Abstract
Background
Globally, colorectal cancer (CRC) is the third and second leading cancer in men and women respectively with 600,000 deaths per year. Traditionally, clinicians have relied solely on nodal disease involvement, and measurements such as lymph node ratio (LNR; the ratio of metastatic/positive lymph nodes to total number of lymph nodes examined), when determining patient prognosis in CRC. The log odds of positive lymph nodes (LODDS) is a logistic transformation formula that uses pathologic lymph node data to stratify survival differences among patients within a single stage of disease. This formula allows clinicians to identify whether patients with clinically aggressive tumours fall into higher-risk groups regardless of nodal positivity and can potentially guide adjuvant treatment modalities. The aim of this study was to investigate whether LODDS in colon cancer provides better prognostication compared to LNR.
Methods
A retrospective study of patients on the prospectively maintained Cabrini Monash University Department of Surgery colorectal neoplasia database, incorporating data from hospitals in Melbourne Australia, identified patients entered between January 2010 and March 2016. Association of LODDS and LNR with clinical variables were analysed. Disease-free (DFS) and overall (OS) survival were investigated with Cox regression and Kaplan–Meier survival analyses.
Results
There were 862 treatment episodes identified in the database (402 male, 47%). The median patient age was 73 (range 22–100 years). There were 799 colonic cancers and 63 rectosigmoid cancers. The lymph node yield (LNY) was suboptimal (< 12) in 168 patients (19.5%) (p = 0.05). The 5-year OS for the different LNR groups were 86, 91 and 61% (p < 0.001) for LNR0 (655 episodes), LNR1 (128 episodes) and LNR2 (78 episodes), respectively. For LODDS, they were 85, 91 and 61% (p < 0.001) in LODDS0 (569 episodes), LODDS1 (217 episodes) and LODDS2 (75 episodes) groups (p < 0.001). Overall survival rates were comparable between the LNR and LODDS group and for LNY < 12 and stage III patients when each were sub-grouped by LODDS and LNR.
Conclusion
This study has shown for that the prognostic impact of LODDS is comparable to LNR for colon cancer patients. Accordingly, LNR is recommended for prognostication given its ease of calculation.
Similar content being viewed by others
Background
Globally, colorectal cancer (CRC) is the third and second leading cancer in men and women respectively with 600,000 deaths per year [1]. Nodal status in surgical oncology can be used to assist in prognostication [2], guide decision making regarding adjuvant chemotherapy [3] and the number of examined lymph nodes examined or the lymph node yield (LNY) can be used as a marker for the quality of an oncological resection [4]. In CRC surgery, harvesting a minimum of 12 lymph nodes has been set as an acceptable benchmark. If the LNY is below 12, this has been suggested to be correlated with under-staging of the disease [5].
Lymph node ratio (LNR) (defined as the ratio of metastatic lymph nodes to the total number of lymph nodes examined) has been investigated as an adjunct parameter to conventional nodal staging. The LNR aids in prognosis and for identifying high-risk patients [6]. However, in node-negative colon cancer, which accounts for approximately 75% of patients who have surgery for colon cancer, LNR is zero and is the same as the pN0 classification and therefore does not provide any additional prognostic information [7].
Traditionally, clinicians have relied solely on nodal disease involvement (including the total number of positive lymph nodes) when determining patient prognosis in CRC [8]. Biologically aggressive tumours however, can initially be placed in the same stage as less clinically aggressive tumours, irrespective of nodal disease. The log odds of positive lymph nodes (LODDS) is a logistic transformation formula that uses pathologic lymph node data to stratify survival differences among patients within a single stage of disease. This formula allows clinicians to identify whether patients with clinically aggressive tumours fall into higher-risk groups regardless of nodal positivity and can potentially guide adjuvant treatment modalities.
Recently LODDS has been proposed as a novel prognostic index in colonic and non-colonic cancers [9,10,11]. In all of these studies, the classification of lymph node status by LODDS proved to be a powerful prognostic indicator with a strong ability to identify patients with a homogeneous prognosis, regardless of lymph node status and count. The aim of this study was to investigate the prognostic impact of LODDS and compare the survival of patients classified in LNR and LODDS groups who underwent a colonic cancer resection.
