Abstract
Background
Hepatoma-derived growth factor (HDGF) participates in angiogenesis and represents a negative prognostic factor in oral cancer. The current study was designed to elucidate the regulatory mechanism between HDGF and vascular endothelial growth factor (VEGF) and the clinical impact of oral cancer.
Methods
TCGA data and surgical samples from oral cancer patients were used for the clinicopathological parameter and survival analysis. Human oral cancer SCC4 and SAS cells were treated with recombinant HDGF protein. VEGF gene expression and protein level were analyzed by RT-PCR, Western blotting, and enzyme-linked immunosorbent assay. The signaling pathways for regulating VEGF expression were investigated. The nucleolin neutralizing antibody and HIF-1α inhibitor were applied to SCC4 cells to investigate their effects on the HDGF-stimulated VEGF pathways.
Results
TCGA and immunohistochemical analysis revealed a positive correlation between HDGF and VEGF expression in oral cancer tissues. Recombinant HDGF significantly increased VEGF gene and protein expression in oral cancer SCC4 cells in a dose-dependent manner. HDGF enhanced the phosphorylation levels of AKT and IkB and the protein level of HIF-1α and NF-κB. The nucleolin-neutralizing antibody abolished HDGF-stimulated HIF-1α, NF-κB and VEGF protein expression in SCC4 cells. The HIF-1α inhibitor antagonized the HDGF-induced VEGF gene expression. High VEGF expression was strongly correlated with HDGF expression, advanced disease, and poor survival.
Conclusion
This study postulated a new pathway in which HDGF activated HIF-1α and then induced VEGF expression through binding to membrane nucleolin under normoxic conditions, leading to poor disease control. The HDGF/HIF-1α/VEGF axis is important for developing future therapeutic strategies.
Similar content being viewed by others
Background
Oral cancer is characterized by its aggressive behavior. Even after radical surgery followed by adjuvant radiotherapy and chemotherapy, the survival rate of oral cancer patients remains poor due to relentless recurrence or metastasis [1, 2].
Angiogenesis is required for tumor growth [3] and facilitates tumor recurrence and metastasis [4, 5] through perturbing the balance of proangiogenic and antiangiogenic factors. Among the proangiogenic factors, vascular endothelial growth factor (VEGF) is the most important one [6]. Angiogenesis plays a critical role in disease progression and mediates treatment resistance [7]. Therefore, understanding angiogenesis, particularly the VEGF pathway, is urgently needed for the risk stratification of oral cancer patients and the development of novel therapeutic targets.
Hepatoma-derived growth factor (HDGF) is a heparin-binding nuclear growth factor purified from the conditioned media of Huh-7 hepatoma cells [8,9,10,11]. HDGF overexpression has been found to correlate with advanced stages and poor prognosis in many types of cancer [12,13,14,15,16,17]. The possibility has been considered that HDGF induces angiogenesis [10, 18] through a direct effect or through the induction of VEGF release by regulating the VEGF upstream genes or VEGF promoters [19].
We have previously demonstrated that HDGF overexpression contributes to oncogenic processes and constitutes a novel negative prognostic factor for oral cancer [20]. HDGF expression has been hypothesized to play an important role in tumorigenesis and angiogenesis in oral cancer, which may be associated with the induction of angiogenic factors, leading to a more aggressive pattern of growth and poor prognosis [21]. However, the possible regulatory mechanism between HDGF and VEGF has not been explored.
Thus, the current study was designed to elucidate the possible interaction or regulatory mechanism between HDGF and VEGF and the possible clinical impact in oral cancer.
Methods
Reagents
Recombinant HDGF protein was generated as previously described [12]. The following reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA): chetomin (C9623), Bay 11–7082 (B5556), Ponceau S solution (P7170), and β-actin (A5441). The following antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA): VEGF (sc-152), p-AKT (sc-33,437), AKT (sc-1619), p-IκB (sc-8404), p65 (sc-372), STAT3 (sc-482) and the nucleolin neutralizing antibody (sc-8031). Other antibodies were obtained as follows: p-STAT3 (4113; Cell Signaling Technology, Inc., Danvers, MA, USA), IκB (ab32518; Abcam plc., Cambridge, UK), and HIF-1α (NB100–479; Novus International Inc., St Louis, MO, USA).
Cell culture
Human tongue squamous carcinoma SCC4 (purchased from the Bioresource Collection and Research Center, Hsinchu, Taiwan) and SAS cells (purchased from Japanese Collection of Research Bioresources Cell Bank, Osaka, Japan) were 13th generations and cultured in DMEM/F12 (Invitrogen; Carlsbad, CA, USA) with 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA), 2 mM glutamine, 100 U/ml penicillin (Invitrogen; Carlsbad, CA, USA) and 100 mg/ml streptomycin (Invitrogen; Carlsbad, CA, USA) at 37 °C in humidified air containing 5% CO2.
Western blotting
Whole cell extracts were prepared and quantified by the Coomassie Plus Assay as previously described [22]. The PVDF membrane was blocked with 5% skim milk in TBS-T for 1 h and then incubated with the indicated primary antibodies and secondary antibodies conjugated with HRP (1:5000; Santa Cruz Inc.; Santa Cruz, CA, USA) for 1 h each. The signals on the membrane were detected using HRP chemiluminescent substrate (Millipore Corporation; Billerica, MA, USA) and exposed to X-ray film for signal detection.
