Abstract
Background
This study aimed to evaluate the feasibility and prognostic accuracy of incorporating the number of positive lymph nodes (PLN) into the TNM staging system for non-small cell lung cancer (NSCLC) patients.
Methods
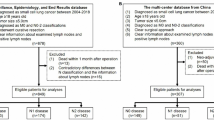
We screened a total of 9539 patients with resected stage IA-IIIB non-small cell cancer between 2010 and 2015 from SEER database. The chi-square test was used to compare patient baseline characteristics and the X-tile model was applied to determine cut-off values for the number of PLN (nN). The X-tile model was used to screen three different cut-off values including nN = 0, nN1–3 and nN4-. Univariate and multivariate Cox proportional hazards regression models were used to analyze the influence of different variables on overall survival (OS). Kaplan-Meier and log-rank test were used to compare survival differences.
Results
Based on the nN cutoffs, we conducted the univariate and multivariate Cox proportional hazards regression. The result showed that nN stage was a significant prognostic factor affecting patients' OS (all P < 0.001). We reclassified the seventh edition TNM stages of the enrolled patients with stage IA-IIIB NSCLC according to the 5-year OS rate. Hypothesized TNM substage based on the location and the number of PLN was further calculated. Then we drew survival curves for each substage, including for the current TNM stage and the hypothesized TNM stage. From the comparison of survival curves, we found that the survival curve of each substage of the hypothesized TNM classification was proportional and well distributed compared with the current TNM classification (P < 0.001).
Conclusion
Revised TNM staging integrating locational pN stage and numerical nN stage was a more accurate prognostic determinant in patients with NSCLC.
Similar content being viewed by others
Background
Lung cancer is the leading cause of malignancy-related deaths in males and is second among female cancer patients worldwide [1, 2]. Non-small cell cancer (NSCLC) makes up approximately 85% of all lung cancers [3, 4]. The tumor-node-metastasis (TNM) staging system is an essential prognostic assessment tool for decision-making about the most appropriate stage-specific therapeutic strategy for NSCLC patients [5].
An accurate assessment of lymph node involvement is essential for the diagnosis and treatment of NSCLC, including the location of the metastatic lymph nodes and the number of positive lymph nodes. Some studies [6, 7] have demonstrated that the number of positive lymph nodes is an important prognostic factor for resected NSCLC. Nwogu CE et al. [8] demonstrated that both the resection of more lymph nodes (LNs) and low ratios of positive LNs to total examined LNs are associated with superior patient survival after NSCLC resection independent of age, sex, grade, tumor size and disease stage. Wei S et al. [9] demonstrated that the nN category was a better prognostic determinant than location-based pN stage classification.
The number of positive lymph nodes is considered as part of the TNM staging system in breast, gastric, and colorectal cancer [10, 11]. However, the seventh edition [12] and the revised eighth edition [13] TNM staging system defined nodal status depending only on the location of the metastatic lymph nodes and does not involve the number of positive lymph nodes. To the best of our knowledge, the number of positive lymph nodes has not been tested as the one criterion of TNM staging.
Accurate staging at the time of initial diagnosis is very important for patients with NSCLC to determine prognosis and guide treatment [14, 15]. The current seventh edition TNM staging system was published in 200912 and the revised eighth edition TNM staging system was published in 2015 [13]. TNM stages include three components: primary tumor (T), nodal status for metastasis (N), and metastasis at the distant organs (M). The eighth TNM staging system included notable changes in the T and M descriptors and in nodal map, while the N descriptor remained the same as in the previous version.
Nodal status is a significant factor in staging, including the location of the metastatic LNs and the number of metastatic LNs, and should predict survival of NCSLC patients after surgery. The nodal status of the current TNM staging assesses tumor burden in the regional hilar and mediastinal nodes [16] and is defined as N0 (no nodal involvement), N1 (peribronchial, interlobar, hilar node involvement), N2 (ipsilateral nodal involvement), N3 (contralateral mediastinal, contralateral hilar or supraclavicular nodal involvement), depending on the location of the metastatic lymph nodes. The number of PLNs has been shown to be a prognostic factor for resected NSCLC [9, 17,18,19]. An accurate PLN metastasis assessment is crucial in determining treatment, as the prognosis of lung cancer is directly proportional to the PLN.