Methods
The prospectively maintained Cabrini Monash University colorectal neoplasia database [12] which contains a representative case mix of patients from both the public and private health sector, was examined for consecutive patients treated for colon adenocarcinoma under the care of 11 colorectal surgeons at Cabrini and Alfred hospitals (Melbourne, Victoria, Australia) between January 2010 and March 2016. Data extracted from the database included patient demographics, tumour characteristics, lymph node yield, medical co-morbidities, and oncological end points (local and distal recurrence, overall survival). Patients were divided into groups according to their LNR and LODDS. Survival analysis was performed and compared for the subgroups within LODDS and LNR. Patients who presented with synchronous colonic tumours, metastatic disease, and ASA 5 (American Society of Anesthesiologists) were excluded.
The LNR was defined as the number of positive lymph nodes divided by the total number of lymph nodes harvested. Patients were divided into three LNR groups based on previous literature [9]: LNR0 (< 0.05), LNR1 (0.05–0.20) and LNR2 (> 0.20). At least 12 harvested lymph nodes were accepted as an adequate number and tumour staging was performed according to the seventh edition of the AJCC TNM manual [13]. Pathological examination of lymph nodes in resected specimens relied on manual dissection by the pathologists. A low LNY was defined as fewer than 12 lymph nodes in the resected specimen.
LODDS is defined as the log of the ratio between the number of positive lymph nodes and the number of negative lymph nodes. LODDS is calculated using an empirical logistic transform formula: log (positive nodes + 0.5)/(total nodal count - positive nodes + 0.5). Patients were divided into three groups based on published LODDS studies specific to colorectal neoplasia [9]: LODDS0 (<− 1.36), LODDS1 (− 1.36 to − 0.53) and LODDS2 (> − 0.53).
Surveillance after surgery involved clinical examination, computed tomography (CT) scan of the chest, abdomen and pelvis, colonoscopic visualisation of the residual colon and carcinoembryonic antigen (CEA) levels, all performed at varying intervals post-surgery. Radiology and/or histological studies were used to diagnose local recurrence or distant metastasis. The follow-up was conducted until July 2016. The primary outcomes for the study were overall survival and disease-free survival.
Statistical analysis
Data analysis was performed using the R 3.5.1 (Windows) statistical package [14]. The effects of clinical variables, LODDS and LNY on disease-free survival (DFS) and overall survival (OS) were investigated using survival analysis techniques such as Kaplan-Meier and log-rank tests. Independent prognostic factors were identified in both univariate and multivariate analyses (Cox regression). The significance level was set at 5%, and terms were included in the models when the p value was below this level. P < 0.05 was considered statistically significant.
Power calculation was carried out based using the R statistical package [14]. The covariates of interest used in the estimation were LNR and LODDS groups together with additional predictors. With the number of episodes of 862, a significance level of 0.05, and a number of different sets of parameters for overall survival and disease-free survival for LNR and LODDS models, the estimated powers were 91.69% for LNR model and 93.39% for LODDS model for the overall survival, while the powers of disease-free survival were 92.29% for the LNR model and 93.04% for the LODDS model. Therefore, there was sufficient power for the study.
Results
Between January 2010 and March 2016, a total of 862 treatment episodes were identified from 856 patients on the colorectal neoplasia database. Patient demographics identified 402 men (47%) and the median age of the cohort was 73 (range 22–100) years. The highest percentage of cancer localization occurred in the sigmoid colon (25.2%) and the ascending colon (20.0%). The LNY was ≥12 in 694 episodes (80.5%). The median duration of follow-up was 27.1 (range 0.1–71) months. Patient characteristics and clinicopathological features are summarised in Table 1.
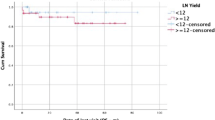
Five-year OS rates for women and men were 86 and 82% respectively (p = 0.4; Table 1). 5-year OS was reduced with increasing age, increasing T stage, N stage, ASA, and with lymphovascular invasion (LVI) (Table 1). Five-year OS rates for the different LNR groups were 85.8% for LNR0, 90.7% for LNR1, and 61.3% for LNR2 (p < 0.0001; Fig. 1). The 5-year OS stratified by nodal stages were 85.2% for pN0, 85.3% for pN1 and 71.2% pN2 (p = 0.01); by LODDS classification were 84.5% for LODDS0, 91.0% for LODDS1 and 61.1% for LODDS2 (p < 0.001; Fig. 1a and b). Five-year OS was not significantly different (p = 0.5) between patients with LNY < 12 and LNY ≥12 (85.4% vs. 83.4%) however, in the subgroup analysis of patients with LNY < 12, both LNR (p < 0.0001) and LODDS (p < 0.002) retained prognostic value for 5-year OS (Fig. 2a and b).