Quantitative real-time PCR
Total RNA purification and quantitative real-time PCR were performed as previously described [23]. The 2X SYBR Green PCR Master Mix (Thermo Fisher Scientific, Waltham, USA) and predesigned gene-specific primers for human VEGFA (NM_001025366.2) and β-actin (NM_007393.3) were used for quantitative real-time PCR. The data were normalized to β-actin and expressed as fold changes with respect to the control group. The primer sequences were as follows: VEGFA forward primer: 5′-CCC TGA TGA GAT CGA GTA CA-3′; VEGFA reverse primer: 5′-AGG AAG CTC ATC TCT CCT AT-3′; β-actin forward primer: 5′-GGA ATC CTG TGG CAT CCA T-3′; and β-actin reverse primer: 5′-GCT CAG GAG GAG CAA TGA T-3′.
Enzyme-linked immunosorbent assay (ELISA)
The VEGF-A concentrations in the supernatants were determined by ELISA using a commercially available kit (Boster Biological Technology, Valley Ave, Pleasanton, CA). Briefly, after supernatant collection, the total cellular proteins were extracted and then measured by the bicinchoninic acid assay to assess the cell number in each group. The secreted VEGFA concentration was normalized to the total cellular protein level and is shown as the mean ± SD.
Immunohistochemical staining and assessment
The surgically resected specimens from 102 oral cancer patients were included with approval from the institutional review board. Immunohistochemical analysis using the tissue microarray (TMA) consisting of surgically resected samples from oral cancer patients was performed as previously described [20] to delineate the correlation between HDGF expression, VEGF expression, and clinic-pathological parameters. Briefly, the slides were incubated with primary HDGF antibody (1:200 dilution) and VEGF antibodies (1:250; Santa Cruz; Santa Cruz, CA, USA) for 30 min and visualized using a peroxidase-conjugated secondary antibody, a polymer detection system (Zymed Laboratories, San Francisco, CA, USA) and 3,3-diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO). The sections were then counterstained with hematoxylin and eosin.
The percentage of tumor cells with definite moderate to intense nuclear or cytoplasmic immunoreactivity was scored, and the median of scores from multiple cores in the same patient was adopted as the labeling index (LI) for each marker, as previously described [12, 20, 24]. A total of 95 patient samples containing at least two preserved-tissue cores were scored and analyzed. Seven patients were excluded due to insufficient TMA samples. The cutoffs of the LIs to define high expression of HDGF were determined as follows: (1) high expression of nuclear HDGF (HDGF-N) if ≥40% of tumor nuclei were stained, (2) high expression of cytoplasmic HDGF (HDGF-C) if ≥40% of tumor cytoplasm was stained and (3) VEGF high expression if ≥50% of tumor cytoplasm was stained.
Immunofluorescent staining of paraffin-embedded tissues
Immunofluorescence staining was performed on surgically resected specimens of oral cancer patients as described previously [25]. To investigate the expression of HDGF and VEGF, tissue sections were incubated with primary HDGF antibody (1:200 dilution) and VEGF antibodies (1:250; Santa Cruz; Santa Cruz, CA, USA). After wash step, tissue sections were incubated with appropriate fluorescent-labeled secondary antibodies then nuclei were stained with DAPI (Sigma-Aldrich, St. Louis, MO, USA). Finally, tissues were mounted with coverslips in fluorescence mounting medium (Dako corporation; Glostrup, Denmark). The fluorescent color of HDGF was Green (AlexaFluor488); VEGF was red (AlexaFluor546); nuclei were stained with blue color (DAPI). The microscope images were captured using Zeiss LSM 510 confocal imaging (200x magnification) and processed with ZEN 2 microscope image analysis software (Carl Zeiss; Jena, Germany).
Computational biology analysis
HDGF and VEGF mRNA expression data were obtained from The Cancer Genome Atlas (TCGA). All the software and graphics for transcriptomics analysis were developed using in-house code implemented in MATLAB (MathWorks, Natick, MA, USA). HDGF and VEGF expression in the TCGA are reported as the fold changes between 1) oral cancer and healthy tissues and 2) head and neck cancer and healthy tissue. The correlation of HDGF and VEGFA mRNA expression in the TCGA dataset was analyzed by UCSC Xena (http://xena.ucsc.edu/).
Statistical analysis
For the Western blotting, RT-PCR, and ELISA data, comparisons were performed by using one-way ANOVA followed by the Newman-Keuls post hoc test or t-test (for multiple comparisons) using Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). All the in vitro experiments in this study were triplicated. A probability value < 0.05 is considered to be statistically significant.
The associations among clinicopathologic factors, HDGF expression, and VEGF expression were evaluated using the X2 test, t-test, and ANOVA as appropriate. Estimates of disease-specific survival (DSS), metastasis-free survival (MFS), and local recurrence-free survival (LRFS) were calculated using the Kaplan–Meier method with log-rank test. The multivariable analyses of DSS, MFS, and LRFS were performed by using the Cox proportional hazards model with a stepwise approach. All tests were two-tailed, with a probability value < 0.05 considered to be statistically significant. Clinical statistical analyses were performed using SPSS 14 software (SPSS, Chicago, IL, USA).
