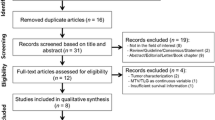
Abstract
Background
Glucose metabolism has been suggested as a therapeutic target in ovarian clear cell carcinoma (CCC). We attempted to clarify 18F-FDG PET/CT-based metabolic metrics in the recurrent ovarian CCC patients and their prognostic values.
Methods
Quantitative metabolic parameters included maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV) and total lesion glycolysis (TLG). Two different methods were employed for defining the threshold SUV to delineate MTV: 1) SUV of 2.5 (designated as MTV); 2) a fixed ratio including 40% (MTV40), 50% (MTV50) and 60% (MTV60) of SUVmax. The Kaplan-Meier model and Cox regression were used in survival analysis.
Results
Among the 35 patients, platinum-resistant recurrence accounted for 34.3% and the median progression-free survival was 13 months (range, 2–135). Fifteen (42.9%) patients presented with single tumor recurrence, while 51 recurrent lesions were identified, with the most common sites in pelvis (29.4%), followed by lymph node metastases (19.6%) and peritoneal carcinomatosis (15.7%). Except four patients with FDG-inavid tumor, the median SUVmax of the 31 patients with high glucose metabolic activity was 7.10 (range, 3.00–20.60). After a median follow-up of 36.5 months (range, 7–155), 22 patients (64.7%) were dead from disease. The median post-relapse survival (PRS) was 17 months (range, 4–126). Platinum-resistant recurrence, peritoneal carcinomatosis and high TLG60 proved to be negative predicators of overall survival after multivariate analysis.
Conclusions
TLG60, platinum-resistant recurrence and peritoneal carcinomatosis were independent negative predicators of overall survival. Whether patients with higher TLG60 required more aggressive treatment warranted further study.
Similar content being viewed by others
Background
Ovarian clear cell carcinoma (CCC) is a distinct histologic subtype, accounting for 5–25% of all epithelial ovarian cancer depending on geographic location [1, 2]. Ovarian CCC represents a great challenge due to its disease aggressiveness and chemotherapy resistance [1, 2]. To be worse, recurrent ovarian CCC is particularly chemoresistant [3]. The response rate to systemic therapy is less than 10% even in the setting of platinum-sensitive recurrence [3]. In two recent publications, biologic agents (sunitinib and cabozantinib) demonstrated minimal activity in the second- and third-line treatment of persistent or recurrent ovarian CCC [4, 5]. Different from other histologic subtype, ovarian CCC has its unique glucose metabolic activity, which might have a future clinical implication of targeting cancer-specific metabolism [6,7,8]. Thus, clarifying the features of metabolic activity and recurrence pattern in ovarian CCC might help to guide new treatment strategies tailored to ovarian CCC.
As a molecular imaging technique, 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is used to evaluate glucose metabolism and tumor distribution in different kinds of cancers including gynecologic malignancy [9]. It has been widely employed in detecting recurrence and restaging in ovarian cancer patients [10]. Several publications have examined the role of 18F-FDG PET/CT quantitative metrics in ovarian cancer, usually lumping different histologic subtypes together [10,11,12,13,14,15,16,17,18,19]. PET/CT-based metabolic parameters include maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV) and total lesion glycolysis (TLG). MTV refers to the estimated volume of tumor with increased tracer uptake while TLG is an estimation of summed metabolic activity inside MTV. In a previous study, metabolic variables at the time of first recurrence were proved to be significant predictors of post-relapse survival (PRS) in ovarian cancer patients [10]. Nevertheless, there has been no publication focusing on the clinical utility of PET/CT-based metabolic variables in patients with recurrent ovarian CCC.
The objective of the present study was to delineate the recurrence pattern of ovarian CCC. A secondary aim was to investigate the possible roles of PET/CT-based metabolic variables in survival prediction in patients with relapsed ovarian CCC.
