Abstract
Background
To examine the effects of recombinant human endostatin combined with radiotherapy on colorectal cancer HCT-116 cell xenografts in nude mice.
Methods
Forty male BALB/c nude mice were injected with human colorectal cancer HCT-116 cells to form xenografts and then randomized into the following 4 groups (each group comprised ten mice): a control group, an endostatin group (20 mg/kg endostatin once a day for 10 days), a radiotherapy group (a 6-Gy dose was administered via a 6-MV X-ray on day 5 post-inoculation), and a combination therapy group (radiotherapy with endostatin treatment). The tumor growth inhibition rate were detected. CD31, vascular endothelial growth factor (VEGF), and hypoxia inducible factor-1α (HIF-1α) expression and microvascular density (MVD) were evaluated by immunohistochemistry. The expression of VEGF protein was also detected by western blotting.
Results
The tumor growth inhibition rate in the radiotherapy with endostatin treatment group was significantly higher than those in endostatin group or radiotherapy group (77.67% vs 12.31% and 38.59%; n = 8 per group, P < 0.05). The results of immunohistochemistry showed that treatment with radiotherapy induced significant increases in CD31, VEGF, and HIF-1α expression and MVD compared with treatment with saline, while treatment with endostatin or radiotherapy with endostatin induced reductions in CD31, VEGF, and HIF-1α expression and MVD compared with treatment with saline (n = 8 per group, P < 0.05). The results of western blotting showed that VEGF protein expression in radiotherapy group was significantly increased compared with that in the control group. However, VEGF protein expression in the endostatin or radiotherapy with endostatin groups was significantly decreased compared with that in the control group (n = 8 per group, P < 0.05).
Conclusions
Endostatin combined with radiotherapy can significantly inhibit HCT-116 cell xenograft growth, possibly by inhibiting angiogenesis and attenuating tumor cell hypoxia.
Similar content being viewed by others
Background
Colorectal cancer is the most common malignant tumor of the digestive system [1]. Dietary changes have resulted in significant increases in the incidence of and mortality associated with colorectal cancer in China in recent years [2], and the age of colorectal cancer onset in China has decreased significantly compared with that in European and American countries (mean age: 45 years). Rectal cancer accounts for 60%–75% of colorectal cancer cases in China and for 45% of colorectal cancer cases in European and American countries [3]. Most patients with colorectal cancer have locally advanced disease at the time of their diagnosis and treatment [4]. However, the overall colorectal cancer survival rate remains poor despite advances in colorectal cancer treatment. Radiotherapy is an important treatment for locally advanced rectal cancer, and many patients respond poorly to RT because of tumor cell hypoxia [5, 6].
Hypoxia is one of the basic characteristics of the solid tumor microenvironment. In tumor cells, the G2/M phase shortens significantly in the setting of hypoxia. Tumor cells in the G2/M phase are more sensitive to radiation than cells in other phases of the cell cycle; thus, hypoxia induces resistance to radiation in tumor cells by shortening the G2/M phase [7].
Folkman first proposed the tumor angiogenesis theory in the New England Journal of Medicine in 1971, the year in which he also demonstrated that antiangiogenesis agents can inhibit tumor growth [8]. In recent decades, a variety of antiangiogenic drugs targeting tumor endothelial cells have been used in combination with radiotherapy and chemotherapy to improve the cure rates of patients with cancer and to prolong survival times in such patients. Antiangiogenesis is an important target in tumor biological therapy, and the idea that antiangiogenic drugs can attenuate tumor cell hypoxia and thus enhance radiosensitivity has become a popular topic in the field in recent years [9].
Recombinant human endostatin (Endostar) is one of the most potent angiogenesis inhibitors of angiogenesis developed independently in China. Endostatin can significantly inhibit the proliferation and migration of vascular endothelial cells and the formation of new blood vessels to prevent tumor cells from receiving the nutrients necessary for growth and metastasis [10]. Some studies have also shown that endostatin can inhibit lymph node metastasis and lymphatic vessel formation [11, 12]. Endostatin combined with chemotherapy has been approved for the treatment of advanced non-small cell lung cancer [13]. Previous studies have shown that endostatin combined with radiotherapy achieved excellent cure rates in various tumors [14, 15]. Li et al. showed that endostatin can normalize tumor vasculature within a short time window to attenuate tumor cell hypoxia [16]. In this study, we determined whether recombinant human endostatin combined with radiotherapy can improve disease outcomes in an in vivo colorectal cancer mouse model.
Methods
Materials
Recombinant human endostatin was provided by Shandong Xiansheng Maidejin Biological Pharmaceutical Co., Ltd. (Shandong, China), and the colorectal cell line HCT-116 was donated by the Sixth Affiliated Hospital, Sun Yat-sen University (Guangzhou, China). The local ethics committee of Sun Yat-sen University ruled that no formal ethics approval was required in this particular case. All laboratory chemicals used herein were of molecular biology grade.
