Abstract
Background
Soft-tissue metastasis (STM) is a relatively rare, but not exceptional, manifestation of lung cancer. The purpose of this study was to evaluate the imaging features of STM from lung cancer using fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT), and assess the impact of STM detected at baseline PET/CT on patient survival.
Methods
Out of 4543 patients with lung cancer who underwent 18F-FDG PET/CT in our hospital between January 2013 and September 2018, 85 were diagnosed with STM (78 at baseline PET/CT and 7 at restaging PET/CT) and included in the imaging study. We conducted a comparative survival analysis between patients with stage 4 lung cancer with and without STM at baseline PET/CT (n = 78 in each group) and performed univariate and multivariate analyses to investigate the factors affecting the prognosis of lung cancer.
Results
A total of 219 lesions were identified by 18F-FDG PET/CT: 215 were detected by PET and 139 by CT. Muscle STM were primarily found in the hip and upper limb muscle, whereas subcutaneous STM were mainly distributed in the chest, abdomen, and back. In 68 patients, STM were found incidentally during routine 18F-FDG PET/CT staging. Isolated STM were detected in 6 patients, whose tumor staging and treatment were affected by PET/CT findings. There were no significant differences in the 1-, 3-, and 5-year survival rates between patients with and without STM at baseline PET/CT. Brain and adrenal metastases, but not STM, were associated with poor prognosis of stage 4 lung cancer.
Conclusions
We described the PET/CT imaging characteristics of STM from lung cancer, and confirmed that PET/CT can detect unsuspected STM to change the staging and treatment of some patients. Our analysis indicates that STM is not a useful prognostic indicator for patients with advanced lung cancer, while brain and adrenal metastases portend a poor prognosis.
Similar content being viewed by others
Background
Lung cancer is one of the most prevalent malignant tumors, and the leading cause of cancer-related death worldwide. In China alone, 700,000 new cases are diagnosed every year, resulting in 600,000 deaths per annum. Increasing environmental pollution has led to a surge in lung cancer incidence in recent years. Nearly 50% of patients are metastatic at diagnosis [1, 2]. Early diagnosis and treatment are essential for improving the survival of affected patients.
Soft-tissue metastasis (STM) refers to the growth of tumor cells in soft tissue that is not connected to the primary tumor or regional lymph nodes, and comprises metastases to skeletal muscle and subcutaneous tissue [3,4,5]. Although skeletal muscle and subcutaneous soft tissue account for more than 50% of the human body weight, STM is relatively rare [3,4,5]. Factors such as changes in local blood flow, presence of various proteases and inhibitors, high partial pressure of oxygen, changes in pH, pressure, and temperature, and local production of lactic acid are not conducive to the growth of tumor cells, making the soft tissue relatively resistant to the malignant penetration [4, 6,7,8,9,10,11,12]. Although infrequent, STM is still encountered in clinical practice and warrants greater attention of radiologists and clinicians [13].
Lung cancer is the most common primary tumor of STM, with adenocarcinoma being the most frequent histological variant [13,14,15,16,17,18,19,20]. The most common sites of distant metastasis of lung cancer include the bones, brain, adrenal glands, and liver, with the STM being much less common [3,4,5]. Usually, when lung cancer progresses to a certain extent, some of the tumor cells break away from the primary tumor and disseminate to remote sites through the bloodstream or lymphatic system [21,22,23]. If local tissue conditions are suitable, the cancer cells begin to divide and proliferate and gradually become metastatic foci [4]. A recent study showed that STM was associated with poor prognosis in lung cancer [7]. However, the prognostic value of specific organ metastases, including STM, is controversial and their effects on lung cancer have not been fully elucidated [24,25,26,27,28].
Magnetic resonance imaging (MRI) is the gold standard for imaging evaluation of soft-tissue diseases owing to its good soft tissue contrast [29]. However, it necessitates long acquisition times and is affected by movement artifacts [30]. Moreover, MRI is less sensitive than fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) in identifying STM [31]. The latter technique uses a radioactive glucose analog, 18F-FDG, to image glucose uptake in tumors and adjacent healthy tissue, enabling improved localization and characterization of tumors. 18F-FDG PET/CT can reveal metabolic changes before the morphological abnormalities occur [15], and it has a high tumor-to-background FDG uptake ratio, allowing the detection of hidden STM [13, 32]. The widespread use of 18F-FDG PET/CT has led to increased detection of STM in various malignancies. However, reports on its use to identify STM from lung cancer are scarce, and most of them represent individual cases.
The purpose of this study was to explore the incidence and imaging characteristics of STM from lung cancer using 18F-FDG PET/CT. We also assessed the impact of 18F-FDG PET/CT findings on tumor staging and treatment, and evaluated the effect of STM detected at baseline PET/CT on the survival prognosis of lung cancer. Lastly, we studied the factors affecting the prognosis of lung cancer.