Methods
Patients
After obtaining the approval by the institutional review board (SCCIRB-090371-2), we included all the ovarian CCC patients who received 18F-FDG PET/CT scan at first recurrence from 2008 to 2017. Patients had to fulfill the following criteria to be included into the study: 1) histologically confirmed diagnosis of ovarian CCC. Patients initially treated in outside hospital were required to have pathology consultation in our institution as a routine practice. 2) 18F-FDG PET/CT performed in our institution at the first relapse. Clinicopathological features were reviewed and retrieved. Data collection included age at first diagnosis, International Federation of Gynecology and Obstetrics (FIGO) stage [20], platinum-free interval, serum cancer antigen 125 (CA 125) and CA199 level at relapse, recurrent tumor location, peritoneal carcinomatosis, type of second-line treatment, and tumor status at the date of last contact. Patients were considered to have platinum-sensitive disease if the interval time was > 6 months from the completion of the last platinum based chemotherapy to disease recurrence. Progression-free survival (PFS) and overall survival (OS) was defined as the time interval from the date of the primary surgery to the date of first recurrence and death or last contact, respectively. Post-relapse survival (PRS) was calculated as the time interval from the date of diagnosis of relapsed disease to the date of death or last contact. Patients with incomplete follow-up information were excluded from the survival analysis. A total of 35 patients were included into the study.
18F-FDG PET/CT protocol and image analysis
The 18F-FDG PET/CT protocol was introduced specifically in our previous publication [18]. A gynecologic oncology dedicated nuclear medicine physician (Dr. Liu) interpreted the images retrospectively, who was blind to the clinicopathologic information. Standardized uptake value (SUV) is defined as [decay corrected activity (kBq) per milliliter of tissue volume]/[injected 18 F-FDG activity (kBq) per gram of body mass]. In line with our previous publication, SUVmax was calculated by placing a spheroid-shaped volume of interest within the primary ovarian tumor. MTV and SUVaverage were evaluated by drawing a contour of the ovarian tumor large enough to encase the tumor in the axial, coronal and sagittal section. In the current work, two methods were used for defining threshold SUV to delineate MTV: 1) a threshold of SUV of 2.5 (designated as MTV); 2) a fixed ratio including 40% (MTV40), 50% (MTV50) and 60% (MTV60) of SUVmax. The boundaries of voxels exceeding the defined threshold were automatically produced and those presenting an SUV greater than the threshold were incorporated to MTV measurement. TLG was calculated as MTV × SUVaverage. The highest SUVmax of all recurrent lesions was determined as SUVmax for one patient, while volumetric metrics (MTV/TLG) were required by summing up all the values of relapsed tumors. PET/CT-based metabolic variables were assessed in 31 patients, given that four (11.4%) presented with low glucose-uptake tumor.
Statistical analysis
Statistical Package for Social Science (SPSS) (Version 20.0, SPSS, Inc., Chicago, IL, USA) and GraphPad Prism (Version 6.0, GraphPad Software, Inc., La Jolla, CA, USA) were used for the analyses. Clinicopathological variables and PET/CT metrics were presented using descriptive statistics. Medians and ranges were used for continuous variables, while proportions were used for categorical data. Survival time was calculated using the Kaplan-Meier model, whereas Cox regression was performed for multivariate analysis. Variables with statistical significance on univariate analysis were included in the multivariate one. To evaluate the relationship between quantitative PET/CT variables and survival outcome, patients were dichotomized based on the median number. All P values reported were two tailed, and P < 0.05 was considered statistically significant.
Result
Patient characteristics and patterns of recurrence
Table 1 shows the clinicopathological characteristics of the 35 patients included in the study. Nearly a half of the patients (44.8%) presented with early-stage disease (FIGO I + II) at initial diagnosis. Overall, platinum-resistant recurrence accounted for 34.3%. The median progression-free survival was 13 months (range, 2–135). At recurrence, normal serum level of CA 125 and CA199 was noted in 17 (48.6%) and 22 (62.3%) patients, respectively. Fifteen (42.9%) patients presented with single tumor recurrence. In terms of treatment, 27 patients (77.1%) received secondary cytoreduction surgery (SCS) in our institution. One patient lost follow-up after SCS, thus excluded from the survival analysis. After a median follow-up time of 36.5 months (range, 7–155), 22 patients (64.7%) were dead from disease and 12 patients (35.3%) were still alive with disease.
A total of 51 recurrent lesions were identified in the 35 patients. The specific recurrent tumor distributions are listed in Table 2. The most common sites of disease were pelvis (n = 15, 29.4%), followed by lymph node metastases (n = 10, 19.6%) and peritoneal carcinomatosis (n = 8, 15.7%). There were six vaginal cuff recurrences. Parenchymal solid organ metastases were noted in lung (n = 2, 3.9%), liver (n = 2, 3.9%) and spleen (n = 1, 2.0%), respectively. It is worthy of mentioning that four patients (7.8%) had abdominal wall lesions.