Cell cultures
HCT-116 cells were cultured in 90% McCoy’s 5a medium with 10% fetal bovine serum (FBS) and 1% double antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) at 37 °C with 5% CO2. The cells were detached using trypsin-EDTA solution when they were approximately 80% confluent, after which some of them were subcultured, while the remaining cells were stored in liquid nitrogen.
In vivo HCT-116 xenograft animal model
Forty BABL/c male nude mice aged 4–6 weeks and weighing 20 ± 2 g were purchased from Shanghai Slac Laboratory Animal Co. Ltd. (Animal Quarantine Conformity Certificate Number: 3,100,159,661, Shanghai, China) and housed under SPF conditions. All animal studies were approved by the Ethics Committee of Qingdao Central Hospital, the Second Affiliated Hospital of Qingdao University Medical College, and were performed according to the local principles of laboratory animal care. HCT-116 tumor cells in the logarithmic phase of growth (1 × 107) were resuspended in 0.2 mL of serum-free medium and then injected into the upper back of each nude mouse. The volume of each implanted tumor was measured every 2 days and was calculated as V = L x W2/2, where L is the longer axis of the tumor, and W is the shorter axis of the tumor. Treatment was initiated when the tumors reached an approximate volume of 308 ± 56 mm3. The mice were randomized into the following 4 groups (each group comprised ten mice): a control group, which received peritumoral subcutaneous injections of 0.2 mL of normal saline every day for 10 days; an endostatin group, which received peritumoral subcutaneous injections of 0.2 mL of endostatin (20 mg/kg) every day for 10 days; a radiotherapy group, which received a single 6-Gy dose of external irradiation (6-MV X ray) on day 5 post-inoculation and a peritumoral subcutaneous injection of 0.2 mLl of normal saline every day for 10 days; and a combination therapy group (radiotherapy with endostatin treatment), in which endostatin was administered as in the endostatin group, and radiotherapy was administered as in the radiotherapy group. The size of the implanted tumor and the weights of the mice were monitored during this period. On day sixteen post-inoculation, all the mice were killed, and the tumors were collected. One half of each tumor was fixed in 4% neutral formalin for use in subsequent immunohistochemistry experiments, and the other half was stored at −80 °C until needed for subsequent western blotting experiments.
Immunohistostaining
Tumor tissue sections were fixed in formalin for 24 h and then embedded in paraffin before being cut into 4-μm thick sections and stained with hematoxylin & eosin (HE). A fully automatized immunohistochemistry assay was performed on a Roche Ventana BenchMark XT (Roche Ventana Medical Systems, Tucson, Arizona,USA) using an Ultra View Universal DAB Detection Kit (Roche Ventana Medical Systems, Tucson, Arizona, USA). The following primary antibodies were used for the experiment: anti-vascular endothelial growth factor (VEGF) antibody (purified rabbit polyclonal antibody, diluted 1:80, WuXi AppTec, AP6290b-400, Suzhou, China), anti-CD31 antibody (purified rabbit polyclonal antibody, diluted 1:80, WuXi AppTec, AP7465B-400, Suzhou, China) and anti-hypoxia inducible factor-1α (HIF-1α) antibody (purified rabbit polyclonal antibody, diluted 1:80, WuXi AppTec, AP7759C-400, Suzhou, China). The tissue sections were subsequently observed under a light microscope by two experienced pathologists.
Western blotting
The tumor tissues that had been stored at −80 °C were weighed, after which total protein was extracted from the tissues on ice using RIPA lysis buffer and a PMSF enzyme inhibitor (RIPA:PMSF = 100:1). Protein concentrations were measured using a BCA Protein Assay Kit (Beyotime Biotechnology, Shanghai, China), after which 50 μg of extracted protein and the appropriate markers were loaded onto a SDS-PAGE gel (containing a 12% separating gel and a 5% stacking gel) and then separated electrophoretically. The protein was subsequently transferred onto a PVDF membrane (Roche Applied Science, Shanghai, China), which was blocked for 1 h using 5% defatted milk diluted in Tris buffered saline Tween (TBST) before being incubated with a primary antibody to VEGF (purified rabbit polyclonal antibody, diluted 1:200, WuXi AppTec, AP6290b-400, Suzhou, China) overnight at 4 °C. The membranes were then incubated with a goat anti-rabbit IgG (H + L) HRP-conjugated secondary antibody (1:3000, LK2001, Sungene Biotech, Tianjin, China) for 1 h at room temperature. After being washed, the membranes were visualized for 1–2 min by ECL luminescence (HB-ECL-025, Hanbio, Shanghai, China), after which the protein signals in the X-ray film were quantified by scanning densitometry and analyzed by ImageJ software. The membrane was subsequently stripped with stripping buffer and then re-blocked to repeat the above steps for GAPDH, which served as an internal control (mouse monoclonal antibody, 1:1000, LK9002, Sungene Biotech, Tianjin, China). The gray value of the experimental band was compared with that of the internal reference band.