Results
Correlation of HDGF and VEGF expression and clinicopathological parameters in oral cancer tissues
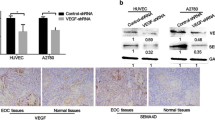
Because HDGF overexpression is correlated with angiogenesis and tumorigenesis, including in oral cancer [26], we investigated whether there was a relationship between HDGF and VEGF expression in oral cancer and head and neck cancer. According to TCGA data analysis (n = 522, oral cancer; n = 566, head and neck cancer [TCGA, Provisional cohort]), the HDGF and VEGF mRNA expression profile exhibited a strong positive correlation (P = 0.0107; R2 = 0.01247, oral cancer; P = 0.0001; R2 = 0.02643, head and neck cancer) (Fig. 1A and B). Therefore, these results suggested that HDGF expression was positively correlated with VEGF expression in human head and neck cancer and oral cancer. An immunohistochemistry assay revealed a positive correlation (P = 0.006) between HDGF-N expression and VEGF expression (Table 1). In addition, high expression of VEGF and HDGF-N were closely linked to advanced status of oral cancer, more advanced primary T stage and poorly differentiated histological grade. Higher VEGF expression also correlated with more advanced nodal status (P = 0.021). Immunohistochemical staining of HDGF and VEGF and immunofluorescence staining of oral cancer patients were shown in Fig. 1c and d.
Correlation of HDGF and VEGF expression in oral cancer. a, b Correlation between HDGF and VEGF mRNA levels in oral cancer and head and neck cancer patients obtained by analysis of data from TCGA. HDGF expression is positively correlated with VEGFA expression in human head and neck squamous cell carcinoma tissues, including oral cancer. c Tissue microarray analysis of the correlation between HDGF and VEGF expression in oral cancer patients. The photographs were from two representative oral cancer patients. Case 1 (pT2N0M0, stage II) exhibited low-expression HDGF and VEGF immunostaining, whereas Case 2 (pT3N2M0, stage III) showed high-expression staining of both HDGF and VEGF. Scale bars, 20 μm. d Immunofluorescence staining of oral cancer patients. The fluorescent color of HDGF was Green (AlexaFluor 488); VEGF was red (AlexaFluor 546); nuclei were stained with blue color (DAPI). Case 3 (pT1N0M0, stage I) exhibited both high-intensity staining of HDGF and VEGF, whereas Case 4 (pT2N0M0, stage II) showed intermediate-intensity HDGF and VEGF immunofluorescence staining, and Case 5 (pT1N0M0, stage II) showed low-intensity staining of HDGF and VEGF. Scale bars, 20 μm
Recombinant HDGF induced VEGF expression and release in oral cancer cells
To investigate whether HDGF regulated VEGF expression in oral cancer cells, SCC4 cells and SAS cells were treated with different concentrations of recombinant HDGF protein and then harvested for subsequent analysis. RT-PCR showed that exogenous HDGF protein significantly increased VEGF gene expression by approximately 1.5-fold compared with the control group in SCC4 cells (Fig. 2a, rHDGF 100 ng/ml, P < 0.01). Western blotting assays showed the protein levels of VEGF were also increased by HDGF stimuli in a dose-dependent manner (Fig. 2b and Additional file 1: Figure S1, rHDGF 100 ng/ml, P < 0.05). We next analyzed the secreted levels of VEGF by Western blotting and ELISA. As expected, more VEGF protein was secreted into the culture medium under HDGF stimulation than in the control group (Fig. 2c, rHDGF 100 ng/ml, P < 0.05). ELISA analysis revealed that HDGF enhanced a small but significant levels of VEGF secreted by SCC4 cells in a dose-dependent manner (Fig. 2d). Approximately additional 50 pg/ml VEGF was secreted in 100 ng/ml-rHDGF-treated group, comparing to the control group (Fig. 2d, P < 0.01). Therefore, these results supported that additional HDGF induced VEGF upregulation and expression in human oral cancer cells. SAS cells were treated with recombinant HDGF protein for 24 h before harvest. Western blotting showed the protein levels of VEGF was upregulated by HDGF stimulation in a dose-dependent manner (Additional file 1: Figure S2A-B).
Effect of HDGF on VEGF expression in oral cancer cells. SCC4 cells were treated with the indicated concentration of recombinant HDGF protein for 24 h before harvest. a Relative gene expression levels of VEGF were analyzed by SYBR green-based RT-PCR. Data are expressed as the fold change with respect to the control group (means ± SD of triplicate experiments). b Cell lysates were analyzed using Western blotting, and the protein levels of VEGF/β-actin were measured and quantified. c The secreted VEGF protein levels in the supernatants were measured by Western blotting. Ponceau S staining was used as a loading control. d Levels of secreted VEGF protein (pg/ml) were detected by enzyme-linked immunosorbent assay (ELISA) in triplicate experiments. Data were mean of three experiments. *, P < 0.05; **, P < 0.01; ns, not statistically significant
HDGF stimulates AKT/HIF-1α/NF-κB signaling in oral cancer cells
Given the well-known signaling pathways for regulating VEGF expression [27, 28], we then focused on the activation of specific transcription factors, including HIF-1α, NF-κB, and STAT3. SCC4 cells were treated with recombinant HDGF, and the levels of HIF-1α, NF-κB, and STAT3 were measured and quantified by Western blotting (Fig. 3a-d and Additional file 1: Figure S3A-D). HDGF enhanced the phosphorylation levels of AKT and IκB in the HDGF-treated group compared with the control group in SCC4 cells (Fig. 3a-b and Additional file 1: Figure S3A-B, rHDGF 10 ng/ml, P < 0.01). In addition, the protein levels of the transcriptional factors HIF-1α and NF-κB p65 were also upregulated under HDGF stimulation (HIF-1α, Fig. 3c and Additional file 1: Figure S3C, rHDGF 1 ng/ml, P < 0.01; NF-κB p65, Fig. 3d and Additional file 1: Figure S3D, rHDGF 10 ng/ml, P < 0.05), indicating that HDGF triggered the AKT/HIF-1α/NF-κB signaling pathway. HIF-1α was upregulated under HDGF stimulation in SAS cells (Additional file 1: Figure S2C, rHDGF 1 ng/ml, P < 0.01). However, HDGF treatment (even at a high dose of 100 ng/ml) did not affect the phosphorylation of STAT3, suggesting that HDGF did not elicit STAT3 activation in SCC4 cells (Fig. 3e and Additional file 1: Figure S3E). Together, these results implied that HDGF stimulated AKT/HIF-1α/NF-κB signaling, thereby modulating VEGF expression in oral cancer cells.