Saji H et al. [17] demonstrated that resection of ≥10 LNs influenced survival and that the number of involved LNs (four and more) was a strong independent prognostic factor in NSCLC. In another relevant study [18], they showed that a combined anatomically based pN stage classification and numerically based nN stage classification was a more accurate prognostic determinant in patients with NSCLC, especially in the prognostically heterogeneous pN1 and pN2 cases. Similarly, other research [19,20,21,22] also showed that the number of lymph nodes and lymph node ratio were important in TNM classification for NSCLC. Furthermore, Asamura H and his colleagues [23] recommended that physicians recorded the number of metastatic lymph nodes (or stations) to further classify N category using new descriptors, such as N1a, N1b, N2a, N2b, and N3, for further testing in the other study.
In NSCLC, similar to colorectal, breast and bladder cancer [24,25,26], the number of positive lymph nodes has been proven to be a prognostic factor and impacts disease survival [21, 27, 28]. In solid tumors, the number of metastatic lymph nodes has been included in the TNM staging system, such as breast, gastric, and colorectal tumors. However, current pN staging of NSCLC depends only on the location of the metastatic lymph nodes.
In this study, we retrospectively evaluated the association between the number of PLN and the prognosis of patients with resected stage IA-IIIB NSCLC and compared this hypothesized TNM staging system with the current TNM stage classification in terms of prognostic accuracy.
Methods
Data source
The SEER Program (www.seer.cancer.gov), a total of 18 population-based cancer registries in the United States (USA), is published annually by the Data Analysis and Interpretation Branch of the National Cancer Institute, MD, USA [29]. The SEER*Stat software (SEER*Stat 8.3.5) was used to identify appropriate patient data. Using this software and according to American Joint Committee on Cancer (AJCC) seventh edition TNM stage, we screened a total of 9539 patients with resected stage IA-IIIB NSCLC between 2010 and 2015. The inclusion criteria was as follows: patients diagnosed IA-IIIB NSCLC and had surgical resection without distant metastasis as well as only one primary tumor and active follow-up. Patients with stage IV NSCLC, incomplete resection, unknown TNM stage, unknown clinical information and benign tumor were excluded. In addition, patients with missing values for positive lymph nodes counts and clinical features were also excluded.
Ethics statement
This study was mainly based on the SEER database and was conducted in compliance with the Helsinki Declaration. The informed consent was not required because personal identifying information was not involved. This study was approved by the ethics committee of the Shandong Cancer Hospital and Institute.
Statistical analysis
For all patients, the following variables were analyzed: age, race, sex, histology, pT stage, pN stage and No. of PLN. OS was regarded as the primary endpoint in this study. Other endpoints were the 1-year, 2-year, 5-year OS rate, especially 5-year OS rate. Differences of patient baseline characteristics were analyzed using the chi-square test. Kaplan-Meier analysis was used to draw the survival curves. Differences in survival were examined using the log-rank test. Additionally, the X-tile model was applied to determine the cut-off values of the number of PLN. Univariate and multivariate Cox proportional hazards regression models were used to evaluate the prognostic factors on OS for NSCLC patients. All statistical analyses were made using Statistical Product and Service Solutions (SPSS) 22.0 software package. All statistical P values were 2-sided and P < 0.05 was considered statistically significant.