Calculations
The tumor inhibition rate was calculated as follows: tumor inhibition rate (%) = (average volume of the control group − average volume of the experimental group)/ average volume of the control group × 100%. Brown-yellow staining indicative of positive CD31 expression was noted in the membrane and cytoplasm of vascular endothelial cells. Microvessel density (MVD) was determined by the Weidner method. First, the entire section was viewed at low power (× 100), after which the numbers of blood vessels in 5 randomly selected visual fields were counted under high power (× 200). The average number of vessels/ field was considered the MVD of the specimen. The presence of yellow or brown granules in the cytoplasm was indicative of VEGF expression. Five different visual fields were randomly selected and then viewed with the high-power microscope (× 400), and the number of positive cells and the total number of cells in each visual field were counted. The rate of positive VEGF expression for each field was calculated as follows: expression rate = number of positive cells/ total number of cells × 100%. The rate of positive VEGF expression for the sample was the mean of the 5 values mentioned above. The presence of brown-yellow granules in the nucleus was indicative of the presence of HIF-1α, whose rate of positive expression was determined in the same manner as the rate of positive VEGF expression.
Statistical analysis
All analyses were performed with SPSS 22.0 statistical software (Softonic, San Francisco, CA, USA). The data were expressed as means ± SEMs. Comparisons among the groups were performed using Student’s t-test. A p-value <0.05 was considered statistically significant, and a p-value <0.01 was considered very significant.
Results
Endostatin combined with radiotherapy significantly inhibited tumor growth
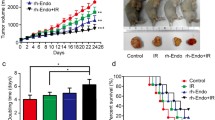
No nude mice died during the experiment, and no differences in nude mouse diet, behavior or mental status were noted among the groups. Moreover, no significant adverse systemic reactions to the above treatments or weight changes were observed in this study. Tumor growth curves were constructed according to the average tumor volume in each group (Fig. 1). Tumor volumes tended to decline in the endostatin or radiotherapy with endostatin treatment groups compared with that in control and radiotherapy groups during the period before radiotherapy administration and after endostatin treatment; however, there was no significant difference in tumor volume among the groups (n = 8 per group, P > 0.05). Tumor growth was significantly inhibited in the radiotherapy or radiotherapy with endostatin treatment after radiotherapy. The average tumor volumes in the above groups on the 16th day of the treatment period were as follows: control group, V = 2029.95 ± 425.59 mm3; endostatin group, V = 1781.75 ± 412.53 mm3; radiotherapy group, V = 1246.60 ± 606.83 mm3; and radiotherapy with endostatin treatment group, V = 453.35 ± 181.43 mm3. We noted a significant difference in tumor volume between the radiotherapy with endostatin treatment group and the other three groups (n = 8 per group, P < 0.01). In addition, the tumor volume was significantly reduced in the radiotherapy group compared with that in the control group (n = 8 per group, P < 0.05); however, there is no significant difference in tumor volume between the endostatin and control groups. The average tumor weights in each group were as follows (g): control group, 1.96 ± 0.56; endostatin group, 1.78 ± 0.45; radiotherapy group, 1.05 ± 0.34; and radiotherapy with endostatin treatment group, 0.51 ± 0.21. There was a significant difference in tumor weight between the radiotherapy with endostatin treatment group and the other three groups (n = 8 per group, P < 0.05) (Fig. 1). On the 16th day of the treatment period, the tumor inhibition rates in the endostatin, radiotherapy and radiotherapy with endostatin treatment groups were 12.31%, 38.59% and 77.67%, respectively. Taken together, these findings indicate that endostatin combined with radiotherapy significantly inhibited HCT-116 xenograft growth.
Endostatin combined with radiotherapy significantly inhibited tumor growth. a Changes in the average body weights of nude mice in each group during treatment (P > 0.05). b Colorectal cancer HCT-116 cell xenograft growth curves. c Average weights of the tumors in each group (n = 8 per group, **P < 0.01), compared with that in the control group. d Images of tumor tissue specimens dissected from mice in each group
Pathological features of the tumor specimens in each group
HE staining showed that many tumor cells were present in the control group and that these cells displayed obvious atypical features. However, less cells were present in the three treatment groups. Some of the cells had been replaced by fibrous tissue, while the remaining cells displayed changes consistent with necrosis, degeneration, and calcification, especially the cells in the radiotherapy with endostatin treatment group (Fig. 2).
