Abstract
Background
Renal impairment (RI) is a negative prognostic factor in Multiple Myeloma (MM) and affected patients are often excluded from autologous stem cell transplantation (ASCT). However, it remains unclear whether historically inferior outcome data still hold true.
Methods
From a total of 475 eligible MM patients who had undergone ASCT between 1998 and 2016, 374 were included in this multi-centric retrospective cohort study. Renal function was determined both at the time of MM diagnosis and ASCT by estimated glomerular filtration rate (eGFR according to the MDRD formula, RI defined as eGFR < 60 ml/min/1.73m2). Patients were categorized into 3 groups: A) no RI diagnosis and ASCT, B) RI at diagnosis with normalization before ASCT and C) RI both at the time of diagnosis and ASCT. Log-rank testing was used for overall and progression-free survival (OS, PFS) analysis.
Conclusion
While severe RI at MM diagnosis confers a risk of shorter OS, MM progression after ASCT is not affected by any stage of renal failure. It can be concluded that ASCT can be safely carried out in MM patients with mild to moderate RI and should be pro-actively considered in those with severe RI.
Results
When comparing all groups, no difference in OS and PFS was found (p = 0.319 and p = 0.904). After further stratification according to the degree of RI at the time of diagnosis, an OS disadvantage was detected for patients with an eGFR < 45 ml/min/m2. PFS was not affected by any RI stage.
Similar content being viewed by others
Background
Multiple Myeloma (MM) is frequently accompanied and complicated by renal impairment (RI) [1, 2]. RI often develops secondary to cast nephropathy where urinary casts consisting of immunoglobulin light chains accumulate in the renal tubules [3]. Other potential causes include monoclonal immunoglobulin deposition disease, interstitial nephritis, tubular necrosis and proximal tubular damage resulting in secondary Fanconi syndrome [4]. Dehydration, hypercalcemia and administration of nephrotoxic medication often add to the development of acute RI [5,6,7]. Furthermore, as many patients are of advanced age at MM diagnosis, other chronic conditions such as arterial hypertension or diabetes mellitus can also underlie a chronic form of RI.
It is known that RI is associated with a higher rate of treatment-related toxicity and reduced overall survival (OS) [8, 9]. Outcomes are even worse when renal failure is advanced and dialysis support is required [10]. Regarding the clinical management, it is of pivotal importance to overcome the negative impact of MM-associated acute RI with prompt institution of anti-myeloma therapy and supportive measures such as adequate hydration, and treatment of metabolic acidosis [11,12,13].
Historically, RI has been defined in MM patients by a serum creatinine value above 2 mg/dL. In line with this classification, approximately 20% of all newly diagnosed MM patients were found to be affected [5]. Yet, as the normal creatinine range varies widely depending on a patients’ age, gender and muscle mass, this imprecise definition made the correct diagnosis and grading of RI difficult. As a result, the classification guidelines for RI in MM were adapted in 2014 [14]. The new criteria include renal function assessment by creatinine clearance measurement. Yet, this measurement also carries pitfalls and is less accurate than other formulas [15]. Alternatively, the estimation of glomerular filtration rate (eGFR) by the widely applied modification of diet in renal disease (MDRD) formula might represent an appropriate classification tool for renal function with a single measurement and is currently recommended by nephrologic guidelines for renal function assessment [16].
Since a higher frailty and transplant-related mortality have historically been postulated in MM patients with RI [17], they still often fail to qualify for high-dose induction chemotherapy and are excluded from autologous stem cell transplantation (ASCT). It seems noteworthy that analyses on which these approaches are based on were carried out applying old classifications of RI and were conducted before the era of immunomodulatory drugs (IMiD) and proteasome inhibitor-based therapy regimens [18]. Newer analyses have concluded that ASCT is safe in MM patients with RI [19].
Since the exclusion from or delay of ASCT results in shorter survival of MM patients per se [20, 21], it seems to be of pivotal clinical importance that MM patients with RI undergo early pro-active evaluation for high-dose immuno-chemotherapy and ASCT. To appraise the question of renal recovery rate following MM diagnosis and evaluate whether patients with initial RI benefit from ASCT, we analyzed the outcome of a multi-center cohort of MM patients with or without RI at diagnosis.