Methods
Patient selection
We retrospectively reviewed medical records of 4543 patients with lung cancer who underwent 18F-FDG PET/CT at the Affiliated Hospital of Southwest Medical University between January 2013 and September 2018. Based on the clinical, imaging, and histopathological data, 85 patients (1.87%) diagnosed with STM at baseline (78 patients) or re-staging (7 patients) 18F-FDG PET/CT were included in the imaging analysis. Sex, age, type, and maximum standardized uptake value (SUVmax) of primary tumor; clinical symptoms; location, size, shape, edge, density, number, SUVmax, and diagnostic method of STM; presence of concomitant distant metastases, including bone, liver, brain, adrenal gland, chest cavity (contralateral pulmonary metastases, pleural effusion/dissemination, and pericardial effusion/dissemination), and other rare metastases, were recorded for all study subjects. From the remaining 4458 subjects, we randomly selected 78 patients with TNM stage M1 lung cancer (regardless of T or N stage) without STM who underwent baseline PET/CT, to act as a control group for patients with STM at baseline PET/CT. The clinical features and distant metastasis of these patients were recorded.
In addition, we evaluated neurological symptoms and/or brain imaging data (MRI or contrast-enhanced CT) of all study subjects to assess brain metastasis. All patients were followed-up via our electronic medical system or telephone until September 2019, to determine health outcomes. Survival time was defined as the period from PET/CT imaging to death due to tumor-related disease.
Inclusion and exclusion criteria
Patients with STM
The inclusion criteria were as follows: 1) underwent 18F-FDG PET/CT and diagnosed with STM for the first time; 2) primary lesion confirmed by puncture biopsy, fiberoptic bronchoscopy, or postoperative pathology. The exclusion criteria were as follows: 1) presence of lymphoma, malignant melanoma, neurofibroma, or other soft-tissue tumor; 2) soft-tissue lesions caused by direct infiltration from primary lesion or bone metastasis; 3) presence of lymph nodes, infection, inflammation, or post-biopsy reactions.
Patients without STM
The inclusion criteria were as follows: 1) underwent baseline 18F-FDG PET/CT and diagnosed with TNM stage M1 lung cancer (regardless of T or N stage) without STM; 2) primary lesion confirmed by puncture biopsy, fiberoptic bronchoscopy, or postoperative pathology. The exclusion criteria were as follows: 1) presence of other primary tumors; 2) lesions caused by direct infiltration from primary lesion.
PET/CT scanning
18F-FDG was prepared using the Siemens Eclipse HD cyclotron and 18F-FDG automated chemical synthesis system, and had radiochemical purity of > 95%. The patients were asked to avoid strenuous physical activity the day before the scan, and fast for at least 6 h prior to intravenous administration of 18F-FDG (5.5 MBq/kg body weight) to ensure a blood glucose level of < 11.1 mmol/L. Following the injection, the patients rested for 40 min-1 h in the dark, drank 300–500 mL of lukewarm water, then underwent PET/CT scanning on a Philips Gemini TF 16 scanner after emptying the bladder. First, a 16-slice spiral CT scan was performed, ranging from the base of the skull to the middle upper thighs, with the arms raised above the head (120 kV, 100 mA, layer thickness 0.5 mm, matrix 512 × 512 pixels, window width 300–500 HU, window level 40–60 HU). If a patient was known to have abnormal lesions in the limbs, they were scanned from the top of the head to the feet, with the arms at the sides of the body. After CT was complete, three-dimensional PET was performed for 70–90 s per bed position, for a total of 7 bed positions. The resulting images were corrected by attenuation and reconstructed iteratively using the ordered subset expectation maximization method (3 iterations, 23 subsets, image size 144 × 144 (matrix)) to obtain transverse, coronal, and sagittal views of the PET/CT scans. Delayed imaging was performed 2 h after 18F-FDG injection, if necessary.
Image analysis and diagnostic criteria
The images were analyzed for the presence of STM and other distant metastases by 3 experienced PET/CT physicians and a radiologist, using a combination of semi-quantitative analysis and visual assessment. Any disagreements were settled through negotiation. For the semi-quantitative analysis, a region of interest was drawn and the SUVmax was measured in the most intense area of focal 18F-FDG accumulation. The soft-tissue lesions were considered PET-positive if their 18F-FDG uptake was focal and greater than that of surrounding healthy muscle and subcutaneous soft tissue. CT-positive soft-tissue lesions were defined as obvious nodules, masses, or abnormal tissue structures. The location, density, maximum diameter, shape, edge, and SUVmax of each soft-tissue lesion were measured, and the number of STM metastases per patient was recorded. Other distant organ metastases were considered “positive” if their 18F-FDG uptake was greater than that of surrounding healthy tissue, or/and if abnormal density changes were noted. Combined with the literature [1, 3, 5, 13, 14, 17, 32], the final diagnostic criteria of STM and other distant organ metastases were histopathological or clinical evaluation (presence of symptoms or diffuse distribution of lesions), concordance between PET/CT results and those of other imaging methods (MRI or contrast-enhanced CT), and evidence of simultaneous remission/progression of primary and metastatic lesion on follow-up PET/CT or other imaging (MRI or contrast-enhanced CT). The patients were followed-up until September 2019.