Pathological features of the tumor specimens in each group. HCT-116 cells in nude mice from the following groups (HE staining, 200×): a: control; b: endostatin; c: radiotherapy; d: radiotherapy with endostatin treatment. The tumor cells in the control group were rich and exhibited obvious atypia, while the tumor cells in other groups were replaced by fibrous tissue and displayed signs of necrosis, degeneration, and calcification
Effects of the indicated treatments on VEGF expression
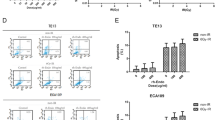
The immunohistochemistry results showed that VEGF was localized mainly in the cytoplasm and that VEGF was positively expressed in all four groups (Fig. 3). Moreover, the results indicated that VEGF was expressed at a particularly high level in the radiotherapy group compared with the other three groups (n = 8 per group, P < 0.05). The rates of positive VEGF expression in each group were as follows: control group, 30.12% ± 0.63%; endostatin group, 6.12% ± 0.18%; radiotherapy group, 40.05% ± 1.27%; and radiotherapy with endostatin treatment group, 12.30% ± 0.25%. These results indicated that radiotherapy can induce VEGF expression in tumor tissues. The intensity at which VEGF stained in the endostatin and radiotherapy with endostatin treatment groups was lower than the intensity at which it stained in the control group. In addition, the endostatin group displayed lighter VEGF staining than the other three groups (n = 8 per group, P < 0.05). The western blotting results also indicated that VEGF expression was significantly increased in the radiotherapy group and significantly decreased in the endostatin and radiotherapy with endostatin treatment groups compared with the control group (n = 8 per group, P < 0.05) (Fig. 4). Taken together, these findings indicate that endostatin can inhibit VEGF expression in tumor tissues.
The expression and localization of VEGF by immunohistochemistry staining. a: control; b: endostatin; c: radiotherapy; d: radiotherapy with endostatin treatment. e: Comparison of the percentages of cells in each group that stained positive for VEGF. VEGF was localized mainly in the cytoplasm and was positively expressed in all four groups. The expression of VEGF was at a particularly high level in the radiotherapy group compared with that in other three groups. The rates of positive VEGF expression in each group were as follows: control group, 30.12% ± 0.63%; endostatin group, 6.12% ± 0.18%; radiotherapy group, 40.05% ± 1.27%; and radiotherapy with endostatin treatment group, 12.30% ± 0.25%. The intensity at which VEGF stained in the endostatin and radiotherapy with endostatin treatment groups was lower than the intensity at which it stained in the control group. In addition, the endostatin group displayed lighter VEGF staining than the other three groups (n = 8 per group, **P < 0.01) (200×)
Results of western blotting. a: control; b: endostatin; c: radiotherapy; d: radiotherapy with endostatin treatment; e: VEGF gray value-to-GAPDH gray value ratio in each group at 16 days after treatment, a P < 0.05 versus control; b P < 0.05 versus radiotherapy (n = 8 per group). The western blotting results also indicated that VEGF expression was significantly increased in the radiotherapy group and significantly decreased in the endostatin and radiotherapy with endostatin treatment groups compared with the control group (n = 8 per group, P < 0.05) (200×)
Effects of each treatment on tumor MVD
Blood vessel proliferation in tumor tissues reflects the ability of a tumor to induce angiogenesis; thus, tumor MVD serves as an index of tumor angiogenesis. The endothelial cell marker CD31 is a new microvascular marker that can be used to identify vascular endothelial cells and to quantitatively evaluate the function of various factors that participate in angiogenesis. Additional information regarding CD31 expression is shown in Fig. 5. The MVD-CD31 values in the four groups were as follows: control group, 18.43 ± 1.58; endostatin group, 9.05 ± 1.46; radiotherapy group, 26.58 ± 1.86; and radiotherapy with endostatin treatment group, 11.22 ± 1.54. The MVD-CD31 value in the radiotherapy group was significantly higher than those in the other three groups (n = 8 per group, P < 0.05), and the MVD-CD31 values in the endostatin and radiotherapy with endostatin treatment groups were significantly decreased compared with that in the control group (n = 8 per group, P < 0.05) (Fig. 5). Taken together, these data showed that radiotherapy can induce tumor angiogenesis and that endostatin may be able to inhibit radiotherapy induced tumor angiogenesis.