Methods
Patient recruitment
The present analysis was carried out as a multi-centric retrospective cohort study. Patients were eligible for inclusion if they had a diagnosis of MM according to the criteria of the International Myeloma Working Group and received a first ASCT between 1998 and 2016. Patients with relapsed disease receiving a second or third ASCT were excluded.
Data of 475 patients from five Austrian Bone Marrow Transplant units (Medical University of Vienna, Medical University of Innsbruck, Medical University of Graz, Hanusch Hospital Vienna, Elisabethinen Hospital Linz) were available for analysis. All centers participate in the Austrian Myeloma Registry (ethics committee number Innsbruck: AN 3252 266/4.2370/5.6 (3997a); further, the ethics committee of the Medical University of Vienna additionally #1085/2017 approved the analysis.
In cases where renal function parameters were unavailable either at the time of diagnosis or ASCT, the respective patient was excluded from the analysis (n = 101). The final analysis was performed on 374 patients.
Renal function assessment
Renal function was assessed by serum creatinine measurement and a subsequent estimation of the GFR by the MDRD formula: eGFR = 175 × standardized serum creatinine− 1.154 × age− 0.203 × 1.212 [if black] × 0.742 [if female].
We then applied the KDIGO (Kidney Disease: Improving Global Outcomes) guidelines for renal failure and staged RI according to eGFR (cut-off for stage 2 at 90 ml/min/1.73m2, stage 3a at 60 ml/min/1.73m2, stage 3b at 45 ml/min/1.73m2 and stage 4 at 30 ml/min/1.73m2) [22].
Further, three subgroups were defined for analysis: A) always normal (eGFR > 60 ml/min/1.73m2 at diagnosis and ASCT), B) improving (eGFR < 60 ml/min/1.73m2 at diagnosis with normalization before ASCT) and C) always impaired (eGFR < 60 ml/min/1.73m2 both at the time of diagnosis and ASCT).
For supplementary analyses, a more stringent RI definition was chosen. Here, an eGFR value < 90 ml/min/1.73m2 defined a patient as being affected by RI.
Induction therapy before ASCT
Prior to ASCT, all patients received induction (immuno)-chemotherapy. As therapy regimens were subject to change during the years of analysis and IMiDs as well as proteasome inhibitors became standard-of-care during the early 2000s, we categorized our patients into either receiving chemotherapy alone versus immuno-chemotherapy including an IMiD and/or proteasome inhibitor. No patients received monoclonal antibodies. Two-hundred ninety patients (77.5%) received the latter (containing either IMiD, proteasome inhibitor or both), while 84 patients (22.5%) received conventional chemotherapy (containing various combinations of cyclophosphamide, etoposide, doxorubicin, idarubicin, vincristine and bendamustin).
Transplant procedure and transplant-related mortality
All ASCT procedures were carried out with peripheral blood stem cell grafts (2–4 × 106 CD34+ cells/kg body weight). Conditioning regimens were melphalan-based in all patients: 84.7% received high-dose melphalan (200 mg/m2), 9.6% received a reduced dose (140 mg/m2), 4.7% received other doses of melphalan and 1.1% received melphalan and total body irradiation. Antimicrobial treatment as well as erythrocyte and platelet support were administered according to best clinical practice guidelines of the respective institution. OS was defined as survival from time of diagnosis, while PFS was defined as the survival free of disease progression or recurrence from the time of ASCT. As only 6 patients died of causes other than MM progression, the time to progression was not calculated separately, but described as PFS instead.
Statistical analysis
We calculated cross-tables and Pearson chi-square tests for categorical variables and means and standard deviations as well as Analysis of Variance (ANOVA) models for continuous variables. Kaplan-Meier curves were generated for survival analyses and Log-rank tests were used to assess differences in OS and progression-free survival (PFS) between the study groups. A p-value ≤0.05 was considered statistically significant. The IBM SPSS System for Mac version 22.0.0 (SPSS, Inc., 2010, Chicago, IL) was used for all analyses.