Survival analysis
To avoid possible bias due to previous treatment, only patients with baseline PET/CT scans were included in this analysis. Patients with unknown survival were excluded. Univariate and multivariate analyses were performed on the STM group and with STM as a variable (i.e. patients with and without STM combined). Lastly, the 1-, 3-, and 5-year survival rates were compared between patients with and without STM.
Statistical analyses
All statistical analyses were performed in the statistical software R 3.6.0. Survival rates were estimated by the Kaplan-Meier estimator and compared between groups using the log-rank or Renyi-type test (the log-rank test was used when the proportional hazards assumption was satisfied; otherwise, a Renyi test was employed). Multivariate Cox proportional hazards regression models were applied to detect potential indicators of survival among patients with lung cancer. The significance level was set at P < 0.05.
Results
Clinical characteristics and PET/CT imaging features
Clinical characteristics of the 85 patients with STM of lung cancer are summarized in Table 1.
Number and imaging characteristics of STM
Muscle STM occurred in 41 cases and subcutaneous STM in 34 cases. In 10 of the patients, both types of STM were present. A total of 219 metastases were located by 18F-FDG PET/CT. Among them, 215 lesions were detected by PET (detection rate = 98.2%; median SUVmax = 6.12 (range 0.8–20.9)). CT identified 139 lesions (detection rate = 63.5%), out of which 109 were isodense and 30 were of low or slightly low density; 96 lesions were nodules or tissue masses, while 43 were accompanied by swelling and had unclear boundaries. Median lesion size was 2.12 cm (range 0.4–13.8).
There were 126 muscle metastases (57.5%), of which 125 were identified as hypermetabolic nodules by PET (detection rate = 99.2%; median SUVmax = 6.79 (range 2.1–20.9)) and 46 were identified as abnormal by CT (detection rate = 36.5%). There were 93 subcutaneous metastases (42.5%), of which 90 were identified as hypermetabolic nodules by PET (detection rate = 96.8%; median SUVmax = 5.36 (range 0.8–19.1)). All subcutaneous STM were identified as abnormal by CT (detection rate = 100%).
Location of STM
Muscle lesions were primarily distributed in the hip muscle, upper limb muscle, and dorsal muscle (Table 2), with the highest frequency in erector spinae, gluteus major muscle, and psoas muscle. Subcutaneous soft-tissue lesions were most commonly located in the chest and abdomen, followed by back, head and neck, hip, and, occasionally, in the extremities (Table 3).
Survival analysis of patients at baseline PET/CT
A total of 4 patients with STM and 5 patients without STM were lost to follow-up. Descriptive characteristics of the remaining patients are listed in Table 4.
Univariate and multivariate analyses of overall survival rate in patients with STM as a variable (patients with and without STM combined)
Results of the univariate analyses demonstrated that adenocarcinoma (ADC) was associated with better prognosis, while small cell lung cancer (SCLC), SUVmax of lung cancer, and brain and adrenal gland metastases were all related with worse prognosis in patients with advanced lung cancer (Table 5). In contrast, presence of STM did not significantly affect the prognosis. Results of multivariate Cox proportional hazards model indicated that SCLC (HR = 2.178, 95% CI 1.044–4.541, P = 0.038), brain metastasis (HR = 2.470, 95% CI 1.240–4.921, P = 0.010), and adrenal gland metastasis (HR = 1.900, 95% CI 1.035–3.488, P = 0.038) were extremely effective at decreasing the lifespan of patients with advanced lung cancer (Table 6).
Univariate and multivariate analyses of overall survival rate in the STM group
Results of univariate analyses demonstrated that the number of STM did not affect the prognosis of patients with advanced lung cancer. ADC was associated with better prognosis, while SCLC, SUVmax of STM, and bone, brain, and adrenal gland metastases were all significantly related to worse prognosis in patients with STM from lung cancer (Table 7).
Furthermore, results of the multivariate Cox proportional hazards model indicated that SCLC (HR = 2.901, 95% CI 1.390–6.053, P = 0.005), bone metastasis (HR = 1.883, 95% CI 1.095–3.237, P = 0.022), and brain metastasis (HR = 2.638, 95% CI 1.316–5.288, P = 0.006) were extremely effective at decreasing the lifespan of patients with STM from lung cancer (Table 8). Patients with STM whose SUVmax was greater than or equal to 5.8 had 2.172 times the hazard faced by patients whose SUVmax of STM was less than 5.8 (95% CI 1.286–3.670, P = 0.004).
Overall 1-, 3-, and 5-year survival rates in the STM and non-STM group
The Renyi test was not significant (Q = 1.372, P = 0.340), suggesting that STM was not related to prognosis in patients with advanced lung cancer (Table 9, Fig. 1).
Discussion
STM are defined as metastases to skeletal muscle and subcutaneous tissue [3,4,5]. Although soft tissue accounts for over 50% of the human body, and has abundant blood supply, it is a relatively rare site of metastasis. Factors such as changes to local blood flow; presence of various proteases and inhibitors; high partial pressure of oxygen; pH, pressure, and temperature changes; and local production of lactic acid are not conducive to the growth of tumor cells, making soft tissue relatively resistant to malignant penetration [4, 6,7,8,9,10,11,12]. Although infrequent, STM are still encountered in clinical practice and warrant greater attention of radiologists and clinicians [13].