Effects of each treatment on tumor MVD. Expression of CD31 in each group at 16 days after treatment (a-d). a: control; b: endostatin; c: radiotherapy; d: radiotherapy with endostatin treatment; e: The MVDs of the four groups were compared by counting the numbers of CD31-positive microvessels in each group. a P < 0.05 versus control; b P < 0.05 versus radiotherapy (n = 8 per group). The MVD-CD31 values in the four groups were as follows: control group, 18.43 ± 1.58; endostatin group, 9.05 ± 1.46; radiotherapy group, 26.58 ± 1.86; and radiotherapy with endostatin treatment group, 11.22 ± 1.54. The MVD-CD31 value in the radiotherapy group was significantly higher than those in the other three groups (n = 8 per group, P < 0.05), and the MVD-CD31 values in the endostatin and radiotherapy with endostatin treatment groups were significantly decreased compared with that in the control group (n = 8 per group, P < 0.05) (200×)
HIF-1α expression in each treatment group
Hypoxia is an initial inducer of malignant transformation and tumor metastasis, and HIF-1α plays an important role in regulating tumor angiogenesis. HIF-1α was strongly expressed in the control and radiotherapy groups but was expressed less strongly in the endostatin and radiotherapy with endostatin treatment groups. The rates of positive HIF-1α expression in each group were as follows: control group, 66.34% ± 3.23%; endostatin group, 16.43% ± 1.14%; radiotherapy group, 82.14% ± 3.65%; and radiotherapy with endostatin treatment group, 31.35% ± 1.51%. The rate of positive HIF-1α expression in the radiotherapy group was significantly higher than those in the other three groups (P < 0.05), while the rates of positive HIF-1α expression in the endostatin and radiotherapy with endostatin treatment groups were significantly decreased compared with that in the control group (P < 0.05) (Fig. 6). Taken together, these results indicated that endostatin may attenuate tumor hypoxia and increase the sensitivity of tumors to radiotherapy.
HIF-1α expression in each treatment group. a: control; b: endostatin; c: radiotherapy; d: radiotherapy with endostatin treatment; e: Comparison of the percentages of cells that stained positive for HIF-1α in the four groups. The rates of positive HIF-1α expression in each group were as follows: control group, 66.34% ± 3.23%; endostatin group, 16.43% ± 1.14%; radiotherapy group, 82.14% ± 3.65%; and radiotherapy with endostatin treatment group, 31.35% ± 1.51%. The rate of positive HIF-1α expression in the radiotherapy group was significantly higher than those in the other three groups (n = 8 per group,P < 0.05), while the rates of positive HIF-1α expression in the endostatin and radiotherapy with endostatin treatment groups were significantly decreased compared with that in the control group (n = 8 per group,P < 0.05) (200×)
Discussion
Neoadjuvant radiotherapy has been used for the treatment of rectal cancer with increasing frequency in recent years. Several studies have shown that neoadjuvant radiotherapy can lower tumor stages, improve radical resection and sphincter preservation rates and decrease local recurrence rates [17,18,19,20]. However, radiotherapy may delay surgery for patients with poor radiotherapy sensitivity. Therefore, researchers and clinicians must devise a means of improving the sensitivity of rectal cancer to radiotherapy. In our study, we found that the tumor inhibition rate in the combination therapy group was significantly higher than those in the other three groups and that no mice experienced significant increases in side effects while receiving recombinant human endostatin combined with radiotherapy as a treatment for colorectal cancer HCT-116 cell xenografts. Therefore, we determined that endostatin combined with radiotherapy is an effective method for increasing the sensitivity of cancer cells to radiotherapy.
Angiogenesis is essential for tumor growth and metastasis. Thus, clinicians and researchers must measure tumor MVD, which serves as an indicator of tumor angiogenesis [21]. VEGF plays an important role in tumor angiogenesis and is also the most important promoter of angiogenesis [22]. Hypoxia is the initial inducer of malignant transformation and tumor metastasis, and HIF-1α plays an important role in regulating tumor angiogenesis [23]. Therefore, in this study, we observed the changes in the levels of several indices of hypoxia and angiogenesis during the course of anti-tumor therapy. We found that treatment with radiotherapy induced significant increases in CD31, VEGF, HIF-1α and MVD expression compared with treatment with saline. However, treatment with endostatin and combination therapy groups induced significant decreases in CD31, VEGF, HIF-1α and MVD expression compared with treatment with saline. VEGF expression levels in the radiotherapy group were significantly increased compared with those in the control group. However, VEGF expression levels were significantly decreased in the endostatin and combination therapy groups compared with the control group. Therefore, we concluded that endostatin can inhibit radiotherapy -induced tumor angiogenesis.
Recombinant human endostatin (Endostar) is an antiangiogenesis drug that was developed independently in China. Endostar inhibits tumor angiogenesis by inhibiting the migration of endothelial cells, which form blood vessels. Endostatin can enhance tumor radiosensitivity when administered in combination with radiotherapy [14], perhaps because it can normalize tumor vasculature and the tumor microenvironment [24,25,26]. Winkler et al. [27] found that VEGFR2 inhibition creates a “normalization window”, i.e., a period during which radiotherapy can yield the best outcome. This window is characterized by an increase in tumor oxygenation, a change that is known to enhance tumor radiation responsiveness. Recombinant human endostatin can normalize tumor vessels for a short time period [28, 29], during which the tumor receives a normal supply of oxygen and thus becomes more sensitive to radiation. In the present study, the time window occurred mainly between days 5 and 7 after the initiation of endostatin therapy [30,31,32,33,34]. Therefore, radiotherapy was administered on the fifth day after the initiation of endostatin therapy in our experiment.