Results
Renal function course between diagnosis and ASCT
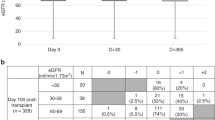
The study cohort’s renal function parameters showed a distinct overall improvement between the time of diagnosis and ASCT, with the mean eGFR increasing from 68.8 ± 26.9 ml/min/1.73m2 to 81.7 ± 27.9 ml/min/1.73m2.
The largest group was constituted of 238 patients (64%) who always had an eGFR above 60 ml/min/1.73m2 (Group A, mean eGFR 83 ± 17 ml/min/1.73m2 at diagnosis and 93 ± 21 ml/min/1.73m2 at the time of ASCT). Group B consisted of 67 patients (18%) whose previously impaired renal function normalized during induction therapy (mean eGFR 42 ± 15 ml/min/1.73m2 at diagnosis and 82 ± 20 ml/min/1.73m2 at time of ASCT). Fifty patients (13%) always had an eGFR below 60 ml/min/1.73m2 (Group C, mean eGFR 33 ± 17 ml/min/1.73m2 to 41 ± 15 ml/min/1.73m2). Nineteen patients (5%) could not be categorized into any of the three pre-defined groups as they exhibited significantly inferior renal function at ASCT (eGFR 43 ± 23 ml/min/1.73m2) compared to diagnosis (eGFR 78 ± 16 ml/min/1.73m2, p < 0.001).
Overall, 13 patients (3%) qualified as having stage 5 renal disease at the time of diagnosis, while 29 (8%) had stage 4 and 75 (20%) were classified as stage 3.
Patient characteristics at diagnosis
Group C patients were significantly older and had more advanced MM disease stages (Table 1). Patients presenting with a free light chain-only paraprotein were more likely to be categorized into Group C. β2 microglobulin was significantly higher in Groups B and C, while hemoglobin levels were lower in these groups (Table 2). Response rates to induction therapy were assessed after first-line therapy and proved comparable in all groups with an achievement of complete remission (CR), very good partial response (VGPR) or partial response (PR) in > 90% of all patients.
Patient characteristics at ASCT
All patient groups showed significant improvement of renal function between MM diagnosis and ASCT (Table 3). β2 microglobulin remained higher in Group C, while it became comparable between Groups A and B. Similarly, hemoglobin levels became comparable in Groups A and B, while they remained lower in Group C. Patients who received a reduced dose of melphalan had lower eGFR rates at ASCT compared to those who received a standard dose (200 mg: 84.6 ± 26.1 compared to 140 mg: 61.6 ± 32.4 ml/min/1.73m2, p < 0.001). Hematological outcome after ASCT, which was assessed after 3 months, was comparable in all three groups.
Thirteen patients required intermittent hemodialysis treatment during their hospital admission for ASCT including four patients from Group B and nine patients from Group C. These patients fared similarly with regard to OS and PFS compared to those who did not require dialysis.
Transplant-related mortality
Three patients died within 100 days after ASCT. One female patient had early infectious complications from Pseudomonas aeruginosa requiring intensive care treatment and subsequently suffered from acute renal failure necessitating hemofiltration. Her eGFR at diagnosis had been 16 ml/min/1.73m2 and had improved to 75 ml/min/1.73m2 at ASCT. The second patient, who was from Group A, developed cholecystitis-related sepsis 3 months after ASCT and also required hemofiltration. However, he also had severe early extra-medullary progression of MM. In the third patient, who died 11 months after ASCT, no cause of death could be determined.
Survival after ASCT according to renal function
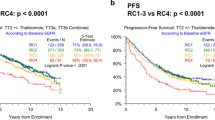
The 1-year OS rate was 94% in Group A, 97% in Group B and 98% in Group C (p = 0.348). It remained comparable after 3 years with rates of 70, 60 and 68%, respectively (p = 0.236). These differences did not amount to statistical significance on Kaplan-Meier survival analysis with Log-rank testing (Fig. 1, Log rank p = 0.319).
PFS rate at 1 year was 74% vs. 64% vs. 71% (p = 0.350), while the freedom of progression dropped to 29% vs. 23% vs. 27% at 3 years (p = 0.658). Again, no differences between the analyzed groups were observed on Log-rank testing (Fig. 2, Log rank p = 0.904).