Results
Patients characteristics
A total of 9539 patients with resected stage IA-IIIB NSCLC from the SEER database were included in our analysis. Most patients were diagnosed at older than 65-year-old (62.5%) and belonged to white race (84.5%). Adenocarcinoma was the main pathology (63.2% vs. 36.8%). According to AJCC stage, patients with T1, 2, 3, 4 stage tumors were 36.2, 43.5, 15.7 and 4.6%, respectively. In addition, patients with pN0, 1, 2, 3 stage were 65.5, 19.4, 14.9 and 0.2%, respectively. Overall, 66.9% patients had no positive LNs and 33.1% patients had more than one PLN. The baseline characteristics of patients were listed in Table 1.
Current TNM staging and survival
Stages were classified according to the seventh TNM staging system based on clinical stage. Survival curves were measured using the Kaplan-Meier and log-rank test to compare survival differences between different stages. Survival analysis was performed according to different T category, N category, and stage group in each substage. The 1-, 2-, 5-year survival of different substages of the current TNM stage were compared. It was found that the 5-year OS rate of T2bN1M0 in stage IIB was better than T1aN1M0 in stage IIA; T1aN2M0 in stage IIIA was better than T3N0M0 in stage IIB; T4N2M0 in stage IIIB was better than T2aN2M0 as well as T4N1M0 in stage IIIA.The specific information about 1-, 2-, 5-year OS rate of the current TNM substages is shown in Fig. 1a.
Finally, survival curves were drawn for each substage of the current TNM stage. We found that partial survival curve of stage IIB was better than that of stage IIA and the survival of stage IIIB was better than that of stage IIIA. Each substage survival curve was shown in Fig. 1b.
So, improvements to the TNM staging system from the view of survival prognosis should be considered.
Cut-off determination for No. of PLN and survival
The cut-off values of nN were determined by the X-tile model. The univariate and multivariate Cox proportional hazards regression model were used to evaluate the prognostic value of baseline characteristics. Using the X-tile model, patients were classified into three nN categories: nN0, no LN metastasis; nN1–3, metastasis in one to three PLNs; and nN4-, metastasis in four or more LNs (Fig. 2a, b). Six thousand three hundred eighty-three patients (66.91%) were stage nN0, 2128 patients (22.31%) were stage nN1–3 and 1028 patients (10.78%) were stage nN4-. More specific information about patients with stage nN was shown in Fig. 2c.
Optimal threshold of No. of PLN count for OS as determined by the X-tile model. a: X-tile plots based on No. of PLN. b: Optimal cut-off point is shown in the blue (No. of PLN = 0), gray (1 ≤ No. of PLN ≤ 3) and violet panel(No. of PLN ≥ 4) for each PLN cutoff respectively. c: specific information about patients with stage nN, blue: 6383 patients (66.91%) with nN0; gray:2128 patients (22.31%) with nN1–3; violet panel: 1028 patients (10.78%) with nN4–61; yellow: a total of 9539 patients
Basing on the cut-off values of nN, univariable and multivariable analysis were completed. The results revealed that age, race, sex, histology, pT stage, nN stage were independent prognostic factors affecting patients' OS (all P < 0.05). In multivariable analysis, we found that nN stage was an independent and significant prognostic factor on OS for patients with resected stage IA-IIIB NSCLC (nN1–3 VS. nN0: HR = 1.728; 95%CI: 1.571–1.901; P < 0.001; nN4- VS. nN0: HR = 2.440; 95% CI: 2.184–2.727; P < 0.001) (Table 2).
Current TNM staging system and the hypothesized TNM staging system
Then, validation of the nN category in terms of the 5-year OS rate for each pathologic tumor (pT) category was executed (Fig. 3a). According to tendency toward the deterioration of the 5-year OS rate, TNM stage was divided based on the location (pN stage) and the number (nN stage) of positive lymph nodes and obtained the hypothesized TNM stages (Fig. 3b).