Lung cancer is the most common primary malignant tumor leading to STM [13,14,15,16,17]. More than half of lung cancer cases are diagnosed at an advanced stage [1, 2]. The most common sites of distant metastasis include the bone, brain, adrenal glands, and liver, with STM being much less common [6, 29, 33]. Usually, when lung cancer progresses to a certain extent, some of the tumor cells break away from the primary tumor and disseminate to remote sites through the bloodstream or lymphatic system [21,22,23]. If local tissue conditions are suitable, the cancer cells begin to divide and proliferate and gradually become metastatic foci [4].
18F-FDG PET/CT can show metabolic changes before morphological abnormalities occur, and is used to screen for extra-pulmonary metastases in patients with lung cancer [15]. It is a whole-body imaging technique, with high tumor-to-background FDG uptake ratio, which allows detection of hidden STM [13, 32]. Despite these advantages, the use of 18F-FDG PET/CT to detect STM of lung cancer has not been widely researched. In previous studies, the prevalence of STM varied from 0.86 to 13% [13, 32]. In our review, we found that approximately 1.87% of patients with lung cancer had STM. Although this proportion is much lower than that for lung, liver, bone, or brain metastases, STM of lung cancer are not exceptional. Importantly, a more widespread use of 18F-FDG PET/CT may allow detection of previously undetected STM.
The median age and sex distribution in our study population was similar to that in previous studies [20, 21] of STM of lung cancer, indicating that the disease is the most prevalent in middle-aged and elderly males. Further, existing literature [16, 18,19,20] suggests that STM mostly occurs in patients with lung adenocarcinoma, which is consistent with our findings. Muscle metastasis is reportedly more common than subcutaneous metastasis, with a ratio of 1.2–3.3:1 [4, 5, 18]. This was also observed in the current study; the overall incidence of skeletal muscle STM was 60%, while that of subcutaneous STM was 51.8%, i.e. a ratio of 1.2:1.
SUVmax is the most widely used parameter to measure the uptake of a radiolabeled tracer by tumor tissue [34]. In this study, the median SUVmax of STM was 6.12 (range 0.8–20.9) while that of skeletal muscle and subcutaneous metastases was 6.79 (range 2.1–20.9) and 5.36 (range 0.8–19.1), respectively. The vast majority of metastatic lesions (98.2%) had high FDG metabolism, and could be detected by visual inspection of PET scans. A total of 80 muscle STM (36.5%) were missed by CT, which was probably related to poor density resolution of low-dose CT, and the isodensity of the lesions. The highest frequency of muscle metastases was in the hip, upper limb, and dorsal muscle, while subcutaneous metastases were mainly distributed in the chest, abdomen, and back. These findings are in line with those reported in the literature, and suggest that the staging of lung cancer should include a thorough examination of soft tissue [14, 16, 21, 35, 36].
Generally, STM are asymptomatic and easy to miss during clinical evaluation [13, 14]. Indeed, most of our patients (80%) did not present with symptoms related to their STM, and if 18F-FDG PET/CT had not been performed, the lesions would have likely remained undetected. If STM is the only metastasis, tumor staging and treatment might change dramatically. In 20% of the patients, the lesions were symptomatic, with local pain or swelling in muscle STM and painless masses in subcutaneous STM. Thus, in patients with lung cancer, unexplained muscle pain or subcutaneous nodules should raise suspicion of STM, and comprehensive physical and imaging examination should be conducted [29]. STM may also be the initial manifestation of lung cancer (Fig. 2), which was observed in 10 of our patients (11.8%). In such cases, in addition to active follow-up of medical history and physical examination, 18F-FDG PET/CT imaging should be performed as soon as possible to locate the primary tumor and ensure optimal patient management.
A case of lung adenocarcinoma with metastasis of right rectus abdominis as the first manifestation. A 64-year-old man presented with a 2-week history of a painful, tough mass in the upper abdomen, which was confirmed as metastatic adenocarcinoma by biopsy. 18F-FDG PET/CT imaging was performed to locate the primary tumor. Maximum intensity projection (MIP, a), chest axial images (b-d), and abdomen axial images (e-g) of PET/CT showed lesions in the upper lobe of the right lung (arrowheads), right rectus abdominis muscle (dotted arrows), multiple lymph nodes (long arrows) and right ilium (short arrow). Lung biopsy confirmed adenocarcinoma of the right lung. Therefore, a diagnosis of right lung cancer with lymph node, bone, and right rectus abdominis metastases was made. The patient survived for 6 months on palliative chemotherapy
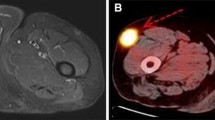
Most patients with STM of lung cancer display multiple organ and lymph node metastases, and since metastasis mostly occurs in patients with a high degree of malignancy, their prognosis is poor [4, 5, 16, 33]. Among the 85 patients in our study, 79 had extensive metastatic diseases. 18F-FDG PET/CT detection of additional STM does not have a significant effect on the staging of lung cancer patients with extensive metastases, but it can help delineate the target area for local radiotherapy [19]. 18F-FDG PET/CT could also guide biopsies of soft-tissue lesions, which usually occur in superficial areas. A small proportion of patients (7.1%) showed solitary STM on 18F-FDG PET/CT (Figs. 3 and 4), which was the only manifestation of metastatic disease. 18F-FDG PET/CT results completely changed tumor staging, treatment plan, and prognosis of these patients.