After further stratification according to RI stage at diagnosis, we found that eGFR < 30 ml/min/1.73m2 (corresponding to renal failure ≥ stage 4) as well as eGFR < 45 ml/min/1.73m2 (renal failure ≥ stage 3b) were significantly correlated with a shorter OS (Fig. 3a and b). Regarding PFS, no association between RI of any stage and survival free of hematological relapse was found (Fig. 4).
Overall survival (months) from the time of MM diagnosis according to renal function at diagnosis. a Stratification for eGFR above (green curve, n = 332) and below (grey curve, n = 42) 30 ml/min/1.73m2. b Stratification for eGFR above (green curve, n = 307) and below (grey curve, n = 64) 45 ml/min/1.73m2. c Stratification for eGFR above (green curve, n = 257) and below (grey curve, n = 117) 60 ml/min/1.73m2. d Stratification for eGFR above (green curve, n = 76) and below (grey curve, n = 298) 90 ml/min/1.73m2
Progression-free survival (months) after ASCT according to renal function at ASCT. a Stratification for eGFR above (green curve, n = 332) and below (grey curve, n = 42) 30 ml/min/1.73m2. b Stratification for eGFR above (green curve, n = 307) and below (grey curve, n = 64) 45 ml/min/1.73m2. c Stratification for eGFR above (green curve, n = 257) and below (grey curve, n = 117) 60 ml/min/1.73m2. d Stratification for eGFR above (green curve, n = 76) and below (grey curve, n = 298) 90 ml/min/1.73m2
When including the small patient group who exhibited worsening of renal function during induction therapy, it was found that these patients did not exhibit different OS or PFS compared to the other groups (Log rank p = 0.066 and p = 0.721, data not shown).
Applying more stringent criteria for the definition of RI (eGFR below 90 ml/min/1.73m2), the group distribution shifted as expected: 14% of all patients were in Group A, 22% in Group B, 58% in Group C and 6% could not be classified. Yet, again, no significant differences between the groups could be determined on comparison of OS and PFS (Additional file 1: Figure S1).
On a further sub-analysis on OS and PFS comparing patients exhibiting an eGFR below 45 ml/min/1.73m2 at the time of ASCT with those above, no disadvantage was found for both outcomes (Log rank p = 0.629 and p = 0.927, data not shown).
Discussion
Despite the high frequency of RI in MM and the extensive knowledge about its underlying pathophysiology, little is known about whether it poses a risk in the treatment with ASCT. The term ‘perceived frailty’, which has been coined by analyses describing hemodialysis patients [23], encompasses why many hematologists are hesitant when it comes to the evaluation for ASCT in patients with moderate to severe RI. Thus far, objective data on this issue remain scarce.
Here, we report on a multi-centric cohort of MM patients with varying degrees of underlying RI who received ASCT.
By definition of RI according to current guidelines, a substantial percentage of MM patients showed renal function impairment both at diagnosis and at the time of ASCT. Approximately one third of all patients had eGFR values below 60 ml/min/1.73m2 at diagnosis. Even though most of them had only mild to moderate RI with eGFR values above 30 ml/min/1.73m2, this finding substantially impacts clinical care of newly diagnosed MM patients. Similar findings have been described previously and correspond well to our results [24]. Early interdisciplinary care including an evaluation for kidney biopsy indication and the diligent treatment of electrolyte and acid-base disorders should be enforced in order to ensure the best treatment for these patients.
Second, a distinct improvement of renal function could be observed in many patients between the initial MM diagnosis and the time of ASCT. It cannot be concluded directly from the present data whether this development was achieved by the applied hematological induction therapy or by supportive care (e.g. discontinuation of pre-existent nephrotoxic medication, acid-base management during acute renal failure at the time of diagnosis, etc.); yet, a combination thereof must be suspected.