Wen et al. [30] concluded that endostatin combined with radiotherapy can enhance the radiosensitivity of human nasopharyngeal carcinoma and human lung adenocarcinoma xenografts, possibly by increasing endothelial cell and tumor cell apoptosis, attenuating tumor cell hypoxia, and modulating proangiogenic factor expression. Jiang et al. [31] showed that radiotherapy combined with weekly endostatin can significantly inhibit tumor growth and induce early tumor regression, phenomena that may be related to the attenuation of tumor cell hypoxia and the inhibition of radiation-induced tumor angiogenesis. Endostatin can inhibit RT-induced increases in the expression of the angiogenic factors HIF-1α and VEGF, effects that may underlie its enhancements of tumor radiosensitivity [30, 35,36,37,38]. Endostatin combined with radiotherapy has seldom been used as a treatment for advanced rectal cancers that are already being treated with well-known antiangiogenesis drugs, such as cetuximab and bevacizumab. What effect does a combination of these drugs and chemoradiotherapy have on locally advanced rectal cancer? Kim performed an analysis of two phase II trials [39] and concluded that preoperative concurrent chemoradiotherapy plus cetuximab did not improve short- or long-term therapeutic outcomes in patients with locally advanced rectal cancer and that KRAS, BRAF and PIK3CA mutations cannot predict the pathological remission rates or disease-free survival (DFS) rates of patients with the disease. Dewdney [40] analyzed 165 patients with locally advanced rectal cancer and concluded that the addition of cetuximab did not improve CR or PFS rates; however, RR and OS rates (both secondary endpoints) were significantly improved in patients with wild-type KRAS/BRAF. Willett et al. [41] conducted a multicenter phase II clinical trial, the results of which showed that bevacizumab combined with preoperative concurrent chemoradiotherapy significantly improved DFS and OS and that the acute and postoperative toxicity induced by the regimen was also acceptable. However, oncologists should be aware that bevacizumab causes surgical wound healing delays, anastomotic fistulae and bleeding. Cetuximab and bevacizumab have been shown to have some therapeutic effects on locally advanced rectal cancer; however, those drugs are so expensive that many patients in China cannot afford them. Endostatin is much cheaper than the above drugs; thus, we conducted this study to determine whether endostatin combined with radiotherapy can have positive effects in an in vivo colorectal cancer mouse model. We observed that endostatin combined with radiotherapy can significantly inhibit HCT-116 cell xenograft growth, possibly by inhibiting angiogenesis and attenuating tumor cell hypoxia. Our study serves a theoretical basis for the application of the combination therapy regimen discussed herein in clinical practice.
Conclusions
Endostatin combined with radiotherapy can significantly inhibit HCT-116 cell xenograft growth, possibly by inhibiting angiogenesis and attenuating tumor cell hypoxia.
Abbreviations
- DFS:
-
Disease-free survival
- Endostar:
-
Recombinant human endostatin
- HIF-1α:
-
Hypoxia inducible factor-1α
- MVD:
-
Microvascular density
- VEGF:
-
Vascular endothelial growth factor
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Byeon JS, Yang SK, Kim TI, Kim WH, Lau JY, Leung WK, Fujita R, Makharia GK, Abdullah M, Hilmi I, et al. Colorectal neoplasm in asymptomatic Asians: a prospective multinational multicenter colonoscopy survey. Gastrointest Endosc. 2007;65(7):1015–22.
Zhang Y, Shi J, Huang H, Ren J, Li N, Dai M. Burden of colorectal cancer in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2015;36(7):709–14.
Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490–502.
Janjan NA, Crane C, Feig BW, Cleary K, Dubrow R, Curley S, Vauthey JN, Lynch P, Ellis LM, Wolff R, et al. Improved overall survival among responders to preoperative chemoradiation for locally advanced rectal cancer. Am J Clin Oncol. 2001;24(2):107–12.
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40.
Li N, Sun M, Wang Y, Lv Y, Hu Z, Cao W, Zheng J, Jiao X. Effect of cell cycle phase on the sensitivity of SAS cells to sonodynamic therapy using low-intensity ultrasound combined with 5-aminolevulinic acid in vitro. Mol Med Rep. 2015;12(2):3177–83.
Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–6.
Cook KM, Figg WD. Angiogenesis inhibitors: current strategies and future prospects. CA Cancer J Clin. 2010;60(4):222–43.