STM is the only manifestation of a small cell lung cancer. A 74-year-old woman presented with a 1-month history of a subcutaneous mass on the right side of her waist, which was confirmed as metastatic small cell carcinoma on biopsy. MIP (a) of 18F-FDG PET/CT showed a soft-tissue mass in the lower lobe of the right lung (arrowheads), with elevated FDG uptake (SUVmax = 8.4). MIP (a), chest axial images (b-d), and pelvis axial images (e-j) revealed multiple nodules and masses throughout subcutaneous tissue and skeletal muscle (short arrows) with increased FDG uptake (SUVmax = 7.5). Subsequently, lung biopsy confirmed small cell lung cancer of the right lung. After 11 months of palliative chemotherapy, the patient died of respiratory failure
STM changed the postoperative stage of a lung squamous cell carcinoma. A 62-year-old woman was referred to our hospital with a 2-month history of cough. Squamous cell carcinoma of the lower lobe of the right lung was diagnosed by chest CT and lung biopsy. The general condition of the patient was good, and no metastases were found in head MRI or thoracic and abdominal CT. The patient underwent surgical resection and received adjuvant chemotherapy after the operation. Three months later, the patient underwent 18F-FDG PET/CT to assess treatment efficacy. MIP (a) showed increased 18F-FDG uptake (SUVmax = 3.8) in the operative area of the right lung (short arrows). MIP (a), axial images of neck and pelvis (e-j) revealed localized reduced-density nodules in the left deltoid muscle, left gluteus medius muscle, and left gluteal muscle (arrowheads), with FDG uptake (SUVmax = 8.0). Therefore, a diagnosis of multiple STM after lung cancer resection was considered. The patient was treated with palliative radiotherapy and chemotherapy to control the disease
Understanding the impact of specific organ metastases, including STM, on the survival of patients with advanced lung cancer is crucial for appropriate treatment and follow-up strategies. However, the effect of different metastatic organs on the prognosis of lung cancer has not been fully elucidated and the prognostic value of STM in advanced lung cancer remains controversial. A recent study by Kanaji et al. [7] showed that STM was associated with poor prognosis and worse response to treatment in lung cancer. Fei-Yu Niu et al. [1] demonstrated that survival time of patients with uncommon metastases from lung cancer (including STM) was significantly shorter than that of patients with common metastases. In other studies, STM did not impact the prognosis [24]. Herein, although the median survival of patients with STM (5 months) was shorter than that of those without STM (6 months), the 1-, 3-, and 5-year survival rates did not differ significantly between the groups (P = 0.340), suggesting that STM does not affect the prognosis of patients with advanced lung cancer. Nevertheless, detection of STM by 18F-FDG PET/CT can be used as an indicator of disease status, because it provides accurate information about tumor load, which could impact treatment decisions. In addition, multivariate analysis showed that SUVmax of STM was associated with poor survival in the STM group, suggesting that SUVmax of STM reflects disease malignancy. When presence of STM was used as a variable, brain and adrenal metastases were related with poor survival. Previous studies investigating whether specific metastatic organs (other than STM) affect survival of patients with lung cancer yielded contrasting conclusions. In Sorensen et al. [37] brain metastasis was an independent prognostic factor in patients with lung cancer, which is consistent with our results, and may be explained by irreversible nerve injury caused by brain metastasis [38, 39]. In other studies [24, 40, 41], bone metastasis portended poor prognosis, possibly owing to bone-related events such as pathological fractures, spinal cord compression, and malignant hypercalcemia [42]. Liver metastasis is also associated with shorter survival in patients with lung cancer [24, 40, 43,44,45,46,47,48]. Since the liver is an important part of the immune system, metastatic cancer cells may inhibit the immune response and induce immune tolerance [49, 50]. In Tamura et al. [2] and Abbas et al. [24], adrenal metastasis implied poor prognosis, which is consistent with our findings. However, adrenal metastases rarely show severe symptoms and their exact cause is unclear [51]. Some researchers believe that specific organ metastases do not affect the prognosis of lung cancer [25,26,27,28]. And some researchers [28, 52] propose that the increase in the number of metastatic organs reflects the ability of tumor cells to adapt to varying tissue microenvironments, resulting in the emergence of drug resistance and shortening of survival time. In our retrospective analysis, we did not assess the impact of the number of metastatic organs on advanced lung cancer. Larger scale studies are needed to confirm the effects of specific organ metastases, and the number of metastatic organs, on patients with this disease.