Third, the analyzed outcomes OS and PFS were highly comparable between patients whose renal function was always undisturbed, those who had RI at diagnosis but improved throughout the induction therapy phase and those whose renal function was always classified as impaired. Comparing these results to a previous analysis by San Miguel et al., a noticeable difference is the fact that they found an OS benefit in the group whose renal function – which at the time was defined by serum creatinine alone – had always been normal [25]. It can now be hypothesized that renal failure – both temporary and persistent – does not result in inferior hematological outcomes anymore as novel medications with fewer nephrotoxic effects, such as advanced immunotherapies, have become standard-of-care. This is in line with previous results by Scheid et al. who found treatment with bortezomib to result in an abrogation of the inferior PFS results in patients with impaired renal function [26]. In the context of significantly reduced OS in patients where ASCT is not considered at all or deemed too hazardous [20], it should be noted that patients with RI – even those with an eGFR below 45 ml/min/1.73m2 at the time of ASCT - clearly benefit from this treatment.
Fourth, the sub-groups of analyzed patients with moderately to severely impaired renal function at the time of diagnosis (defined as an eGFR below 45 ml/min/1.73m2 corresponding to renal failure stage 3b or worse) were found to have a decreased OS. Interestingly, this finding did not extend to hematological outcomes, as RI did not influence PFS. This confirms previously described results from Raab et al., who analyzed OS and PFS in a small cohort of 17 dialysis-dependent patients and compared them with a matched control group [27]. Similarly to our results, no difference in PFS was described. Although not statistically significant, OS was longer in dialysis-free patients in their analysis. Yet, this non-significance might be attributable to their very small sample size. Additionally, further data supporting the idea that severe RI is associated with shorter survival in MM patients has previously been delivered by analyses that defined RI by a serum creatinine value above 2 mg/dl, which can safely be interpreted as severe RI nowadays [28]. Considering our results and the well-known fact that renal failure is generally associated with a reduced life expectancy [29], an increased risk of earlier death in MM patients with severe RI should be acknowledged. However, our results cannot provide a new threshold definition for renal impairment in MM due to the limited sample size.
Some further limitations of this study warrant discussion: as the analysis was of retrospective nature and as only patients who actually received ASCT were included, patient selection bias cannot be ruled out. Further, the number of patients presenting with an eGFR < 60 ml/min/1.73m2 was small overall (n = 117), leading to limited power of the study. Additionally, induction therapy prior to ASCT was heterogeneous and we only analyzed effects of conventional chemotherapy versus immunochemotherapy including IMiDs and/or proteasome inhibitors. It must be suspected from previous studies that the choice of agent exerts a certain influence on renal function. Furthermore, as the graded measurement of spot urine albuminuria was only included into the KDIGO guidelines in 2009, we did not have enough proteinuria measurements at hand to provide substantial information on this aspect of renal impairment. Last, the definition and classification of RI in MM should remain a subject of critical discussion. Nowadays, nephrologic guidelines include eGFR measurements in their definition of chronic renal failure. The KDIGO grading of chronic kidney disease stages represents a simple and well-established tool; yet, other calculations besides the here-applied MDRD formula might be even more accurate in the estimation of renal function [30]. Furthermore, certain forms of renal failure, such as acute renal failure, are defined by different criteria (e.g. RIFLE, AKIN criteria [31, 32]), which makes a correct classification of MM patients, who can either be affected by acute or chronic renal disease, difficult. For our analysis, we consciously decided to use eGFR values and the grade of RI according to the KDIGO guidelines as a differentiation between acute and chronic RI was not fully possible in this cohort and, further, many patients actually did fulfill criteria for chronic renal failure (eGFR below 60 ml/min/m2 for ≥3 months).
Conclusions
In conclusion, our data show that ASCT can be carried out safely in patients who present with mild to moderate renal failure at the time of diagnosis. Patients presenting with severe impairment of renal function should be pro-actively evaluated for ASCT, since hematological outcomes are comparable to those of patients with normal renal function. Further, while amelioration of renal function represents a highly desirable treatment goal, the lack of response should not preclude patients from autologous transplantation. Interdisciplinary care should be enforced in order to improve not only hematological, but also overall outcomes.