Lin K, Ye P, Liu J, He F, Xu W. Endostar inhibits hypoxia-induced cell proliferation and migration via the hypoxia-inducible factor-1alpha/vascular endothelial growth factor pathway in vitro. Mol Med Rep. 2015;11(5):3780–5.
Shang L, Zhao J, Wang W, Xiao W, Li J, Li X, Song W, Liu J, Wen F, Yue C. Inhibitory effect of endostar on lymphangiogenesis in non-small cell lung cancer and its effect on circulating tumor cells. Zhongguo Fei Ai Za Zhi. 2014;17(10):722–9.
Brideau G, Makinen MJ, Elamaa H, Tu H, Nilsson G, Alitalo K, Pihlajaniemi T, Heljasvaara R. Endostatin overexpression inhibits lymphangiogenesis and lymph node metastasis in mice. Cancer Res. 2007;67(24):11528–35.
Wang J, Sun Y, Liu Y, Yu Q, Zhang Y, Li K, Zhu Y, Zhou Q, Hou M, Guan Z, et al. Results of randomized, multicenter, double-blind phase III trial of rh-endostatin (YH-16) in treatment of advanced non-small cell lung cancer patients. Zhongguo Fei Ai Za Zhi. 2005;8(4):283–90.
Itasaka S, Komaki R, Herbst RS, Shibuya K, Shintani T, Hunter NR, Onn A, Bucana CD, Milas L, Ang KK, et al. Endostatin improves radioresponse and blocks tumor revascularization after radiation therapy for A431 xenografts in mice. Int J Radiat Oncol Biol Phys. 2007;67(3):870–8.
Luo X, Slater JM, Gridley DS. Radiation and endostatin gene therapy in a lung carcinoma model: pilot data on cells and cytokines that affect angiogenesis and immune status. Technology in cancer research & treatment. 2006;5(2):135–46.
Li N, Zheng D, Wei X, Jin Z, Zhang C, Li K. Effects of recombinant human endostatin and its synergy with cisplatin on circulating endothelial cells and tumor vascular normalization in A549 xenograft murine model. J Cancer Res Clin Oncol. 2012;138(7):1131–44.
Coco C, Valentini V, Manno A, Mattana C, Verbo A, Cellini N, Gambacorta MA, Covino M, Mantini G, Micciche F, et al. Long-term results after neoadjuvant radiochemotherapy for locally advanced resectable extraperitoneal rectal cancer. Dis Colon rectum. 2006;49(3):311–8.
Shoji H, Motegi M, Takakusagi Y, Asao T, Kuwano H, Takahashi T, Ogoshi K. Chemoradiotherapy and concurrent radiofrequency thermal therapy to treat primary rectal cancer and prediction of treatment responses. Oncol Rep. 2017;37(2):695–704.
Wang XJ, Chi P, Lin HM, XR L, Huang Y, ZB X, Huang SH, Sun YW, Ye DX. Effects of neoadjuvant chemoradiotherapy on the rates of sphincter preserving surgery in lower rectal cancer and analysis of their prognostic factors. Zhonghua Wai Ke Za Zhi. 2016;54(6):419–23.
Kuan FC, Lai CH, HY K, CF W, Hsieh MC, Liu TW, Yeh CY, Lee KD. The survival impact of delayed surgery and adjuvant chemotherapy on stage II/III rectal cancer with pathological complete response after neoadjuvant chemoradiation. Int J Cancer. 2017;140(7):1662–9.
Deliu IC, Neagoe CD, Bezna M, Genunche-Dumitrescu AV, Toma SC, Ungureanu BS, Uscatu CD, Bezna MC, Lungulescu CV, Padureanu V, et al. Correlations between endothelial cell markers CD31, CD34 and CD105 in colorectal carcinoma. Rom J Morphol Embryol. 2016;57(3):1025–30.
Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20(17):4762–73.
Yang T, Yao Q, Cao F, Liu Q, Liu B, Wang XH. Silver nanoparticles inhibit the function of hypoxia-inducible factor-1 and target genes: insight into the cytotoxicity and antiangiogenesis. Int J Nanomedicine. 2016;11:6679–92.
Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64(11):3731–6.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62.
Lin MI, Sessa WC. Antiangiogenic therapy: creating a unique "window" of opportunity. Cancer Cell. 2004;6(6):529–31.
Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553–63.
Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74(2–3):72–84.
Huang G, Chen L. Recombinant human endostatin improves anti-tumor efficacy of paclitaxel by normalizing tumor vasculature in Lewis lung carcinoma. J Cancer Res Clin Oncol. 2010;136(8):1201–11.
Wen QL, Meng MB, Yang B, LL T, Jia L, Zhou L, Xu Y, Lu Y. Endostar, a recombined humanized endostatin, enhances the radioresponse for human nasopharyngeal carcinoma and human lung adenocarcinoma xenografts in mice. Cancer Sci. 2009;100(8):1510–9.