a: 5-year OS rate of different substages combined nN stage in current TNM staging system. b: 5-year OS rate of different substages combined nN stage according to tendency toward deterioration in hypothesized TNM staging system. c: Subclassifications of the hypothesized TNM staging system and the comparison between 1-, 2-, 5-year OS rate of stage IA-IIIB
The number of stage pN3 cases in the database were too few so we ignored this population. Combined nN stage and pN stage in hypothesized TNM staging, stage II was re-subdivided into IIA, IIB and stage III into IIIA, IIIB. The main changes compared with the classic TNM staging system included reclassification of T2aN1M0 to stage IIB from stage IIA, T1 N2(1–3)M0 and T2aN2(1–3)M0 to stage IIB from IIIA, T4 N2(1–3)M0 to stage IIIA from stage IIIB. Other changes included reclassification of T2bN1(4-)M0 to stage IIIB from stage IIIA and T3 N1(4-)M0, T3N2M0, T4 N1(4-)M0 to stage IIIB from stage IIIA (Fig. 3c).
Comparison of survival between different TNM staging system
Survival curves were measured using the Kaplan-Meier and compared by log-rank test. A survival curve was drawn for each substage IA - IIIB with the current TNM classifications (Fig. 4a) and the hypothesized classifications (Fig. 4b), respectively. We found that the hypothesized TNM stages were proportional and well distributed among the survival curves. These results revealed that each survival curve (P < 0.001) of the hypothesized subclassification had significant tendency and proportional compared with the survival curve (P < 0.001) of the current TNM subclassifications.
Discussion
In the present study, 1-, 2-, 5-year OS rate were the endpoints. Using the X-tile models, we classified involved the number of PLN into the three nN categories as follows: nN0, no PLN metastasis; nN1–3, metastasis in one to three PLNs; and nN4-, metastasis in four or more PLNs. The outcomes of multivariable analysis had shown that 1 ≤ No. of PLN ≤ 3 and No. of PLN ≥ 4 were independent factors associated with OS (all P < 0.001) in patients with stage IA - IIIB NSCLC after surgery. We compared the 1-, 2-, 5-year OS rate for different substages of the current TNM stages, respectively. From this comparison, we found that the current TNM staging system was irrational to guide clinical treatment.
In this study, there were only 20 cases with stage pN3 which accounted for 0.2% of the total patient population. So, we ignored this population and only examined those with stage pN1 and pN2 as the focus of our research. According to nN category and 5-year OS rate, we re-divided the TNM stages and obtained the hypothesized TNM stages. The main changes compared with the current TNM stages included the reclassification of T2aN1M0 to stage IIB from stage IIA, T1 N2(1–3)M0 and T2aN2(1–3)M0 to stage IIB from IIIA, T4 N2(1–3)M0 to stage IIIA from stage IIIB and reclassification of T2bN1(4-)M0 to stage IIIB from stage IIIA and T3 N1(4-)M0, T3N2M0, T4 N1(4-)M0 to stage IIIB from stage IIIA. Survival curves were drawn for both staging system. A comparison showed that the patients with the hypothesized TNM stages had better OS than those with classic TNM staging, although both of P < 0.001.
For patients with stage IA-IIIA NSCLC, the first choice treatment is curative tumor resection via surgery [30, 31]. Postoperative chemotherapy and radiotherapy have improved outcomes for these patients and decreased the risk of locoregional recurrence [32,33,34]. However, Andre F et al. [35] have reported that many patients who have N2+ disease are a heterogeneous group. And the treatment should be individualized for these patients. Surgery alone may be a more limited for patients with stage IIIA-N2 NSCLC [36]. Chemotherapy and radiation are main treatment methods for these patients. In this study, patients with pN2 NSCLC who account for 14.9% were treated with surgery from SEER database. We analyzed the impact of these patients on staging and we did not analyze those patients who did not undergo surgical treatment. This may have an impact on staging system. So, we need to validate this result in future studies.