HDGF triggered AKT/HIF-1α/NF-κB signaling in SCC4 oral cancer cells. a-d Cells were treated with recombinant HDGF (1–100 ng/ml) for 24 h and then harvested for total protein extraction. The cell lysates were separated by SDS-PAGE and detected by Western blotting with the indicated primary antibodies. β-actin was used as an internal control for loading and transfer. Data were mean of three experiments. *, P < 0.05; **, P < 0.01; ns, not statistically significant
Antibody neutralization of surface nucleolin abolished HDGF-stimulated AKT/HIF1α/NF-κB/VEGF signaling in oral cancer cells
Because the surface nucleolin/AKT axis has been found to participate in transmitting the oncogenic signaling of HDGF [22], we investigated whether blockage of the HDGF/nucleolin axis by antibody neutralization affected HDGF-stimulated HIF-1α, NF-κB and VEGF expression in SCC4 cells. Western blotting analysis demonstrated that the additional recombinant HDGF was unable to enhance phosphorylation levels of AKT and HIF-1α protein under the co-treatment with the neutralizing antibodies against nucleolin in SCC4 cells (Fig. 4a-b and Additional file 1: Figure S4A-B). Moreover, blocking the HDGF/nucleolin axis not only diminished the HDGF-stimulated phosphorylation of IκB and NF-κB p65 but also significantly reduced VEGF protein expression (Fig. 4c-d and Additional file 1: Figure S4C-E, P < 0.05). These results suggested that the nucleolin-mediated signaling pathway is important for HDGF-modulated VEGF expression.
The Neutralizing antibody against nucleolin eliminate HDGF-stimulated AKT/HIF-1α/NF-κB/VEGF signaling in SCC4 oral cancer cells. a-d SCC4 cells were treated with recombinant HDGF protein (100 ng/ml) in the presence of anti-NCL or anti-IgG antibody (5 μg/ml) for 24 h before total protein extraction. Cell lysates were subjected to Western blotting with the indicated antibodies. β-actin was used as an internal control for loading and transfer. Data were mean of three experiments. *, P < 0.05; **, P < 0.01; ns, not statistically significant
Application of the HIF-1α inhibitor chetomin antagonized HDGF-induced VEGF upregulation in oral cancer cells
To further investigate which of the transcription factors HIF-1α and NF-κB were dominant in HDGF-induced VEGF gene expression, we employed the HIF-1α inhibitor chetomin and the NF-κB inhibitor Bay 11–7082. RT-PCR analysis showed no significant difference in VEGF mRNA levels with or without additional HDGF in the chetomin group (Fig. 5a, P, not statistically significant). The application of chetomin potently suppressed the HDGF-induced VEGF gene expression. On the other hand, HDGF treatment was able to induce VEGF upregulation even in the presence of Bay 11–7082 (Fig. 5a). Western blot assays demonstrated that chetomin suppressed the VEGF protein expression induced by HDGF (Fig. 5b). Although Bay 11–7082 could inhibit the basal level of VEGF, VEGF was still enhanced in HDGF-treated cells (Fig. 5b). Moreover, ELISA also revealed that chetomin eliminated the increased secretion of VEGF protein induced by HDGF (Fig. 5c, P, not statistically significant). Thus, HIF-1α signaling plays a critical role in HDGF-induced VEGF gene regulation.
Effects of chetomin and Bay 11–7082 on HDGF-induced VEGF upregulation in SCC4 oral cancer cells. Cells were treated with recombinant HDGF protein (100 ng/ml) in the presence of Bay 11–7082 (10 nM) or chetomin (10 nM) for 24 h. a Relative gene expression levels of VEGF were analyzed by SYBR Green-based RT-PCR. Data are expressed as the fold change with respect to the control group (means ± SD of triplicate experiments). b The protein levels of VEGF were analyzed by Western blotting and normalized to β-actin expression. (c) the levels of secreted VEGF protein (pg/ml) were detected by ELISA in triplicate experiments. d Scheme for HDGF-regulated VEGF transcription in oral cancer cells. Data were mean of three experiments. *, P < 0.05; **, P < 0.01; ns, not statistically significant
Univariate log-rank analyses of survival
According to univariate survival analysis, postoperative concurrent chemoradiotherapy (Post-OP CCRT), histological grade, and high expression of HDGF-N and VEGF were statistically significant prognostic predictors for DSS, MFS, and LRFS. The univariate survival analysis is summarized in Additional file 1: Table S1. High VEGF expression predicted a higher rate of local and distant recurrence and shorter DSS in the Kaplan–Meier survival analysis (Fig. 6).