Abbreviations
- ASCT:
-
Autologous stem cell transplantation
- CR:
-
Complete remission
- eGFR:
-
estimated glomerular filtration rate
- IMiD:
-
Immunomodulatory drug
- KDIGO:
-
Kidney disease improving global outcomes
- MDRD:
-
Modification of diet in renal disease
- MM:
-
Multiple Myeloma
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PR:
-
Partial response
- RI:
-
Renal impairment
- VGPR:
-
Very good partial response
References
Winearls CG. Acute myeloma kidney. Kidney Int. 1995;48(4):1347–61.
Chow CC, Mo KL, Chan CK, Lo HK, Wong KS, Chan JC. Renal impairment in patients with multiple myeloma. Hong Kong Med J. 2003;9(2):78–82.
Nasr SH, Valeri AM, Sethi S, Fidler ME, Cornell LD, Gertz MA, Lacy M, Dispenzieri A, Rajkumar SV, Kyle RA, et al. Clinicopathologic correlations in multiple myeloma: a case series of 190 patients with kidney biopsies. Am J Kidney Dis. 2012;59(6):786–94.
Korbet SM, Schwartz MM. Multiple myeloma. J Am Soc Nephrol. 2006;17(9):2533–45.
Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fonseca R, Rajkumar SV, Offord JR, Larson DR, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33.
Dimopoulos MA, Kastritis E, Rosinol L, Bladé J, Ludwig H. Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia. 2008;22(8):1485–93.
Eleutherakis-Papaiakovou V, Bamias A, Gika D, Simeonidis A, Pouli A, Anagnostopoulos A, Michali E, Economopoulos T, Zervas K, Dimopoulos MA, et al. Renal failure in multiple myeloma: incidence, correlations, and prognostic significance. Leuk Lymphoma. 2007;48(2):337–41.
Knudsen LM, Hjorth M, Hippe E. Renal failure in multiple myeloma: reversibility and impact on the prognosis. Nordic Myeloma Study Group. Eur J Haematol. 2000;65(3):175–81.
Carlson K, Hjorth M, Knudsen LM, Group NMS. Toxicity in standard melphalan-prednisone therapy among myeloma patients with renal failure--a retrospective analysis and recommendations for dose adjustment. Br J Haematol. 2005;128(5):631–5.
Martín Reyes G, Valera A, Frutos MA, Ramos B, Ordóñez V, López de Novales E. Survival of myeloma patients treated with dialysis. Nefrologia. 2003;23(2):131–6.
Kleber M, Ihorst G, Terhorst M, Koch B, Deschler B, Wäsch R, Engelhardt M. Comorbidity as a prognostic variable in multiple myeloma: comparative evaluation of common comorbidity scores and use of a novel MM-comorbidity score. Blood Cancer J. 2011;1(9):e35.
Dimopoulos MA, Terpos E, Chanan-Khan A, Leung N, Ludwig H, Jagannath S, Niesvizky R, Giralt S, Fermand JP, Bladé J, et al. Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the international myeloma working group. J Clin Oncol. 2010;28(33):4976–84.
Bladé J, Fernández-Llama P, Bosch F, Montolíu J, Lens XM, Montoto S, Cases A, Darnell A, Rozman C, Montserrat E. Renal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institution. Arch Intern Med. 1998;158(17):1889–93.
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E, Richardson P, et al. International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–48.
Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010;5(6):1003–9.
Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO controversies conference report. Kidney Int. 2011;80(1):17–28.
Mohty M, Harousseau JL. Treatment of autologous stem cell transplant-eligible multiple myeloma patients: ten questions and answers. Haematologica. 2014;99(3):408–16.
Dimopoulos MA, Sonneveld P, Leung N, Merlini G, Ludwig H, Kastritis E, Goldschmidt H, Joshua D, Orlowski RZ, Powles R, et al. International myeloma working group recommendations for the diagnosis and Management of Myeloma-Related Renal Impairment. J Clin Oncol. 2016;34(13):1544–57.
Mahindra A, Hari P, Fraser R, Fei M, Huang J, Berdeja J, Callander N, Costa L, Diaz MA, Freytes C, et al. Autologous hematopoietic cell transplantation for multiple myeloma patients with renal insufficiency: a center for international blood and marrow transplant research analysis. Bone Marrow Transplant. 2017;52(12):1616–22.
van Rhee F, Giralt S, Barlogie B. The future of autologous stem cell transplantation in myeloma. Blood. 2014;124(3):328–33.