In addition, Scagliotti GV et al. [37] have reported that patients with stage IIA-IIIB NSCLC could be benifit from neoadjuvant treatment including preoperative chemotherapy, preoperative radiotherapy and targeted treatment. Among stage IIIA-IIIB patients, we screened from the database, neoadjuvant therapy may or may not be accepted. However, due to the limitation of the database, we could not obtain information on neoadjuvant therapy for stage IIIA and IIIB patients and these data may have an impact on clinical prognosis. Even if this information is not available, we could reflect some problems by incorporating the number of lymph nodes into the revised staging compared with conventional staging.
However, this study has any other limitations that should be noted. First, this was a retrospective study. Moreover, it was difficult for physicians to evaluate the number of positive lymph nodes at the time of diagnosed NSCLC. Some other variables, including smoking history, type of surgery, other treatment affecting the prognosis were not included in the analysis. In addition, all data originated from SEER database according to the seventh edition TNM staging system rather than the eighth edition TNM staging system. This may have some influence on the final results. So, the results require further large-scale prospective clinical study to confirm these recommendations.
Conclusion
This study demonstrated that hypothesized TNM stage based pN stage and nN stage could more accurately reflect survival for patients with NSCLC. If applied, these results may guide clinicians and surgeon in choose more appropriate oncological treatments for NSCLC but further large-scale prospective clinical studies are required to confirm these recommendations.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AJCC:
-
American Joint Committee on Cancer
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- LNs:
-
Lymph nodes
- nN:
-
Number of positive lymph nodes
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PLN:
-
Positive lymph nodes
- pN:
-
Pathological lymph nodes
- pT:
-
Pathological tumor
- SEER:
-
Surveillance Epidemiology and End Results
- TNM:
-
Tumor-node-metastasis
References
Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. https://doi.org/10.1007/978-3-319-24223-1_1.
Jernal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52(1):23–47.
Le Péchoux C. Role of postoperative radiotherapy in resected non-small cell lung cancer: a reassessment based on new data. Oncologist. 2011;16(5):672–81.
Ihde DC, Minna JD. Non-small cell lung cancer: part I. biology, diagnosis and staging. Curr Probl Cancer. 1991;15:61–104.
Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC lung Cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung Cancer. J Thorac Oncol. 2016;11:39–51.
Fukui T, Mori S, Yokoi K, et al. Significance of the number of positive lymph nodes in resected non-small cell lung cancer. J Thorac Oncol. 2006;1:120–5.
Lee JG, Lee CY, Park IK, et al. Number of metastatic lymph nodes in resected non-small cell lung cancer predicts patient survival. Ann Thorac Surg. 2008;85:211–5.
Nwogu CE, Groman A, Fahey D, Yendamuri S, Dexter E, Demmy TL, Miller A, Reid M. Number of lymph nodes and metastatic lymph node ratio are associated with survival in lung cancer. Ann Thorac Surg. 2012;93(5):1614–9. https://doi.org/10.1016/j.athoracsur.2012.01.065 discussion 1619-20 Epub 2012 Mar 20.
Wei S, Asamura H, Kawachi R, Sakurai H, Watanabe S-i. Which is the better prognostic factor for resected non-small cell lung Cancer: the number of metastatic lymph nodes or the currently used nodal stage classification? J Thorac Oncol. 2011;6(2):310–8. https://doi.org/10.1097/JTO.0b013e3181ff9b45
Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumours. 7th ed. Oxford: Wiley-Blackwell; 2009. p. 181–93. Google Scholar
Karpeh MS, Leon L, Klimstra D, Brennan MF. Lymph node staging in gastric cancer: is location more important than number? An analysis of 1,038 patients. Ann Surg. 2000;232(3):362–71.
Goldstraw P, Crowley J, Chansky K, et al. The IASLC lung Cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–14.
Nicholson AG, Chansky K, Crowley J, Beyruti R, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the Clinical and Pathologic Staging of Small Cell Lung Cancer in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11(3):300–11. https://doi.org/10.1016/j.jtho.2015.10.008.
