Abstract
Background
Eribulin is a non-taxane, microtubule dynamics inhibitor that increases survival of patients with metastatic breast cancer. Although eribulin is well tolerated in patients with heavily pretreated disease, eribulin-induced liver dysfunction (EILD) can occur, resulting in treatment modification and subsequent poor disease control. We aimed to clarify the effect of EILD on patient survival.
Methods
The medical records of 157 metastatic breast cancer patients treated with eribulin between July 2011 and November 2013 at Cancer Institute Hospital were retrospectively analyzed. EILD was defined as 1) an increase in alanine aminotransferase or aspartate aminotransferase levels >3 times the upper limit of normal, and/or 2) initiation of a liver-supporting oral drug therapy such as ursodeoxycholic acid or glycyron. Fatty liver was defined as a decrease in the liver-to-spleen attenuation ratio to <0.9 on a computed tomography scan.
Results
EILD occurred in 42 patients, including one patient for whom eribulin treatment was discontinued due to severe EILD. The patients who developed EILD had significantly higher body mass indices (BMIs) than those who did not develop EILD (24.5 vs. 21.5, respectively; P < 0.0001), with no difference in the dose intensity of eribulin between the two groups (P = 0.76). Interestingly, the patients with EILD exhibited significantly longer progression-free survival (PFS) and overall survival (OS) than those without EILD (P = 0.010 and P = 0.032, respectively). Similarly, among 80 patients without liver metastasis, 19 with EILD exhibited significantly longer PFS and OS than the others (P = 0.0012 and P = 0.044, respectively), and EILD was an independent prognostic factor of PFS (P = 0.0079) in multivariate analysis. During eribulin treatment, 18 patients developed fatty liver, 11 of whom developed EILD, with a median BMI of 26.7.
Conclusions
Although EILD and fatty liver occurred at a relatively high frequency in our study, most of the patients did not experience severe adverse effects. Surprisingly, the development of EILD was positively associated with patient survival, especially in patients without liver metastases. EILD may be a clinically useful predictive biomarker of survival, but further studies are needed to confirm these findings in another cohort of patients.
Similar content being viewed by others
Background
Eribulin mesylate is a synthetic analog of halichondrin B, which is a natural product isolated from the marine sponge Halichondria okadai. Eribulin is a non-taxane, microtubule dynamics inhibitor belonging to the halichondrin class of antineoplastic agents [1].
Eribulin was demonstrated to provide significant survival benefits in patients with locally advanced and metastatic breast cancer in a randomized phase III trial that compared the use of eribulin with the treatment of the physician’s choice (TPC) [2], and its use was therefore approved in Japan for breast cancer in July 2011. Currently, it is widely used to treat locally advanced and metastatic breast cancer patients in daily practice.
Although eribulin is well tolerated in patients with heavily pretreated disease [2–5], eribulin-induced liver dysfunction (EILD) can occur in daily practice and may result in treatment modification such as dose-delay, dose-reduction, and treatment discontinuation, leading to poor disease control. Therefore, we conducted a retrospective study to clarify the effects of EILD on the survival of patients with metastatic breast cancer.
Methods
Patients
The medical records of 157 metastatic breast cancer patients treated with eribulin at the Cancer Institute Hospital between July 2011 and November 2013 were retrospectively analyzed. This study was approved and the need to obtain informed consent was waived by the Institutional Review Board of Cancer Institute Hospital (2015-1048).
Definition of EILD and fatty liver
EILD was defined as follows: 1) an increase in alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels more than three times above the upper limit of normal, and/or 2) initiation or dose-escalation of liver-supporting oral drug therapy, such as ursodeoxycholic acid or glycyron, during eribulin treatment. If patients already had transaminase levels more than 3 times the upper limit of the normal range at the beginning of eribulin treatment, and showed a further increase apparently due to eribulin, they were diagnosed with EILD.
Fatty liver was defined as a liver-to-spleen attenuation ratio of <0.9 on an unenhanced computed tomography (CT) scan [6]. The mean CT attenuation values of the liver and spleen were determined in the parenchyma of the right and left lobes of the liver and of the spleen by using a 100-mm2 region of interest cursor, avoiding liver metastases and vessels. CT was performed every 2 or 3 months to evaluate the efficacy of eribulin in most cases.