Limitations
First of all, our study was retrospective and spanned a relatively long period of time. Diagnosis of metastatic organs mostly depends on clinical evaluation and imaging data, and most STM and other distant metastases lacked detailed pathology. In fact, only 17.6% of patients were confirmed to have STM by histopathology. While in line with patient care standards (most metastases do not need pathological diagnosis), it might have caused deviation in the results [13, 14, 17]. In addition, a variety of physiological and pathological factors, including hyperactivity, infectious/inflammatory processes, post-surgical reactions, primary soft-tissue tumors, and lymphoma, may increase 18F-FDG uptake in soft tissue [18, 53], leading to false positive results. Conversely, factors that decrease 18F-FDG uptake by soft tissue (small lesions, tumors with low metabolic activity, elevated blood glucose levels, etc.) could lead to false negative results.
Second, the density resolution of low-dose CT for attenuation correction is relatively poor, which may have failed to detect lesions with small density changes.
Third, the vast majority of our patients were scanned from the base of the skull to the middle upper thighs, which is not a true whole-body (TWB) scan. In previous studies, 18F-FDG PET/CT detected limb STM in 51.8% (9/12) - 75% (14/27) patients with STM of lung cancer [3, 54], and approximately 11.7–46.8% of STM lesions located in the extremities [3, 5, 13, 32]. Nguyen et al. [3] used TWB PET/CT to evaluate STM and found that approximately 46% of the lesions occurred outside the field of vision of limited whole-body (LWB) PET/CT. In our study, 14.1% (12/85) patients with limb STM, 15.9% (35/219) of STM were located in the extremities. These proportions are lower than those reported in the literature, suggesting that many lesions outside the LWB scan range may have been missed. Missed diagnosis of limb metastases can underestimate the extent of STM, leading to under-staging and mis-management of the disease. Newer PET/CT technology allows fast whole-body scanning without affecting imaging accuracy. In our future work, we will gradually adopt the whole-body approach to PET/CT imaging (from the top of the head to the soles of the feet) to prevent missed lesions.
Fourth, some preclinical brain metastases might have been missed as not all patients with lung cancer underwent head MRI or contrast-enhanced CT, possibly affecting the results of the study. In addition, not all patients underwent thoracic and abdominal CT enhancement. Therefore, we could not compare the diagnostic performance of PET/CT and contrast-enhanced CT in the detection of STM.
Finally, due to the small number of SCLC cases, we were unable to reliably compare patients with SCLC and NSCLC. Therefore, we did not study the two groups separately. Further, since not all patients received systematic treatment, and, in many cases, the information about treatment was limited, we did not analyze the effects of various treatments in this study.
Conclusions
STM is a relatively rare, but not exceptional, manifestation of lung cancer. There are few studies on 18F-FDG PET/CT detection of STM from lung cancer, and most of the existing data is derived from case reports. Thus, our results make a valuable contribution to the literature. We assessed the incidence and imaging characteristics of STM from lung cancer using 18F-FDG PET/CT, which will help clinical and nuclear medicine doctors deepen their understanding of the disease and guide timely assessment of patients with lung cancer. Further, we confirmed that 18F-FDG PET/CT can detect unsuspected STM, and thus change the staging and treatment in some cases. Although PET/CT-detected STM were not a useful prognostic indicator, other metastatic diseases, such as brain and adrenal gland metastases, were associated with poor prognosis of advanced lung cancer.
Availability of data and materials
The datasets and materials during the present study are available from the corresponding author on reasonable request.
Abbreviations
- 18F-FDG:
-
Fluorine-18 fluorodeoxyglucose
- PET/CT:
-
Positron emission tomography/computed tomography
- STM:
-
Soft-tissue metastasis
- SUVmax:
-
Maximum standardized uptake value
- MRI:
-
Magnetic resonance imaging
- TNM:
-
Tumor-node-metastasis
- SD:
-
Standard deviation
- ADC:
-
Adenocarcinoma
- SCLC:
-
Small cell lung cancer
- SqCC:
-
Squamous cell carcinoma
- NSCLC:
-
Non-small cell lung carcinoma
- NSCLC-NOS:
-
Non-small cell lung carcinoma- not otherwise specified
- ASCC:
-
Adenosquamous carcinoma
- LCC:
-
Large cell carcinoma
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- PH:
-
Proportional hazards
- MIP:
-
Maximum intensity projection
- TWB:
-
True whole-body
- LWB:
-
Limited whole-body
References
Niu F-Y, Zhou Q, Yang J-J, et al. Distribution and prognosis of uncommon metastases from non-small cell lung cancer. BMC Cancer. 2016. https://doi.org/10.1186/s12885-016-2169-5.
Tamura T, Kurishima K, Nakazawa K, et al. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol Clin Oncol. 2015;3(1):217–21.
Nguyen NC, Chaar BT, Osman MM. Prevalence and patterns of soft tissue metastasis: Detection with true whole-body F-18 FDG PET/CT. BMC Med Imaging. 2007. https://doi.org/10.1186/1471-2342-7-8.
Pretorius ES, Fishman EK. Helical CT of skeletal muscle metastases from primary carcinomas. AJR Am J Roentgenol. 2000;174(2):401–4.