Jiang XD, Dai P, Wu J, Song DA, JM Y. Inhibitory effect of radiotherapy combined with weekly recombinant human endostatin on the human pulmonary adenocarcinoma A549 xenografts in nude mice. Lung Cancer. 2011;72(2):165–71.
Meng MB, Jiang XD, Deng L, Na FF, He JZ, Xue JX, Guo WH, Wen QL, Lan J, Mo XM, et al. Enhanced radioresponse with a novel recombinant human endostatin protein via tumor vasculature remodeling: experimental and clinical evidence. Radiother Oncol. 2013;106(1):130–7.
Jiang XD, Dai P, Wu J, Song DA, JM Y. Effect of recombinant human endostatin on radiosensitivity in patients with non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;83(4):1272–7.
Peng F, Xu Z, Wang J, Chen Y, Li Q, Zuo Y, Chen J, Hu X, Zhou Q, Wang Y, et al. Recombinant human endostatin normalizes tumor vasculature and enhances radiation response in xenografted human nasopharyngeal carcinoma models. PLoS One. 2012;7(4):e34646.
Jia Y, Liu M, Cao L, Zhao X, Wu J, Lu F, Li Y, He Y, Ren S, Ju Y, et al. Recombinant human endostatin, Endostar, enhances the effects of chemo-radiotherapy in a mouse cervical cancer xenograft model. Eur J Gynaecol Oncol. 2011;32(3):316–24.
Hsu HW, Gridley DS, Kim PD, Hu S. De Necochea-campion R, Ferris RL, Chen CS, Mirshahidi S: Linifanib (ABT-869) enhances radiosensitivity of head and neck squamous cell carcinoma cells. Oral Oncol. 2013;49(6):591–7.
Meijer TW, Kaanders JH, Span PN, Bussink J. Targeting hypoxia, HIF-1, and tumor glucose metabolism to improve radiotherapy efficacy. Clin Cancer Res. 2012;18(20):5585–94.
Zhang L, Ge W, Hu K, Zhang Y, Li C, Xu X, He D, Zhao Z, Zhang J, Jie F, et al. Endostar down-regulates HIF-1 and VEGF expression and enhances the radioresponse to human lung adenocarcinoma cancer cells. Mol Biol Rep. 2012;39(1):89–95.
Kim SY, Shim EK, Yeo HY, Baek JY, Hong YS, Kim DY, Kim TW, Kim JH, Im SA, Jung KH, et al. KRAS mutation status and clinical outcome of preoperative chemoradiation with cetuximab in locally advanced rectal cancer: a pooled analysis of 2 phase II trials. Int J Radiat Oncol Biol Phys. 2013;85(1):201–7.
Dewdney A, Cunningham D, Tabernero J, Capdevila J, Glimelius B, Cervantes A, Tait D, Brown G, Wotherspoon A, Gonzalez de Castro D, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(14):1620–7.
Willett CG, Duda DG, di Tomaso E, Boucher Y, Ancukiewicz M, Sahani DV, Lahdenranta J, Chung DC, Fischman AJ, Lauwers GY, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(18):3020–6.
Acknowledgements
The authors thank Xiaofeng Mu (Clinical Laboratory, Qingdao Central Hospital, The Second Affiliated Hospital of Medical College of Qingdao University, Qingdao, Shandong 266042, P.R. China) for help with preparing the manuscript. The authors acknowledge the editorial assistance of American Journal Experts, Durham, North Carolina, USA.
Funding
This study was supported by the National Natural Science Foundation of China (81,370,990, 81,670,822) and Shangdong Medical and Health Technology Development Program (2014WS0229). The National Natural Science Foundation of China (81,370,990, 81,670,822) had no role in the design of the study and collection, analysis, and interpretation of data, and in writing the manuscript, but solely provided financial means to perform the described experiments. We acknowledge support by Shangdong Medical and Health Technology Development Program (2014WS0229) within the funding program Open Access Publishing.
Availability of data and materials
The datasets generated and analysed during the current study are not publicly available due this study is undergoing further research but are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
KZ, XY and YS performed experiments and wrote the manuscript; YW designed the experiments and was a major contributor in writing the manuscript; YY and XW analyzed and interpreted the data. XM provided valuable technical advice for conducting the experiments and discussed the manuscript. XM approved for the final version and submission. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal studies were approved by the Ethics Committee of Qingdao Central Hospital, the Second Affiliated Hospital of Medical College of Qingdao University, and were performed according to the local principles of laboratory animal care.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhang, K., Wang, Y., Yu, X. et al. Recombinant human endostatin combined with radiotherapy inhibits colorectal cancer growth. BMC Cancer 17, 899 (2017). https://doi.org/10.1186/s12885-017-3903-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-017-3903-3