Statistical analysis
Comparisons between the treatment groups were evaluated using the chi-square test, Fisher exact test, or Mann-Whitney U-test. Progression-free survival (PFS) and overall survival (OS) curves were generated using the Kaplan-Meier method and compared using a log-rank test. Univariate and multivariate Cox proportional hazards models were used to explore the associations of specific clinical variables with PFS and OS. For all tests, differences with P < 0.05 were considered statistically significant. All analyses were performed using the JMP 6.0 software package for Windows (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics and EILD status
The patient characteristics are summarized in Table 1. The median follow-up duration for our cohort was 43.4 weeks (range, 6.0–121.7 weeks). Among the 157 patients treated with eribulin, the median treatment duration was 16.0 weeks (range, 1.9–112.4), the median age was 56 years (range, 25–81), the median disease-free interval was 2.8 years (range, 0–26.5), and the median body mass index (BMI) was 22.2 (range, 15.8–36.3). All patients had a performance status (PS) of 2 or less. Forty-one patients had comorbid diseases including diabetes (n = 7), hypertension (n = 22), hyperlipidemia (n = 6), autoimmune disorders (n = 6), cardiovascular disorders (n = 6), asthma (n = 1), and Parkinson’s disease (n = 1). However, these diseases had been controlled well during our observational period. Four patients had a history of other malignancies (2 uterine cervical cancer, one renal cancer, and one Hodgkin lymphoma), but they did not relapse during the follow-up period. Total mastectomy was performed in 142 patients, 35 patients underwent breast-conserving surgery, and 6 patients underwent bilateral breast surgery. A total of 93, 95, and 69 % patients, respectively, had been treated previously with anthracycline, taxane, and capecitabine in the adjuvant or metastatic setting. Among 25 patients with human epidermal growth factor receptor 2 (HER2)-positive tumors, 13 patients had received trastuzumab concurrently with eribulin. Therefore, among the 157 patients treated with eribulin, 144 received eribulin monotherapy. Seventy-seven patients had liver metastases at the start of eribulin treatment.
EILD occurred in 42 (27 %) patients, including ten patients who required dose-delays or dose-reductions, and one patient for whom eribulin treatment was discontinued due to EILD. Among the patients with EILD, aminotransferase levels were the highest at a median of 21 days (range, 8–154) after the first eribulin administration. The median AST and ALT levels at the beginning of the eribulin treatment were 34 IU/L (range, 17–78) and 23 IU/L (range, 10–65), respectively, and the medians of the highest AST and ALT levels during eribulin treatment were 93 IU/L (range, 39–300) and 91 IU/L (range, 37–268), respectively.
The patients who developed EILD had significantly higher BMIs than those who did not develop EILD (24.5 vs. 21.5, respectively; P < 0.0001; Table 1), while there was no difference in the dose intensity of eribulin between the two groups (0.72 vs. 0.72 mg/m2/week, respectively; P = 0.76; Table 1). Interestingly, patients with a good PS exhibited a higher frequency of EILD than those with a poor PS (P = 0.05, Table 1). The development of EILD was not associated with other clinical factors such as patient age, comorbid disease status, history of other malignancy, primary surgical procedure, disease-free interval (DFI), hormone-receptor (HR) status, HER2 status, previous chemotherapy, previous endocrine therapy, type of prior treatment, and liver metastatic status (Table 1).
Prognostic value of EILD for patient survival
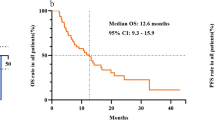
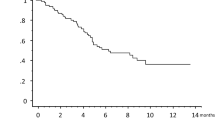
Patients with EILD had significantly longer PFS and OS than those without EILD (P = 0.010 and P = 0.032, respectively; Fig. 1a and b). Interestingly, this difference appeared to be specific for patients without liver metastases. Among the 80 patients without liver metastases, patients with EILD (n = 19) exhibited significantly longer PFS and OS compared patients without EILD (n = 61) (P = 0.0012 and P = 0.044, respectively; Fig. 1c and d). By contrast, EILD was not significantly associated with PFS or OS in patients with liver metastases (P = 0.58 and P = 0.28, respectively; Fig. 1e and f).
Progression-free survival (PFS) and overall survival (OS) analyses. a-b: PFS (a) and OS (b) for patients with and without eribulin-induced liver dysfunction (EILD) among all patients. c-d: PFS (c) and OS (d) for the patients with and without EILD among the patients without liver metastasis. e-f: PFS (e) and OS (f) for the patients with and without EILD among the patients with liver metastasis
In order to clarify the prognostic role of EILD, we conducted multivariate analyses of clinically significant factors including age, PS, comorbid disease status, DFI, BMI, HR status, HER2 status, liver metastatic status, and development of EILD. In the whole cohort (n = 157), multivariate analyses identified only PS as an independent prognostic indicator of better OS (P = 0.0039, Table 2), whereas no clinical factors were significantly associated with PFS. In contrast, in the subset of the patients without liver metastases (n = 80), EILD was the only significant independent predictor of PFS (P = 0.0079, Table 3), and no clinical factors were significantly associated with OS.