We were particularly interested in the relapse pattern of patients with initially early-stage disease, which are presented in Table 3. The median progression-free survival was 20 months (range, 6–108). Overall, three (23%) patients developed platinum-resistant recurrences. Interestingly, a pelvic component of relapse was observed in more than half the patients (7/13, 53.8%) and five patients (5/13, 38.5%) had a solitary pelvic lesion. On the whole, eight patients (61.5%) had solitary lesions. The majority of the patients underwent secondary debulking surgery. The median PRS was 26.5 months (range, 6–126). The estimated 5-year survival was 59.4% for the entire cohort.
Glucose uptake quantification metrics and prognostic implication
Among 35 patients, four presented with low glucose-uptake tumor: two vaginal cuff lesions, one pelvic tumor and one hepatorenal recess tumor. Therefore, PET/CT-based metabolic parameters were calculated for a total of 31 patients, which is presented in Table 4. The median SUVmax was 7.10 (range, 3.00–20.60). The median values of metabolic parameters were used as cut-offs for subsequent survival analysis, which is illustrated in Table 5.
Platinum-resistant recurrence, peritoneal carcinomatosis and high TLG60 proved to be negative predicators of overall survival after multivariate analysis. In terms of post-relapse survival, only peritoneal carcinomatosis retained statistic significance on multivariate analysis. Figure 1 shows representative PET/CT images of two patients and Fig. 2 presents that patients with higher TLG60 had worse survival compared to those with lower level of TLG60.
Representative PET/CT images. a:The patient had vaginal cuff recurrence. The SUV max, MTV60 and TLG60 was 4.1, 0.98 mL and 3. 19 g, respectively. The overall survival (OS) and post-relapse survival (PRS) was 41 and 39 months, respectively. b:In another patient, the recurrent tumors were located in the abdominal wall and liver. The SUV max, MTV60 and TLG60 was 13.4, 9.03 mL and 82.76g, respectively. The OS and PRS was 12 and 6 months, respectively. Abbreviations: MTV: metabolic tumor volume; TLG: Total lesion glycolysis
Discussion
The study depicted the relapse pattern of a cohort of ovarian CCC patients treated in our institution. The median PFS was 13 months (range, 2–135) for the entire population and 20 months (range, 6–108) for patients with early-stage tumor. The most commonly reported site of recurrence was pelvis (29.4%), followed by lymph node (19.6%) and peritoneal carcinomatosis (15.7%). An even higher rate (53.8%) of pelvic recurrence was noted in patients with initially early-stage disease, which supports the concept that ovarian CCC has a predilection for pelvic failure in line with previous publications [21, 22]. Importantly but not surprisingly, nearly a half of the patients had normal level of serum CA125 at recurrence.
Publications assessing the clinical utility of 18F-FDG PET/CT particularly in ovarian CCC patients are limited and mainly focused on patients with primary disease [23]. Ovarian CCC tumor was usually assumed to be low FDG uptake. According to a Japanese study with small cases (n = 11), positive FDG accumulation was shown in 54.5% patients and the median SUVmax was 3.52 before primary surgery [23]. In our previous work (under submission), we evaluated the role of preoperative PET/CT-based metabolic variables in ovarian CCC patients at first diagnosis. We found that 90% (20/22) of the patients had high-FDG tumors and the median SUVmax was 7.25. In the present study, we specifically focused on the PET/CT imaging in patients with first relapse. Again, the majority patients had FDG-avid recurrent lesions and the median SUVmax was 7.10 (range, 3.00–20.60). Further, we assessed the prognostic implications of PET/CT-based metabolic parameters and found that higher TLG60 was negative predictor for overall survival. SUVmax represented the highest SUVmax of all recurrent tumors. However, its utility in representing the metabolic activity is incomplete [12]. On the contrary, TLG might better represent the tumor burden in that it combines both volumetric and metabolic information [10]. Based on the study, we found that patients with higher level of TLG60 at first relapse had worse overall survival with statistic significance. It is noteworthy that different threshold definitions have been used in delineating metabolic tumors and no consensus has ever been achieved. In published literature, three major criteria have been employed: the absolute (SUV 2.5), relative (a fixed ratio such as 40% of SUVmax) and background-related relative thresholds (mediastinal background SUV plus two standard deviations) [12, 24]. As an exploratory study, we used two methods including absolute and relative thresholds, with the hope of finding a more appropriate threshold method in ovarian CCC patients.