Rami-Porta R, Bolejack V, Giroux DJ, Chansky K, Crowley J, Asamura H, Goldstraw P. International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Board Members and Participating Institutions. The IASCL Lung Cancer Staging Project: The new database to inform the eight edition of the TNM classification of lung cancer. J Thorac Oncol. 2014;9:1618–24. https://doi.org/10.1097/JTO.0000000000000334.
Cerfolio RJ, Buddhiwardhan O, Bryant AS, et al. The accuracy of integrated PET/CT compared with dedicated PET alone for the staging of patients with non-small cell lung cancer. Ann Thorac Surg. 2004;78:1017–23.
Mirsadraee S, Oswal D, Alizadeh Y, Caulo A, van Beek E Jr. The 7th lung cancer TNM classification and staging system: review of the changes and implications. World J Radiol. 2012;4:128–34. https://doi.org/10.4329/wjr.v4.i4.128.
Saji H, Tsuboi M, Yoshida K, et al. Prognostic impact of number of resected and involved lymph nodes at complete resection on survival in non-small cell lung cancer. J Thorac Oncol. 2011;6(11):1865–71.
Saji H, Tsuboi M, Shimada Y, et al. A proposal for combination of total number and anatomical location of involved lymph nodes for nodal classification in non-small cell lung cancer. Chest. 2013;143:1618–25. https://doi.org/10.1378/chest.12-0750.
Ding X, Hui Z, Dai H, Fan C, Men Y, Ji W, Liang J, Lv J, Zhou Z, Feng Q, Xiao Z, Chen D, Zhang H, Yin W, Lu N, He J, Wang L. A proposal for combination of lymph node ratio and anatomic location of involved lymph nodes for nodal classification in non-small cell lung Cancer. J Thorac Oncol. 2016;11(9):1565–73. https://doi.org/10.1016/j.jtho.2016.05.004. Epub 2016 May 17.
Matsuguma H, Oki I, Nakahara R, Ohata N, Igarashi S, Mori K, Endo S, Yokoi K. Proposal of new nodal classifications for non-small-cell lung cancer based on the number and ratio of metastatic lymph nodes. Eur J Cardiothorac Surg. 2012;41(1):19–24.
Bria E, Milella M, Sperduti I, Alessandrini G, Visca P, Corzani F, Giannarelli D, Cerasoli V, Cuppone F, Cecere FL, Marchetti A, Sacco R, Mucilli F, Malatesta S, Guetti L, Vitale L, Ceribelli A, Rinaldi M, Terzoli E, Cognetti F, Facciolo F. A novel clinical prognostic score incorporating the number of resected lymph-nodes to predict recurrence and survival in non-small-cell lung cancer. Lung Cancer. 2009;66(3):365–71.
Dehing-Oberije C, De Ruysscher D, van der Weide H, Hochstenbag M, Bootsma G, Geraedts W, Pitz C, Simons J, Teule J, Rahmy A, Thimister P, Steck H, Lambin P. Tumor volume combined with number of positive lymph node stations is a more important prognostic factor than TNM stage for survival of non-small-cell lung cancer patients treated with (chemo)radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70(4):1039–44 Epub 2007 Sep 24.
Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer lung Cancer staging project: proposals for the revision of the N descriptors in the forthcoming 8th edition of the TNM classification for lung Cancer. J Thorac Oncol. 2015;10:1675–84.
Tepper JE, O’Connell MJ, Niedzwiecki D, et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol. 2001;19(1):157–63.
Weir L, Speers C, D’yachkova Y, Olivotto IA. Prognostic signifi cance of the number of axillary lymph nodes removed in patients with node-negative breast cancer. J Clin Oncol. 2002;20(7):1793–9.
Herr HW, Bochner BH, Dalbagni G, Donat SM, Reuter VE, Bajorin DF. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. J Urol. 2002;167(3):1295–8.
Darling GE, Allen MS, Decker PA, et al. Number of lymph nodes harvested from a mediastinal lymphadenectomy: results of the randomized, prospective American College of Sur geons oncology group Z0030 trial. Chest. 2011;139(5):1124–9.