Multivariate analyses of survival
In the multivariate comparison (Table 2), advanced primary T stage (P = 0.0001; RR, 5.98), higher histological grade (P = 0.0014; RR, 7.50), lack of Post-OP CCRT (P < 0.0001; RR, 6.89), high expression of HDGF-N (P = 0.028; RR, 3.04) and high expression of VEGF (P = 0.0183; RR, 4.09) represented independent negative prognostic factors for DSS. For MFS, strong independent prognostic factors were advanced primary T stage (P = 0.0003; RR, 4.39), higher histological grade (P = 0.0009; RR, 6.70), lack of Post-OP CCRT (P < 0.0001; RR, 5.61), and high expression of VEGF (P = 0.0153; RR, 4.01). Lack of Post-OP CCRT (P = 0.0117; RR, 2.00), high expression of VEGF (P = 0.0461; RR, 2.10) and HDGF-N (P = 0.0285; RR, 2.14) were predictive of inferior LRFS.
Discussion
Angiogenesis is essential for cancer progression, metastasis and treatment resistance. The regulation of angiogenesis involves a number of critical growth factors, cytokines, signaling cascades and cellular processes that are triggered in response to either a hypoxic or an inflammatory stimulus [29]. Hypoxia- and inflammation-driven angiogenesis are regulated via distinctly different and yet overlapping pathways [30].
Through correlation analysis of the immunohistochemistry assay and TCGA data, these results provided support for the interaction between HDGF and VEGF expression in oral cancer. In this study, for the first time, we demonstrated that HDGF enhanced VEGF expression in oral cancer cells at the mRNA level, protein level and secretion level with a dose-dependent manner.
The mechanism through which HDGF induces or regulates VEGF expression in tumor cells remains unclear. HDGF has been reported to stimulate the proliferation and invasion of hepatocellular carcinoma cells via PI3K/AKT signaling [22, 31]. Indeed, activation of the PI3K/AKT pathway in both tumor and endothelial cells increases VEGF secretion by both HIF-1α-dependent and HIF-1α-independent mechanisms [32,33,34].
In hypoxia-driven angiogenesis, hypoxia activates the PI3K/AKT pathway to prevent the posttranslational hydroxylation and the subsequent degradation of HIF-1α, allowing it to accumulate and then translocate to the nucleus, where it upregulates VEGF production pathways [29, 35,36,37]. The inflammatory stimulus activates the PI3K/AKT pathway, leading to the phosphorylation of IκBα. IκBα is degraded, allowing NF-κB subunits p50 and p65 to translocate into the nucleus and activate VEGF production [29, 38].
HIF-1 has been shown to essentially control the cellular response to hypoxia. Evidence has emerged that HIF-1α is also responsive to stimuli under normoxic conditions [39]. One important mechanism underlying these normoxic conditions is the transcriptional regulation of HIF-1α by NF-κB [40], which is the key promoter in the inflammatory angiogenic pathway [29, 39]. Recently, HIF-1α has been reported that directly bound to the HDGF promoter region, which was highly correlated with pancreatic cancer-associated fibrosis under normoxic condition [41].
Our data have shown that exogenous HDGF protein not only stimulated the phosphorylation levels of AKT and IκB but also increased the protein levels of the transcriptional factors HIF-1α and NF-κB p65 in oral cancer cells. The Western blotting results (Fig. 3) showed HDGF in a dose of 10 ng/ml was able to enhance more than two folds of phosphorylation levels of AKT and IκB; additional HDGF in the low-dose of 1 ng/ml could induce two folds of the protein levels of HIF-1α. The upregulation of phosphorylated IκB implied a loss of NF-κB blockage by IκB, leading to NF-κB activation and subsequently to modulated HIF-1α expression or VEGF production. This finding offered a rationale for how HDGF simultaneously triggered the AKT/HIF-1α and NF-κB signaling pathways in oral cancer cells.
HDGF has been demonstrated to bind directly to surface nucleolin (NCL) and activate the NCL/PI3K/AKT axis in hepatoma cells during liver carcinogenesis [22]. Here, we applied a neutralizing antibody against nucleolin that was able to abolish the HDGF-stimulated phosphorylation levels of AKT, IκB and NF-κB p65 and the HDGF-stimulated protein levels of HIF-1α VEGF. These results suggest that surface nucleolin plays a pivotal role in mediating the HDGF-induced AKT/HIF-1α signaling and NF-κB signaling pathways, ultimately modulating VEGF expression in oral cancer cells.
Studies have shown that the binding of both STAT3 and HIF-1α to the VEGF promoter is essential for the maximum transcription of VEGF mRNA under hypoxia [42]. STAT3 signaling is required for VEGF- and PI3K/AKT-mediated HIF-1α expression. Blocking STAT3 abolished both HIF-1 and VEGF expression [43]. However, whether STAT3 contributes to HIF-1 expression/activity independently of AKT remains to be determined. Here, HDGF did not modulate the phosphorylation levels of the transcription factor STAT3 even at a high dose (100 ng/ml), suggesting that STAT3 activation was not modulated by recombinant HDGF in SCC4 cells. This result implied that HDGF-stimulated VEGF expression might act through alternative AKT/HIF-1α and NF-κB signaling pathways but not the STAT3 pathway in oral cancer cells.