According to a review article with profound influence, reprogramming energy metabolism especially glucose metabolism is considered as an emerging hallmark of cancer [25]. Quite a few publications have evaluated the clinical implication of 18F-FDG PET/CT based quantitative variables in ovarian cancer, irrespective of histologic subtype [10,11,12,13,14,15,16,17]. Most studies including ours focused on the prognostic implication of metabolic volumetric parameters (MTV, TLG) in ovarian cancer [10, 11, 14, 15, 17]. Besides, Vargas and colleagues investigated the predictive value of metabolic tumor volume in optimal debulking of secondary cytoreductive surgery [15]. In summary, most studies confirm that ovarian cancer patients with higher metabolic volumetric variables tended to have worse survival [10,11,12,13,14,15,16,17]. We postulated that higher metabolic volume might be a potential surrogate biomarker for disease aggressiveness and warranted more aggressiveness treatment, which requires further study.
The present study has several limitations. Firstly, selection bias might be one important problem. The cases were included by searching both the inpatient medical record system (mainly for surgery patients) and the PET/CT database in nuclear department. This is partly the reason why most patients underwent secondary surgery. Those patients who were not candidates for surgery might be missed out. Secondly, the clinicopathological information was not complete given that some patients received primary surgery at outside hospital. Thirdly, the specific details of second-line chemotherapy were not presented in the study. A part of the patients received chemotherapy in local hospital due to several reasons. Therefore, cautions should be taken when interpreting the data of study.
Conclusion
In the study, we presented the relapse pattern of 35 ovarian CCC patients. TLG60, along with platinum-resistant recurrence and peritoneal carcinomatosis, proved to be negative predicators of overall survival after multivariate analysis. Whether patients with higher TLG60 required more aggressive treatment warranted further assessment.
Abbreviations
- 18F-FDG PET/CT:
-
18F-fluorodeoxyglucose positron emission tomography/computed tomography
- CCC:
-
Clear cell carcinoma
- MTV:
-
Metabolic tumor volume
- SUVmax:
-
Maximum standardized uptake value
- TLG:
-
Total lesion glycolysis
- FIGO:
-
The International Federation of Gynecology and Obstetrics
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- PRS:
-
Post-relapse survival
- CA:
-
Cancer antigen
- SCS:
-
Secondary cytoreduction surgery
References
Anglesio MS, Carey MS, Kobel M, Mackay H, Huntsman DG. Clear cell carcinoma of the ovary: a report from the first ovarian clear cell symposium, June 24th, 2010. Gynecol Oncol. 2011;121:407–15.
del Carmen MG, Birrer M, Schorge JO. Clear cell carcinoma of the ovary: a review of the literature. Gynecol Oncol. 2012;126:481–90.
Crotzer DR, Sun CC, Coleman RL, Wolf JK, Levenback CF, Gershenson DM. Lack of effective systemic therapy for recurrent clear cell carcinoma of the ovary. Gynecol Oncol. 2007;105:404–8.
Chan JK, Brady W, Monk BJ, et al. A phase II evaluation of sunitinib in the treatment of persistent or recurrent clear cell ovarian carcinoma: an NRG oncology/gynecologic oncology group study (GOG-254). Gynecol Oncol. 2018;150:247–52.
Konstantinopoulos PA, Brady WE, Farley J, Armstrong A, Uyar DS, Gershenson DM. Phase II study of single-agent cabozantinib in patients with recurrent clear cell ovarian, primary peritoneal or fallopian tube cancer (NRG-GY001). Gynecol Oncol. 2018;150:9–13.
Yamaguchi K, Mandai M, Oura T, et al. Identification of an ovarian clear cell carcinoma gene signature that reflects inherent disease biology and the carcinogenic processes. Oncogene. 2010;29:1741–52.
Mandai M, Amano Y, Yamaguchi K, Matsumura N, Baba T, Konishi I. Ovarian clear cell carcinoma meets metabolism; HNF-1beta confers survival benefits through the Warburg effect and ROS reduction. Oncotarget. 2015;6:30704–14.
Okamoto T, Mandai M, Matsumura N, et al. Hepatocyte nuclear factor-1beta (HNF-1beta) promotes glucose uptake and glycolytic activity in ovarian clear cell carcinoma. Mol Carcinog. 2015;54:35–49.
Amit A, Schink J, Reiss A, Lowenstein L. PET/CT in gynecologic Cancer: present applications and future prospects-a Clinician's perspective. PET Clin. 2010;5:391–405.
Kim CY, Jeong SY, Chong GO, et al. Quantitative metabolic parameters measured on F-18 FDG PET/CT predict survival after relapse in patients with relapsed epithelial ovarian cancer. Gynecol Oncol. 2015;136:498–504.