The univariate Cox regression analysis identified ten variables associated with survival that were statistically significant (Table 2): age ≥ 80 (p < 0.001), sigmoid colon tumours (p < 0.001), T3 stage (p = 0.012), T4 stage (p < 0.001), N2 stage (p = 0.002), ASA 3 (p = 0.003), ASA 4 (p < 0.001), lymphovascular invasion (LVI) (p = 0.017), LNR ≥0.2 (p < 0.001) and LODDS2 (p < 0.001). In multivariate analysis, age ≥ 80, hepatic flexure tumour site, sigmoid colon tumour site, T4 stage, and LNY ≥ 12 were identified as independent prognostic factors of OS when data was sub-grouped into LNR and LODDS categories (Table 3).
OS rates over 5 years decreased with advancing ASA; 96% OS survival for ASA I and 46% for ASA 4 (p < 0.001). 619 patients (72.3%) were lymph node negative (pN0) and thus all the patients were inherently in the LNR0 group. Overall survival rates of node-negative patients were not significantly different between the different LODDS0 and LODDS1 groups. When OS was analysed for the 243 patients with stage III colon cancer it was observed that both LNR (p = 0.047) and LODDS (p = 0.019) were associated with decreased survival (Fig. 3a and b). During the study period 56 patients died (5.6%), 55 experienced a recurrence of their cancer (6.4%), and the mean follow-up time was 26.5 months (SD 16.2 months).
In univariate analyses of disease free survival (DFS), positive circumferential margins (p = 0.006), and the presence of lymphovascular invasion (p = 0.016) were significant, however no predictors were significant on multivariate analyses. Log rank tests of DFS survival curves showed no significance for patients staged by LNR groups (p = 0.13), LODDS groups (p = 0.77), < 12LNY (LNR groups; p = 0.12), < 12LNY (LODDS groups; p = 0.9), stage III colon cancer (LNR groups; p = 0.79), stage III colon cancer (LODDS groups; p = 0.73).
Discussion
The current study compares the prognostic impact between LODDS and LNR in the surgical management of colon cancer. In the present study of patients with non-metastatic colonic and rectosigmoid cancers, overall survival rates were comparable between LNR and LODDS groups. Patient factors (age > 80 and ASA 3/4) and tumour factors (tumour location, tumour stage, nodal stage, LVI, LNR and LODDS) were related to 5-year OS in univariate analysis. Our finding of LNR being related to 5-year OS is similar to a Danish cohort study of 8901 patients, where LNR was superior to N-stage in differentiating overall survival in stage III colon cancer [15]. Data on LODDS in colorectal cancer remains limited and accordingly LNR is more frequently used.
A systematic review from Ceelan et al., showed that LNR is a more accurate prognostic method for colorectal cancer patients and gives a superior prediction of survival to the TNM system [16, 17]. Although the LNR classification has been proven to be superior to the pN classification, there are limitations in using this for prognostic assessment. LNR has no prognostic value in node-negative cancer patients because of having the same definition of a LNR0 classification as pN0 classification. If there are inadequate lymph nodes harvested, then LNR is not prognostically accurate [18, 19].
LODDS is a novel indicator that improves the accuracy of lymph node evaluation for prognostic assessment irrespective of nodal positivity status and has been identified in many malignancies as a superior prognostic marker compared to LNR [10, 11]. In an analysis of 2547 curative gastric cancer patients treated with radical resection, LODDS was identified as a better prognostic indicator for overall survival than the LNR [20]. Similar findings have been mirrored for breast cancer patients [21]. In colonic cancers, LODDS was found to be an independent prognostic factor which has prognostic superiority compared to LNR or pN disease [22].
It has been proposed that the lymph node count can be used as a measure of the quality of surgery [23], however adequate lymph node harvesting cannot be achieved in approximately half of patients [24]. In the present study, 19.5% of all colon cancer resections were below the current benchmark of a minimum harvest of 12 lymph nodes; this is comparable to contemporary data from specialist centres [8, 25], but more favourable than that from other population-based studies [26, 27]. This variation in nodal harvesting can be due to patient factors (older age), operative factors (left sided/rectal operations) or the quality of the histopathological examination [28]. Studies have shown that colorectal surgeons have a higher LNY compared to those operations performed by non-specialists [29, 30]. The LNY was adequate in 80% of cases and the eleven surgeons contributing patients to our database are specialist colorectal surgeons.