Varlotto JM, Recht A, Nikolov M, Flickinger JC, Decamp MM. Extent of lymphadenectomy and outcome for patients with stage I nonsmall cell lung cancer. Cancer. 2009;115(4):851–8.
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2018 Sub (1975-2016) - Linked To County Attributes - Total U.S., 1969–2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission.
Ettinger DS, Wood DE, Akerley W, Bazhenova LA, Borghaei H, Camidge DR, Cheney RT, Chirieac LR, D’Amico TA, Demmy TL, Dilling TJ, Govindan R, Grannis FW Jr, Horn L, Jahan TM, Komaki R, Kris MG, Krug LM, Lackner RP, Lanuti M, Lilenbaum R, Lin J, Loo BW Jr, Martins R, Otterson GA, Patel JD, Pisters KM, Reckamp K, Riely GJ, Rohren E, Schild S, Shapiro TA, Swanson SJ, Tauer K, Yang SC, Gregory K, Hughes M. Non-small cell lung cancer, version 1.2015. J Natl Compr Cancer Netw. 2014;12(12):1738–61.
Van Schil PE, Balduyck B, De Waele M, Hendriks JM, Hertoghs M, Lauwers P. Surgical treatment of early-stage non-small-cell lung cancer. EJC Suppl. 2013;11(2):110–22.
Bradley JD, Paulus R, Graham MV, Ettinger DS, Johnstone DW, Pilepich MV, Machtay M, Komaki R, Atkins J, Curran WJ, Groupet RTO. Phase II trial of postoperative adjuvant paclitaxel/carboplatin and thoracic radiotherapy in resected stage II and IIIA non-small-cell lung cancer: promising long-term results of the radiation therapy oncology group-- RTOG 9705. J Clin Oncol. 2005;23(15):3480–7.
Hui Z, Dai H, Liang J, Lv J, Zhou Z, Feng Q, Xiao Z, Chen D, Zhang H, Yin W, Wang L. Selection of proper candidates with resected pathological stage IIIA-N2 non-small cell lung cancer for postoperative radiotherapy. Thorac Cancer. 2015;6(3):346–53.
Gomez DR, Komaki R. Postoperative radiation therapy for non-small cell lung cancer and thymic malignancies. Cancers (Basel). 2012;4(1):307–22.
Andre F, Grunenwald D, Pignon JP, Dujon A, Pujol JL, Brichon PY, et al. Survival of patients with resected N2 non-small-cell lung cancer: evidence for subclassification and implications. J Clin Oncol. 2000;18:2981–9.
Reif M, Socinski MA, Rivera MP. Evidence-based medicine in the treatment of non-small cell lung cancer. Clin Chest Med. 2000;21:107–20.
Scagliotti GV, Pastorino U, Vansteenkiste JF, et al. Randomized phase III study of surgery alone or surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non-small-cell lung cancer. J Clin Oncol. 2012;30:172–8.
Acknowledgements
Not applicable.
Funding
This study was supported jointly by National Natural Science Foundation of China (Grant no. 81603348), Special funds for Taishan Scholars Project (Grant no. tsqn201812149 ), Shandong Province key R & D Plan (Grant no. 2018GSF119014).
Author information
Authors and Affiliations
Contributions
X-L S in charge of analysis and wrote the article. J L, Z-X L and J-M L helped with acquisition and analysis of the data. H-Y W, the Corresponding author, was in charge of guidance of the design and analysis the whole research. H-Y W and X-L S were major contributor in the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We were approved by the National Cancer Institute to use patient data from the SEER database. Participants consent was not necessary because the study involved using a previously published identity database based on the SEER database.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Shang, X., Liu, J., Li, Z. et al. A hypothesized TNM staging system based on the number and location of positive lymph nodes may better reflect the prognosis for patients with NSCLC. BMC Cancer 19, 591 (2019). https://doi.org/10.1186/s12885-019-5797-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-019-5797-8