Chung HH, Kwon HW, Kang KW, et al. Prognostic value of preoperative metabolic tumor volume and total lesion glycolysis in patients with epithelial ovarian cancer. Ann Surg Oncol. 2012;19:1966–72.
Liao S, Lan X, Cao G, Yuan H, Zhang Y. Prognostic predictive value of total lesion glycolysis from 18F-FDG PET/CT in post-surgical patients with epithelial ovarian cancer. Clin Nucl Med. 2013;38:715–20.
Konishi H, Takehara K, Kojima A, et al. Maximum standardized uptake value of fluorodeoxyglucose positron emission tomography/computed tomography is a prognostic factor in ovarian clear cell adenocarcinoma. Int J Gynecol Cancer. 2014;24:1190–4.
Lee JW, Cho A, Lee JH, et al. The role of metabolic tumor volume and total lesion glycolysis on (1)(8)F-FDG PET/CT in the prognosis of epithelial ovarian cancer. Eur J Nucl Med Mol Imaging. 2014;41:1898–906.
Vargas HA, Burger IA, Goldman DA, et al. Volume-based quantitative FDG PET/CT metrics and their association with optimal debulking and progression-free survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery. Eur Radiol. 2015;25:3348–53.
Yamamoto M, Tsujikawa T, Fujita Y, et al. Metabolic tumor burden predicts prognosis of ovarian cancer patients who receive platinum-based adjuvant chemotherapy. Cancer Sci. 2016;107:478–85.
Gallicchio R, Nardelli A, Venetucci A, et al. F-18 FDG PET/CT metabolic tumor volume predicts overall survival in patients with disseminated epithelial ovarian cancer. Eur J Radiol. 2017;93:107–13.
Liu S, Feng Z, Jiang Z, et al. Prognostic predictive value of preoperative intratumoral 2-deoxy-2-(18F)fluoro-D-glucose uptake heterogeneity in patients with high-grade serous ovarian cancer. Nucl Med Commun. 2018. https://doi.org/10.1097/mnm.0000000000000861.
Liu S, Feng Z, Wen H, et al. (18)F-FDG PET/CT can predict chemosensitivity and proliferation of epithelial ovarian cancer via SUVmax value. Jpn J Radiol; 2018. https://doi.org/10.1007/s11604-018-0755-y.
FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int J Gynaecol Obstet. 2014;125:97–8.
Hoskins PJ, Le N, Gilks B, et al. Low-stage ovarian clear cell carcinoma: population-based outcomes in British Columbia, Canada, with evidence for a survival benefit as a result of irradiation. J Clin Oncol. 2012;30:1656–62.
Macrie BD, Strauss JB, Helenowski IB, et al. Patterns of recurrence and role of pelvic radiotherapy in ovarian clear cell adenocarcinoma. Int J Gynecol Cancer. 2014. https://doi.org/10.1097/igc.0000000000000270.
Tanizaki Y, Kobayashi A, Shiro M, et al. Diagnostic value of preoperative SUVmax on FDG-PET/CT for the detection of ovarian cancer. Int J Gynecol Cancer. 2014;24:454–60.
Mayoral M, Fernandez-Martinez A, Vidal L, et al. Prognostic value of (18)F-FDG PET/CT volumetric parameters in recurrent epithelial ovarian cancer. Rev Esp Med Nucl Imagen Mol. 2016;35:88–95.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Acknowledgements
Not applicable.
Funding
The study was supported by grants from National Natural Science Foundation of China (81702558) and Fudan University Shanghai Cancer Center (YJ201603). The funding bodies didn’t participate in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The dataset supporting the conclusions of this article is available upon request. Please contact Prof. Huijuan Yang (huijuanyang@hotmail.com).
Author information
Authors and Affiliations
Contributions
SY, SL, LX, XW and HY contributed to the contraception and design of the study. SY and LX collected and analyzed patients’ clinicopathological data. SL interpreted the 18F-FDG PET/CT images. SY, SL and LX were major contributors in writing the manuscript. SY, SL, LX, XW and HY read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Fudan University Shanghai Cancer Center review board and the requirement for written informed consent was waived due to its retrospective design.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ye, S., Liu, S., Xiang, L. et al. 18F-FDG PET/CT-based metabolic metrics in recurrent tumors of ovarian clear cell carcinoma and their prognostic implications. BMC Cancer 19, 226 (2019). https://doi.org/10.1186/s12885-019-5441-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-019-5441-7