Arslan et al, found that LODDS was better than LNR at providing more oncologically relevant information as it is less influenced by the LNY. Furthermore, LNR was not sufficient to stage patients when LNY was < 12 [9]. The prognostic values of both LODDS and LNR in this study were independent of the number of harvested nodes. In sub-group analysis of the patients with < 12 LNY in the present study, both LODDS and LNR were significant predictors of 5-year OS. When comparing 5-year OS between LNY < 12 to LNY ≥12, no statistical differences were found when adjusted for either LNR or LODDS.
Conclusion
The study is the first study to examine LODDS in the Australian region (with Australia having one of the highest rates of colorectal cancer in the world) and is one of the largest published single centre series examining LODDS. This study has shown that the prognostic impact of LODDS is comparable to LNR for overall colon cancers and when stratified for stage III patients and patients with a LNY < 12. Since the prognostic information provided between the two is equivalent, LNR may be more clinically practical due to the simple calculation required. Further research is needed to assess whether the addition of the LODDS to the N category defined by the TNM would affect the selection of colon cancer patients who may most benefit from adjuvant treatments.
Availability of data and materials
The datasets generated during and/or analysed during the current study are not publicly available as study participants were assured raw data would remain confidential and not be shared.
Abbreviations
- AJCC:
-
American Joint Committee on Cancer
- ASA:
-
American Society of Anesthesiologists;
- CEA:
-
Carcinoembryonic antigen
- CI:
-
Confidence interval
- CRC:
-
Colorectal cancer
- CRM:
-
Circumferential resection margin
- CT:
-
Computed tomography
- DFS:
-
Disease-free survival
- LNR:
-
Lymph node ratio
- LNY:
-
Lymph node yield
- LODDS:
-
Log odds of positive lymph nodes
- LVI:
-
Lymphovascular invasion
- OS:
-
Overall survival
- TNM:
-
Tumour nodes metastasis.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29.
Lee HY, Choi HJ, Park KJ, et al. Prognostic significance of metastatic lymph node ratio in node-positive colon carcinoma. Ann Surg Oncol. 2007;14:1712–7.
Carrato A. Adjuvant treatment of colorectal Cancer. Gastrointestinal Cancer Res. 2008;2:S42–S6.
McDonald JR, Renehan AG, O’Dwyer ST, Haboubi NY. Lymph node harvest in colon and rectal cancer: current considerations. World J Gastrointestinal Surg. 2012;4:9–19.
Wong SL. Lymph node counts and survival rates after resection for colon and rectal cancer. Gastrointest Cancer Res. 2009;3:S33–5.
Galizia G, Orditura M, Ferraraccio F, et al. The lymph node ratio is a powerful prognostic factor of node-positive colon cancers undergoing potentially curative surgery. World J Surg. 2009;33:2704–13.
Ricciardi R, Madoff RD, Rothenberger DA, Baxter NN. Population-based analyses of lymph node metastases in colorectal cancer. Clin Gastroenterol Hepatol. 2006;4:1522–7.
Lee CHA, Wilkins S, Oliva K, Staples MP, McMurrick PJ. Role of lymph node yield and lymph node ratio in predicting outcomes in non-metastatic colorectal cancer. BJS Open. 2019;3:95–105.
Arslan NC, Sokmen S, Canda AE, Terzi C, Sarioglu S. The prognostic impact of the log odds of positive lymph nodes in colon cancer. Color Dis. 2014;16:O386–92.
Calero A, Escrig-Sos J, Mingol F, et al. Usefulness of the log odds of positive lymph nodes to predict and discriminate prognosis in gastric carcinomas. J Gastrointest Surg. 2015;19:813–20.
Chen L-J, Chung K-P, Chang Y-J, Chang Y-J. Ratio and log odds of positive lymph nodes in breast cancer patients with mastectomy. Surg Oncol. 2015;24:239–47.
McMurrick PJ, Oliva K, Carne P, et al. The first 1000 patients on an internet-based colorectal neoplasia database across private and public medicine in Australia: development of a binational model for the colorectal surgical Society of Australia and new Zealand. Dis Colon Rectum. 2014;57:167–73.
American Joint Committee on Cancer. AJCC Cancer staging manual (7th edn). New York: Springer; 2010.
R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing URL https://www.R-project.org/; 2018.
Lykke J, Roikjaer O, Jess P. The relation between lymph node status and survival in stage I-III colon cancer: results from a prospective nationwide cohort study. Color Dis. 2013;15:559–65.