Fatty liver disease during eribulin treatment
During eribulin treatment, 18 (11 %) patients who developed fatty liver disease had a median BMI of 26.7, which was significantly higher than that of patients who did not develop fatty liver disease (26.7 vs. 21.7, respectively; P < 0.0001). Eleven (61 %) of these 18 patients developed EILD.
Discussion
In the present study, we found that EILD occurred with a relatively high frequency, but most patients, except one, did not experience severe liver damage. In this particular patient, eribulin treatment was terminated because the patient’s aminotransferase levels increased to more than ten times the upper limit of normal and PS decreased to and remained at 3 even after aminotransferase levels peaked in the early phase of eribulin treatment. While liver toxicity was uncommon in the global phase III trial of eribulin vs. TPC, in which 93 % of the patients were Caucasian [2], a Japanese phase II trial demonstrated liver toxicity in approximately 30 % of patients [5]. These studies suggest that ethnicity may influence eribulin-induced liver toxicity.
To our knowledge, this is the first study to demonstrate that eribulin induces fatty liver disease, and that the development of both EILD and fatty liver disease is significantly associated with higher BMI. These results indicate that eribulin might precipitate liver damage and latent fatty liver in patients with obesity. The main mechanism of chemotherapy-induced liver injury is thought to be secondary to the production of reactive oxygen species (ROS), and steatotic livers are more susceptible to chemotherapy-induced injury [7]. Presumably, eribulin might also produce ROS in hepatocytes in a similar manner, resulting in EILD and fatty liver disease. In fact, other microtubule-targeted agents such as paclitaxel and vinorelbine were shown to induce accumulation of ROS in cancer cell lines, and their anti-tumor effects partially depend on this mechanism [8, 9].
Paradoxically, we observed a positive correlation between the development of EILD and patient survival, especially in patients without liver metastases. Therefore, EILD may be a clinically useful and easily available biomarker that can be used to predict the efficacy of eribulin in the early stages of treatment. One possible reason for this finding may be that patients with EILD had a better nutritional status, and hence, they could survive longer. However, previous studies showed that obesity was a poor prognostic factor for patients with metastatic breast cancer [10, 11]. Furthermore, most adjuvant clinical trials showed the same results [12].
Another possible cause of our paradoxical results is that eribulin-induced ROS production might result in the eradication of minute metastases consisting mainly of cancer stem cells (CSCs). The CSC hypothesis has been widely accepted, and CSCs are considered to play an important role in the initiation of tumor metastasis [13–16]. Moreover, ROS have a dual role in cancer progression. Although ROS are thought to play an important role in carcinogenesis initiation, malignant transformation, and cell proliferation, excess ROS production can also trigger apoptosis of malignant cells [17]. Cellular ROS metabolism is tightly regulated by the redox mechanism, and ROS concentrations are maintained lower especially in CSCs compared to non-CSCs [18–20]. ROS elevation by exogenous drugs may be a potential treatment strategy to selectively kill CSCs [21], and in fact, some chemotherapeutic drugs have been shown to elicit such an effect on leukemic stem cells [22–24].
Taken together, these previous evidences described above support our hypothesis. In fact, we found that, among the 70 patients without liver metastasis at the beginning of eribulin treatment who were evaluated for liver metastasis at the final follow-up, the appearance of new metastatic liver lesions was less frequent in those who developed EILD than in those who did not (2 of 19 [10.5 %] and 12 of 51 [23.5 %], respectively). This is consistent with our hypothesis that eribulin-induced ROS production eradicates minute disseminated CSCs in the liver. However, the difference between these frequencies was not significant (P = 0.32); therefore, larger studies are needed to further evaluate this hypothesis.
Conclusions
In summary, although EILD occurred with a relatively high frequency in eribulin-treated breast cancer patients, it was generally well tolerated in heavily pretreated patients in clinical practice. To our knowledge, this is the first study to show that eribulin may induce fatty liver disease, and that EILD and fatty liver disease occur more frequently in obese patients.
We found that EILD was a significant positive prognostic factor for breast cancer patient survival, especially among patients without liver metastasis. EILD may be a clinically useful and easily available biomarker that can be used to predict the efficacy of eribulin in the early stages of treatment. However, our study was limited by its small size, retrospective design, and restriction to a single institute. Therefore, further studies are needed to confirm our findings in other patient cohorts and to elucidate the mechanism of EILD.
Abbreviations
BMI, body mass index; CSC, cancer stem cell; CT, computed tomography; DFI, disease-free survival; EILD, eribulin-induced liver dysfunction; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; OS, overall survival; PFS, progression-free survival; TPC, treatment of physician’s choice
References
Okouneva T, Azarenko O, Wilson L, Littlefield BA, Jordan MA. Inhibition of centromere dynamics by eribulin (E7389) during mitotic metaphase. Mol Cancer Ther. 2008;7(7):2003–11.