To confirm the signaling pathway between HDGF and VEGF, a HIF-1α inhibitor (chetomin) and an NF-κB inhibitor (Bay 11–7082) were used. Mild upregulation in VEGF mRNA level, protein level, and secreted protein level were noted in the chetomin alone group. In the cotreatment group of HDGF and chetomin, the VEGF levels were reduced without further enhancement that suggested chetomin was able to eliminate HDGF-induced VEGF expression pathway in SCC4 cells. In the other hand, Bay 11–7082 has some suppressive effects on the VEGF mRNA level, protein level, and secreted protein level. The HDGF cotreatment with Bay 11–7082 was able to upregulate the VEGF mRNA and protein levels even under the possible suppression caused by Bay 11–7802. Therefore, the current study revealed the pivotal role of HIF-1α signaling in the HDGF-mediated upregulation of VEGF (Fig. 5d). There are some limitations to this study. We only analyzed three common signal transduction pathways for the possible regulatory VEGF pathways by Western blotting. Western blotting can only identify a single protein-protein interaction but not for weak or transient interactions and evaluate numerous pathways will be time-consuming. However, the two cell lines in the current study showed consistent results. Also, we validated the correlation between HDGF and VEGF in the clinical data.
In the current study, the cohort of patients with oral cancer who received radical treatment was selected to assess the prognostic value of VEGF immunohistochemical staining. High expression of VEGF was strongly correlated with HDGF-N expression, primary T stage, nodal status and histological grade. In a previous study, high expression of HDGF seemed limited to locally aggressive behavior only [20]. Here, high expression of VEGF was associated with a greater likelihood of both local and distant recurrence. VEGF is capable of increasing vascular permeability in both blood and lymphatic vessels and helps cancer cells enter lymphatic or blood vessels and become established in both local lymph nodes and at distant sites [4, 44]. In multivariate analysis, high expression of VEGF was the most significant predictor for all survival endpoints (LRFS, DMS, and DSS).
Antiangiogenic agents can potentially modulate the tumor microenvironment and induce radiosensitivity and chemosensitivity. Using antiangiogenic agents alone or in combination with conventional therapies in oral cancer is a promising new approach [45]. In the current study, HDGF activated HIF-1α and then induced VEGF expression, leading to poor disease control. The combination of antiangiogenic agents and HIF-1 inhibitors might be efficacious, as antiangiogenic agents would cut off the tumor’s blood supply, and HIF-1α inhibitors could potentiate the effect of antiangiogenic agents and reduce the potential for the development of drug resistance [46]. Therefore, the HDGF/nucleolin/HIF-1α/VEGF axis is a very attractive target for oral cancer treatment.
Conclusions
In summary, this study is the first to report the association between HDGF and VEGF and prognosis in oral cancer. Our study postulated a new pathway in which HDGF activated HIF-1α and the NF-κB signaling pathway and then increased VEGF expression through binding to membrane NCL under normoxic conditions. The HDGF/HIF-1α/VEGF axis is important for developing future therapeutic strategies.
Availability of data and materials
All data analyzed during this study are included in this published article.
Abbreviations
- DSS:
-
Disease-specific survival
- ELISA:
-
Enzyme-linked immunosorbent assay
- HDGF:
-
Hepatoma-derived growth factor
- HDGF-C:
-
Cytoplasmic HDGF
- HDGF-N:
-
Nuclear HDGF
- LRFS:
-
Local recurrence-free survival
- MFS:
-
Metastasis-free survival
- Post-OP CCRT:
-
Postoperative concurrent chemoradiotherapy
- TCGA:
-
The Cancer Genome Atlas
- TMA:
-
Tissue microarray
- VEGF:
-
Vascular endothelial growth factor
References
Funk GF, Karnell LH, Robinson RA, Zhen WK, Trask DK, Hoffman HT. Presentation, treatment, and outcome of oral cavity cancer: a National Cancer Data Base report. Head Neck. 2002;24(2):165–80.
Brown JS, Shaw RJ, Bekiroglu F, Rogers SN. Systematic review of the current evidence in the use of postoperative radiotherapy for oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 2012;50(6):481–9.
Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82(1):4–6.
Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324(1):1–8.
Sauter ER, Nesbit M, Watson JC, Klein-Szanto A, Litwin S, Herlyn M. Vascular endothelial growth factor is a marker of tumor invasion and metastasis in squamous cell carcinomas of the head and neck. Clin Cancer Res. 1999;5(4):775–82.
Boonkitticharoen V, Kulapaditharom B, Leopairut J, Kraiphibul P, Larbcharoensub N, Cheewaruangroj W, Chintrakarn C, Pochanukul L. Vascular endothelial growth factor a and proliferation marker in prediction of lymph node metastasis in oral and pharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2008;134(12):1305–11.
Vassilakopoulou M, Psyrri A, Argiris A. Targeting angiogenesis in head and neck cancer. Oral Oncol. 2015;51(5):409–15.
Sasaki H, Hoshi H, Hong YM, Suzuki T, Kato T, Sasaki H, Saito M, Youki H, Karube K, Konno S, et al. Purification of acidic fibroblast growth factor from bovine heart and its localization in the cardiac myocytes. J Biol Chem. 1989;264(29):17606–12.