Wang J, Hassett JM, Dayton MT, Kulaylat MN. Lymph node ratio: role in the staging of node-positive colon cancer. Ann Surg Oncol. 2008;15:1600–8.
Ceelen W, Van Nieuwenhove Y, Pattyn P. Prognostic value of the lymph node ratio in stage III colorectal cancer: a systematic review. Ann Surg Oncol. 2010;17:2847–55.
Rosenberg R, Engel J, Bruns C, et al. The prognostic value of lymph node ratio in a population-based collective of colorectal cancer patients. Ann Surg. 2010;251:1070–8.
Zhang M-R, Xie T-H, Chi J-L, et al. Prognostic role of the lymph node ratio in node positive colorectal cancer: a meta-analysis. Oncotarget. 2016;7:72898–907.
Sun Z, Xu Y, Li DM, et al. Log odds of positive lymph nodes. Cancer. 2010;116:2571–80.
Vinh-Hung V, Verschraegen C, Promish DI, et al. Ratios of involved nodes in early breast cancer. Breast Cancer Res. 2004;6:R680.
Wang J, Hassett JM, Dayton MT, Kulaylat MN. The prognostic superiority of log odds of positive lymph nodes in stage III Colon Cancer. J Gastrointest Surg. 2008;12:1790–6.
Parsons HM, Tuttle TM, Kuntz KM, Begun JW, McGovern PM, Virnig BA. Association between lymph node evaluation for colon cancer and node positivity over the past 20 years. Jama. 2011;306:1089–97.
Veen T, Nedrebo BS, Stormark K, Soreide JA, Korner H, Soreide K. Qualitative and quantitative issues of lymph nodes as prognostic factor in colon cancer. Dig Surg. 2013;30:1–11.
Dent OF, Newland RC, Chan C, Bokey L, Chapuis PH. Trends in pathology and long-term outcomes after resection of colorectal cancer: 1971-2013. ANZ J Surg. 2017;87:34–8.
Nathan H, Shore AD, Anders RA, Wick EC, Gearhart SL, Pawlik TM. Variation in lymph node assessment after colon cancer resection: patient, surgeon, pathologist, or hospital? J Gastrointest Surg. 2011;15:471–9.
Ahmadi O, Stringer MD, Black MA, McCall JL. Clinico-pathological factors influencing lymph node yield in colorectal cancer and impact on survival: analysis of New Zealand cancer registry data. J Surg Oncol. 2015;111:451–8.
Tekkis PP, Smith JJ, Heriot AG, Darzi AW, Thompson MR, Stamatakis JD. A national study on lymph node retrieval in resectional surgery for colorectal cancer. Dis Colon Rectum. 2006;49:1673–83.
Hsu C-W, Lin C-H, Wang J-H, Wang H-T, Ou W-C, King T-M. Factors that influence 12 or more harvested lymph nodes in early-stage colorectal Cancer. World J Surg. 2009;33:333–9.
Lykke J, Jess P, Roikjaer O. Increased lymph node yield is associated with improved survival in rectal Cancer irrespective of Neoadjuvant treatment: results from a National Cohort Study. Dis Colon Rectum. 2015;58:823–30.
Acknowledgements
The authors thank colorectal surgeons S. Bell, M. Chin, P. Carne, K. C. Farmer, A. Polglase, P. Ranchod, P. Simpson, S. Skinner, R. Wale and S. Warrier for contributing their patients to this study. The authors thank Dr. Margaret Staples for additional statistical support during this project. They also thank Let’s Beat Bowel Cancer www.letsbeatbowelcancer.com. a benevolent fundraising and public awareness organization, for financial support during this project.
Funding
This study was funded in part by “Let’s Beat Bowel Cancer” a benevolent fund raising and public awareness foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
ARB, SW, and PM designed the study, carried out prospective data collection, carried out retrospective data collection, analysed the data, and prepared the manuscript. KO carried out prospective data collection, carried out retrospective data collection, analysed the data, and prepared the manuscript. WW designed the study, analysed the data, and prepared the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Human Research ethics committee approval was obtained prior to commencement of the study (#02–10–04-06). Informed written consent was obtained from all patients entered on the Cabrini Monash colorectal neoplasia database.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Baqar, A.R., Wilkins, S., Wang, W. et al. Log odds of positive lymph nodes is prognostically equivalent to lymph node ratio in non-metastatic colon cancer. BMC Cancer 20, 762 (2020). https://doi.org/10.1186/s12885-020-07260-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-020-07260-y