Qiu DA-S, Xu L-Y, Shames S. The value of 18F-fluorodeoxyglucose positron emission tomography combined with computed tomography in the detection and characterization of soft tissue metastasis. Mol Clin Oncol. 2014;2(5):761–6.
Sersar SI. Skeletal muscle metastasis secondary to lung Cancer. South Med J. 2009;102(1):14–5.
Kanaji N, Tadokoro A, Watanabe N, et al. Association of specific metastatic organs with the prognosis and chemotherapeutic response in patients with advanced lung cancer. Respir Investig. 2019;57(5):472–80.
Koike Y, Hatori M, Kokubun S. Skeletal muscle metastasis secondary to cancer--a report of seven cases. Ups J Med Sci. 2005;110(1):75–83.
Plaza JA, Perez-Montiel D, Mayerson J, et al. Metastases to soft tissue: a review of 118 cases over a 30-year period. Cancer. 2008;112(1):193–203.
Stein-Werblowsky R. Skeletal muscle and tumour metastasis. Experientia. 1974;30(4):423–4.
Strauss JB, Shah AP, Chen SA, et al. Psoas muscle metastases in non-small cell lung cancer. J Thorac Dis. 2012;4(1):83–7.
Weiss L. Biomechanical destruction of cancer cells in skeletal muscle: a rate-regulator for hematogenous metastasis. Clin Exp Metastasis. 1989;7(5):483–91.
Khandelwal AR, Takalkar AM, Lilien DL, et al. Skeletal Muscle Metastases on FDG PET/CT Imaging. Clin Nucl Med. 2012;37(6):575–9.
So Y, Yi JG, Song I, et al. Detection of skeletal muscle metastasis: torso FDG PET-CT versus contrast-enhanced chest or abdomen CT. Acta Radiol. 2015;56(7):860–6.
Yilmaz M, Elboga U, Celen Z, et al. Multiple muscle metastases from lung Cancer detected by FDG PET/CT. Clin Nucl Med. 2011;36(3):245–7.
Arpaci T, Ugurluer G, Akbas T, et al. Imaging of the skeletal muscle metastases. Eur Rev Med Pharmacol Sci. 2012;16(15):2057–63.
Haygood TM, Wong J, Lin JC, et al. Skeletal muscle metastases: a three-part study of a not-so-rare entity. Skelet Radiol. 2012;41(8):899–909.
Karunanithi S, Soundararajan R, Sharma P, et al. Spectrum of physiologic and pathologic skeletal muscle 18F-FDG uptake on PET/CT. AJR Am J Roentgenol. 2015;205(2):W141–9.
Kwas HH, Zendah I, Ghedira H. Skeletal muscle metastases from lung cancer. Asian Cardiovasc Thorac Ann. 2013;21(6):741–3.
Tuoheti Y, Okada K, Osanai T. Skeletal muscle metastases of carcinoma: a Clinicopathological study of 12 cases. Jpn J Clin Oncol. 2004;34(4):210–4.
Surov A, Hainz M, Holzhausen H-J, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol. 2010;20(3):649–58.
Nagao E, Nishie A, Yoshimitsu K. Gluteal muscular and sciatic nerve metastases in advanced urinary bladder carcinoma. Abdom Imaging. 2004;29(5):619–22.
Batson OV. The function of the vertebral veins and their role in the spread of metastases. Ann Surg. 1940;112(1):138–49.
Omar A, Bae S, Arafat W, et al. Site of metastases as prognostic factors in unselected population of stage IV non-small cell lung Cancer. Asian Pac J Cancer Prev. 2018;19(7):1907–10.
Sakurai M, Shinkai T, Eguchi K, et al. Prognostic factors in non-small cell lung cancer: multiregression analysis in the National Cancer Center Hospital (Japan). J Cancer Res Clin Oncol. 1987;113(6):563–6.
Borges M, Sculier JP, Paesmans M, et al. Prognostic factors for response to chemotherapy containing platinum derivatives in patients with unresectable non-small cell lung cancer (NSCLC). Lung Cancer. 1996;16(1):21–33.
Paralkar VR, Li T, Langer CJ. Population characteristics and prognostic factors in metastatic non-small-cell lung cancer: a Fox Chase Cancer Center retrospective. Clin Lung Cancer 9. 2008;9(2):116–21.
Gibson AJW, Li H, D’Sliva A, et al. Impact of number versus location of metastases on survival in stage IV M1b non-small cell lung cancer. Med Oncol. 2018;35(9):117.
Di Giorgio A, Sammartino P, Cardini CL, et al. Lung cancer and skeletal muscle metastases. Ann Thorac Surg. 2004;78(2):709–11.
Emmering J, Vogel WV, Stokkel MPM. Intramuscular metastases on FDG PET-CT: a review of the literature. Nucl Med Commun. 2012;33(2):117–20.
Pfannenberg C, Aschoff P, Schanz S, et al. Prospective comparison of 18F-fluorodeoxyglucose positron emission tomography/computed tomography and whole-body magnetic resonance imaging in staging of advanced malignant melanoma. Eur J Cancer. 2007;43(3):557–64.