Wildes TM, Finney JD, Fiala M, Gao F, Vij R, Stockerl-Goldstein K, Carson KR, Mikhael J, Colditz G. High-dose therapy and autologous stem cell transplant in older adults with multiple myeloma. Bone Marrow Transplant. 2015;50(8):1075–82.
Group KDIGOKC-MW. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2009;113:S1–130.
Salter ML, Gupta N, Massie AB, McAdams-DeMarco MA, Law AH, Jacob RL, Gimenez LF, Jaar BG, Walston JD, Segev DL. Perceived frailty and measured frailty among adults undergoing hemodialysis: a cross-sectional analysis. BMC Geriatr. 2015;15:52.
Kleber M, Ihorst G, Deschler B, Jakob C, Liebisch P, Koch B, Sezer O, Engelhardt M. Detection of renal impairment as one specific comorbidity factor in multiple myeloma: multicenter study in 198 consecutive patients. Eur J Haematol. 2009;83(6):519–27.
San Miguel JF, Lahuerta JJ, García-Sanz R, Alegre A, Bladé J, Martinez R, García-Laraña J, De La Rubia J, Sureda A, Vidal MJ, et al. Are myeloma patients with renal failure candidates for autologous stem cell transplantation? Hematol J. 2000;1(1):28–36.
Scheid C, Sonneveld P, Schmidt-Wolf IG, van der Holt B, el Jarari L, Bertsch U, Salwender H, Zweegman S, Blau IW, Vellenga E, et al. Bortezomib before and after autologous stem cell transplantation overcomes the negative prognostic impact of renal impairment in newly diagnosed multiple myeloma: a subgroup analysis from the HOVON-65/GMMG-HD4 trial. Haematologica. 2014;99(1):148–54.
Raab MS, Breitkreutz I, Hundemer M, Benner A, Klaus J, Hegenbart U, Moehler T, Ho AD, Zeier M, Goldschmidt H. The outcome of autologous stem cell transplantation in patients with plasma cell disorders and dialysis-dependent renal failure. Haematologica. 2006;91(11):1555–8.
Krejci M, Hajek R, Buchler T, Krivanova A, Svobodnik A, Pour L, Adam Z, Mayer J, Vorlicek J. Simple variables predict survival after autologous transplantation: a single Centre experience in 181 multiple myeloma patients. Neoplasma. 2007;54(2):143–8.
Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17(7):2034–47.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Workgroup ADQI. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute Dialysis quality initiative (ADQI) group. Crit Care. 2004;8(4):R204–12.
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Network AKI. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The full datasets are available from the corresponding author at reasonable request.
Author information
Authors and Affiliations
Contributions
MA and MTK designed the research, gathered data, performed the statistical analysis and wrote the paper. TD, TR, AB, WWL, MG, EW, DN, RW, SRR, DL and HA gathered data and critically revised the manuscript. WR, NW, HG1, FK and HG2 interpreted the data and critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All centers participate in the Austrian Myeloma Registry (ethics committee number Innsbruck: AN 3252 266/4.2370/5.6 (3997a); further, the ethics committee of the Medical University of Vienna additionally #1085/2017 approved the analysis. No consent to participate was sought from the patients as this analysis represents a retrospective data accrual.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Figure S1. Overall survival from MM diagnosis (S1A) and progression-free survival from ASCT (S1B) in months according to renal function groups. RI was defined as eGFR < 90 ml/min/1.73m2. Group A: eGFR always > 90 ml/min/1.73m2; Group B: eGFR < 90 ml/min/1.73m2 at diagnosis improving to > 90 ml/min/1.73m2 before ASCT; Group C: eGFR always < 90 ml/min/1.73m2. (TIF 1521 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Antlanger, M., Dust, T., Reiter, T. et al. Impact of renal impairment on outcomes after autologous stem cell transplantation in multiple myeloma: a multi-center, retrospective cohort study. BMC Cancer 18, 1008 (2018). https://doi.org/10.1186/s12885-018-4926-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-018-4926-0