Nakamura H, Izumoto Y, Kambe H, Kuroda T, Mori T, Kawamura K, Yamamoto H, Kishimoto T. Molecular cloning of complementary DNA for a novel human hepatoma-derived growth factor. Its homology with high mobility group-1 protein. J Biol Chem. 1994;269(40):25143–9.
Everett AD, Stoops T, McNamara CA. Nuclear targeting is required for hepatoma-derived growth factor-stimulated mitogenesis in vascular smooth muscle cells. J Biol Chem. 2001;276(40):37564–8.
Kishima Y, Yamamoto H, Izumoto Y, Yoshida K, Enomoto H, Yamamoto M, Kuroda T, Ito H, Yoshizaki K, Nakamura H. Hepatoma-derived growth factor stimulates cell growth after translocation to the nucleus by nuclear localization signals. J Biol Chem. 2002;277(12):10315–22.
Hu TH, Huang CC, Liu LF, Lin PR, Liu SY, Chang HW, Changchien CS, Lee CM, Chuang JH, Tai MH. Expression of hepatoma-derived growth factor in hepatocellular carcinoma. Cancer. 2003;98(7):1444–56.
Yoshida K, Tomita Y, Okuda Y, Yamamoto S, Enomoto H, Uyama H, Ito H, Hoshida Y, Aozasa K, Nagano H, et al. Hepatoma-derived growth factor is a novel prognostic factor for hepatocellular carcinoma. Ann Surg Oncol. 2006;13(2):159–67.
Ren H, Tang X, Lee JJ, Feng L, Everett AD, Hong WK, Khuri FR, Mao L. Expression of hepatoma-derived growth factor is a strong prognostic predictor for patients with early-stage non-small-cell lung cancer. J Clin Oncol. 2004;22(16):3230–7.
Yamamoto S, Tomita Y, Hoshida Y, Takiguchi S, Fujiwara Y, Yasuda T, Doki Y, Yoshida K, Aozasa K, Nakamura H, et al. Expression of hepatoma-derived growth factor is correlated with lymph node metastasis and prognosis of gastric carcinoma. Clin Cancer Res. 2006;12(1):117–22.
Matsuyama A, Inoue H, Shibuta K, Tanaka Y, Barnard GF, Sugimachi K, Mori M. Hepatoma-derived growth factor is associated with reduced sensitivity to irradiation in esophageal cancer. Cancer Res. 2001;61(15):5714–7.
Yamamoto S, Tomita Y, Hoshida Y, Morii E, Yasuda T, Doki Y, Aozasa K, Uyama H, Nakamura H, Monden M. Expression level of Hepatoma-derived growth factor correlates with tumor recurrence of esophageal carcinoma. Ann Surg Oncol. 2007.
Everett AD, Narron JV, Stoops T, Nakamura H, Tucker A. Hepatoma-derived growth factor is a pulmonary endothelial cell-expressed angiogenic factor. Am J Physiol Lung Cell Mol Physiol. 2004;286(6):L1194–201.
Okuda Y, Nakamura H, Yoshida K, Enomoto H, Uyama H, Hirotani T, Funamoto M, Ito H, Everett AD, Hada T, et al. Hepatoma-derived growth factor induces tumorigenesis in vivo through both direct angiogenic activity and induction of vascular endothelial growth factor. Cancer Sci. 2003;94(12):1034–41.
Lin YW, Li CF, Chen HY, Yen CY, Lin LC, Huang CC, Huang HY, Wu PC, Chen CH, Chen SC, et al. The expression and prognostic significance of hepatoma-derived growth factor in oral cancer. Oral Oncol. 2012;48(7):629–35.
Lin Y, Li C, Lin L, Yen C, Tai M: Expression of Hepatoma-derived Growth Factors is Correlated with Prognosis of Oral Cancer and Vascular Endothelial Growth Factor. International Journal of Radiation Oncology • Biology • Physics 2009, 75(3):S533.
Chen SC, Hu TH, Huang CC, Kung ML, Chu TH, Yi LN, Huang ST, Chan HH, Chuang JH, Liu LF, et al. Hepatoma-derived growth factor/nucleolin axis as a novel oncogenic pathway in liver carcinogenesis. Oncotarget. 2015;6(18):16253–70.
Liu GS, Wu JC, Tsai HE, Dusting GJ, Chan EC, Wu CS, Tai MH. Proopiomelanocortin gene delivery induces apoptosis in melanoma through NADPH oxidase 4-mediated ROS generation. Free Radic Biol Med. 70:14–22.
Li SH, Li CF, Sung MT, Eng HL, Hsiung CY, Huang WW, Lin CN, Yu SC, Huang HY. Skp2 is an independent prognosticator of gallbladder carcinoma among p27(Kip1)-interacting cell cycle regulators: an immunohistochemical study of 62 cases by tissue microarray. Mod Pathol. 2007;20(4):497–507.
Chu TH, Chan HH, Kuo HM, Liu LF, Hu TH, Sun CK, Kung ML, Lin SW, Wang EM, Ma YL, et al. Celecoxib suppresses hepatoma stemness and progression by up-regulating PTEN. Oncotarget. 2014;5(6):1475–90.
