgements
Not applicable.
Funding
Not applicable. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
SS has made substantial contributions to the conception/design of the work, acquisition, analysis, interpretation of data, and drafted the work. SS has approved the submitted version (and any substantially modified version that involves the author’s contribution to the study) and has agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. MS has made substantial contributions to the conception/design of the work, acquisition, analysis, interpretation of data, and drafted the work. MS has approved the submitted version (and any substantially modified version that involves the author’s contribution to the study) and has agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. JM has made substantial contributions to the conception/design of the work, analysis, interpretation of data and substantively revised the draft. JM has approved the submitted version (and any substantially modified version that involves the author’s contribution to the study) and has agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. OJ has made substantial contributions to the analysis and interpretation of data, and substantively revised the draft. OJ has approved the submitted version (and any substantially modified version that involves the author’s contribution to the study) and has agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. MB has made substantial contributions to the analysis and interpretation of data, and substantively revised the draft. MB has approved the submitted version (and any substantially modified version that involves the author’s contribution to the study) and has agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the responsible ethics committee of the Ruhr-University Bochum, Germany. All patients gave their verbal informed consent before examination. Written informed consent was waived because of the.
retrospective nature of the study and the analysis of anonymous clinical data.
The consent was noted in a general patient informed consent sheet for all patients before examination.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sedaghat, S., Sedaghat, M., Meschede, J. et al. Diagnostic value of MRI for detecting recurrent soft-tissue sarcoma in a long-term analysis at a multidisciplinary sarcoma center. BMC Cancer 21, 398 (2021). https://doi.org/10.1186/s12885-021-08113-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-08113-y