Nocuń A, Chrapko B. Multiple and solitary skeletal muscle metastases on 18F-FDG PET/CT imaging. Nucl Med Commun. 2015;36(11):1091–9.
Savas K, Pinar KZ, Sevda KS, et al. Haematogenous muscular metastasis of non-small cell lung cancer in F-18 fluorodeoxyglucose positron emission tomography/computed tomography. Contemp Oncol. 2015;19(3):241–5.
Hagi T, Nakamura T, Sugino Y, et al. Is FDG-PET/CT useful for diagnosing pulmonary metastasis in patients with soft tissue sarcoma? Anticancer Res. 2018;38(6):3635–9.
Glockner JF, White LM, Sundaram M, et al. Unsuspected metastases presenting as solitary soft tissue lesions: a fourteen-year review. Skelet Radiol. 2000;29(5):270–4.
Surov A, Pawelka MK, Wienke A, et al. PET/CT imaging of skeletal muscle metastases. Acta Radiol. 2014;55(1):101–6.
Sorensen JB, Hansen HH, Hansen M, et al. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol. 1988;6:1474–80.
Jacot W, Quantin X, Boher JM, et al. Brain metastases at the time of presentation of non-small cell lung cancer: a multi-centric AERIO analysis of prognostic factors. Br J Cancer. 2001;84:903–9.
Sanchez de Cos J, Sojo Gonzalez MA, Montero MV, et al. Non-small cell lung cancer and silent brain metastasis. Survival and prognostic factors. Lung Cancer. 2009;63:140–5.
Finkelstein DM, Ettinger DS, Ruckdeschel JC. Long-term survivors in metastatic non-small-cell lung cancer: an eastern cooperative oncology group study. J Clin Oncol. 1986;4:702–9.
Bauml J, Mick R, Zhang Y, et al. Determinants of survival in advanced non-small-cell lung cancer in the era of targeted therapies. Clin Lung Cancer. 2013;14:581–91.
Saad F, Lipton A, Cook R, et al. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110:1860–7.
Riihimaki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86:78–84.
McKay RR, Kroeger N, Xie W, et al. Impact of bone and liver metastases on patients with renal cell carcinoma treated with targeted therapy. Eur Urol. 2014;65:577–84.
Riihimaki M, Thomsen H, Hemminki A, et al. Comparison of survival of patients with metastases from known versus unknown primaries: survival in metastatic cancer. BMC Cancer. 2013;13:36.
Hemminki K, Riihimaki M, Sundquist K, et al. Site-specific survival rates for cancer of unknown primary according to location of metastases. Int J Cancer. 2013;133:182–9.
Li J, Zhu H, Sun L, et al. Prognostic value of site-specific metastases in lung cancer: a population based study. J Cancer. 2019;10:3079–86.
Hoang T, Xu R, Schiller JH, et al. Clinical model to predict survival in chemonaive patients with advanced non-small-cell lung cancer treated with third-generation chemotherapy regimens based on eastern cooperative oncology group data. J Clin Oncol. 2005;23:175–83.
Li F, Tian Z. The liver works as a school to educate regulatory immune cells. Cell Mol Immunol. 2013;10:292–302.
Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996–1006.
Mohammad K, Sadikot RT. Adrenal insufficiency as a presenting manifestation of nonsmall cell lung cancer. South Med J. 2009;102:665–7.
Kanaji N, Mizoguchi H, Inoue T, et al. Clinical features of patients with lung cancer accompanied by thromboembolism or disseminated intravascular coagulation. Ther Clin Risk Manag. 2018;14:1361–8.
Shin D-S, Shon O-J, Han D-S, et al. The clinical efficacy of 18F-FDG-PET/CT in benign and malignant musculoskeletal tumors. Ann Nucl Med. 2008;22(7):603–9.
Pop D, Nadeemy AS, Venissac N, et al. Skeletal muscle metastasis from non-small cell lung Cancer. J Thorac Oncol. 2009;4(10):1236–41.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding
This work is supported by the Nuclear Medicine and Molecular Imaging Key Laboratory of Sichuan Province.
Author information
Authors and Affiliations
Contributions
X-TT and Z-XY contributed to the study design and X-TT wrote the manuscript. X-TT and Z-XY collected and analyzed the clinical data of patients and they contributed equally to this paper. Z-SM, L-CF, F-WH and Z-CR were responsible for the integrity of the data and the accuracy of the data analysis. C-Y was responsible for revising for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures were carried out in accordance with the principles of the Helsinki Declaration. This study was approved by the ethics committee of the Affiliated Hospital of Southwest Medical University (Luzhou, China). Because the study was retrospective, and included data from deceased patients, informed consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, T., Zhang, X., Zhang, S. et al. Imaging features and prognostic value of 18F-FDG PET/CT detection of soft-tissue metastasis from lung cancer: a retrospective study. BMC Cancer 20, 596 (2020). https://doi.org/10.1186/s12885-020-07080-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-020-07080-0