Li SZ, Zhao YB, Cao WD, Qu Y, Luo P, Zhen HN, Chen XY, Yan ZF, Fei Z. The expression of hepatoma-derived growth factor in primary central nervous system lymphoma and its correlation with angiogenesis, proliferation and clinical outcome. Med Oncol. 2013;30(3):622.
Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6(3):203–8.
Goradel NH, Asghari MH, Moloudizargari M, Negahdari B, Haghi-Aminjan H, Abdollahi M. Melatonin as an angiogenesis inhibitor to combat cancer: mechanistic evidence. Toxicol Appl Pharmacol. 2017;335:56–63.
Ridiandries A, Tan JT, Bursill CA. The Role of CC-Chemokines in the Regulation of Angiogenesis. Int J Mol Sci. 2016:17(11).
Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6(4):389–95.
Kung ML, Tsai HE, Hu TH, Kuo HM, Liu LF, Chen SC, Lin PR, Ma YL, Wang EM, Liu GS, et al. Hepatoma-derived growth factor stimulates podosome rosettes formation in NIH/3T3 cells through the activation of phosphatidylinositol 3-kinase/Akt pathway. Biochem Biophys Res Commun. 2012;425(2):169–76.
Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci U S A. 2000;97(4):1749–53.
Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci. 2011;4:51.
Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–32.
Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16(9):4604–13.
Levy AP, Levy NS, Wegner S, Goldberg MA. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995;270(22):13333–40.
Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci U S A. 1997;94(9):4273–8.
Tong Q, Zheng L, Lin L, Li B, Wang D, Huang C, Li D. VEGF is upregulated by hypoxia-induced mitogenic factor via the PI-3K/Akt-NF-kappaB signaling pathway. Respir Res. 2006;7:37.
Gorlach A, Bonello S. The cross-talk between NF-kappaB and HIF-1: further evidence for a significant liaison. Biochem J. 2008;412(3):e17–9.
van Uden P, Kenneth NS, Rocha S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem J. 2008;412(3):477–84.
Chen YT, Chen FW, Chang TH, Wang TW, Hsu TP, Chi JY, Hsiao YW, Li CF, Wang JM. Hepatoma-derived growth factor supports the antiapoptosis and profibrosis of pancreatic stellate cells. Cancer Lett. 2019;457:180–90.
Gray MJ, Zhang J, Ellis LM, Semenza GL, Evans DB, Watowich SS, Gallick GE. HIF-1alpha, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene. 2005;24(19):3110–20.
Xu Q, Briggs J, Park S, Niu G, Kortylewski M, Zhang S, Gritsko T, Turkson J, Kay H, Semenza GL, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24(36):5552–60.
Kyzas PA, Cunha IW, Ioannidis JP. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: a meta-analysis. Clin Cancer Res. 2005;11(4):1434–40.
Hsu HW, Wall NR, Hsueh CT, Kim S, Ferris RL, Chen CS, Mirshahidi S. Combination antiangiogenic therapy and radiation in head and neck cancers. Oral Oncol. 2014;50(1):19–26.
Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8(4 Suppl):S62–7.
Acknowledgements
Part of this manuscript was presented at the 59th Annual Meeting of the American Society for Radiation Oncology, San Diego, September 2017. This study was supported by National Sun Yat-sen University-Kaohsiung Veterans General Hospital Joint Research Center, Kaohsiung, Taiwan.
Funding
This work was supported in parts by grants from Kaohsiung Veterans General Hospital, Taiwan (VGHKS 106–112 & VGHNSU108-001) for the design of the study and collection, analysis, and interpretation of data; the Ministry of Science and Technology, Taiwan (MOST 107–2320-B-110-003; 108-2314-B-110-003-MY2) for collection, analysis, and interpretation of data; Kaohsiung Armed Forces General Hospital, Taiwan (102–5) for analysis and interpretation of data and in writing.
Author information
Authors and Affiliations
Contributions
Study concepts: YWL, CCL, MHT. Study design: MHT, YWL. Data acquisition: STH, JCW, THC. Quality control of data and algorithms: STH, JCW, THC, SCH. Data analysis and interpretation: YWL, STH. Statistical analysis: JCW, THC, SCH. Manuscript preparation: YWL, CCL. Manuscript editing: CCL, MHT. Manuscript review: YWL, STH, CCL, MHT. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Board (IRB) at Chi Mei Medical Center approved this study (IRB09703–001). The IRB waived the need for written informed consent from the participants because this was a retrospective review study. No ethics approval is required for these purchased cell lines (SCC4 and SAS) from the bioresource centers.
Consent for publication
Not applicable.
Competing interests
All the authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1:
Table S1. Univariate log-rank analyses of HDGF and VEGF. Figure S1. Effect of HDGF on VEGF expression in oral cancer cells. Figure S2. Effect of HDGF on VEGF expression in oral cancer cells. Figure S3. HDGF triggered AKT/HIF-1α/NF-κB signaling in SCC4 oral cancer cells. Figure S4. The Neutralizing antibody against nucleolin eliminates HDGF-stimulated AKT/HIF-1α/NF-κB/VEGF signaling in SCC4 oral cancer cells.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lin, YW., Huang, ST., Wu, JC. et al. Novel HDGF/HIF-1α/VEGF axis in oral cancer impacts disease prognosis. BMC Cancer 19, 1083 (2019). https://doi.org/10.1186/s12885-019-6229-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-019-6229-5