Cortes J, O’Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Dieras V, Delozier T, et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377(9769):914–23.
Vahdat LT, Pruitt B, Fabian CJ, Rivera RR, Smith DA, Tan-Chiu E, Wright J, Tan AR, Dacosta NA, Chuang E, et al. Phase II study of eribulin mesylate, a halichondrin B analog, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2009;27(18):2954–61.
Cortes J, Vahdat L, Blum JL, Twelves C, Campone M, Roche H, Bachelot T, Awada A, Paridaens R, Goncalves A, et al. Phase II study of the halichondrin B analog eribulin mesylate in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2010;28(25):3922–8.
Aogi K, Iwata H, Masuda N, Mukai H, Yoshida M, Rai Y, Taguchi K, Sasaki Y, Takashima S. A phase II study of eribulin in Japanese patients with heavily pretreated metastatic breast cancer. Ann Oncol. 2012;23(6):1441–8.
Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, Park SW, Ha HK, Lee MG, Hwang S, Lee SG, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239(1):105–12.
Maor Y, Malnick S. Liver injury induced by anticancer chemotherapy and radiation therapy. Int J Hepatol. 2013;2013:815105.
Thomas-Schoemann A, Lemare F, Mongaret C, Bermudez E, Chereau C, Nicco C, Dauphin A, Weill B, Goldwasser F, Batteux F, et al. Bystander effect of vinorelbine alters antitumor immune response. Int J Cancer. 2011;129(6):1511–8.
Alexandre J, Hu Y, Lu W, Pelicano H, Huang P. Novel action of paclitaxel against cancer cells: bystander effect mediated by reactive oxygen species. Cancer Res. 2007;67(8):3512–7.
von Drygalski A, Tran TB, Messer K, Pu M, Corringham S, Nelson C, Ball ED. Obesity is an independent predictor of poor survival in metastatic breast cancer: retrospective analysis of a patient cohort whose treatment included high-dose chemotherapy and autologous stem cell support. Int J Breast Cancer. 2011;2011:523276.
Jung SY, Rosenzweig M, Sereika SM, Linkov F, Brufsky A, Weissfeld JL. Factors associated with mortality after breast cancer metastasis. Cancer Causes Control. 2012;23(1):103–12.
Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, Navarro Rosenblatt D, Thune I, Vieira R, Norat T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901–14.
Brabletz T. EMT and MET in metastasis: where are the cancer stem cells? Cancer Cell. 2012;22(6):699–701.
Charafe-Jauffret E, Ginestier C, Iovino F, Tarpin C, Diebel M, Esterni B, Houvenaeghel G, Extra JM, Bertucci F, Jacquemier J, et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 2010;16(1):45–55.
Balic M, Lin H, Young L, Hawes D, Giuliano A, McNamara G, Datar RH, Cote RJ. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12(19):5615–21.
Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009;11(4):R46.
Gupta SC, Hevia D, Patchva S, Park B, Koh W, Aggarwal BB. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid Redox Signal. 2012;16(11):1295–322.
Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell. 2011;19(3):387–400.
Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, Lin Y, Yao J, Shi J, Kang T, et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013;23(3):316–31.
Kim HM, Haraguchi N, Ishii H, Ohkuma M, Okano M, Mimori K, Eguchi H, Yamamoto H, Nagano H, Sekimoto M, et al. Increased CD13 expression reduces reactive oxygen species, promoting survival of liver cancer stem cells via an epithelial-mesenchymal transition-like phenomenon. Ann Surg Oncol. 2012;19 Suppl 3:S539–48.
Shi X, Zhang Y, Zheng J, Pan J. Reactive oxygen species in cancer stem cells. Antioxid Redox Signal. 2012;16(11):1215–28.
Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, Jordan CT. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105(11):4163–9.
Ito K, Bernardi R, Morotti A, Matsuoka S, Saglio G, Ikeda Y, Rosenblatt J, Avigan DE, Teruya-Feldstein J, Pandolfi PP. PML targeting eradicates quiescent leukaemia-initiating cells. Nature. 2008;453(7198):1072–8.
Jin Y, Lu Z, Ding K, Li J, Du X, Chen C, Sun X, Wu Y, Zhou J, Pan J. Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: inactivation of the NF-kappaB pathway and generation of reactive oxygen species. Cancer Res. 2010;70(6):2516–27.
Acknowledgments
We would like to thank Editage [http://www.editage.com] for editing and reviewing this manuscript for English language.
Funding
This study was supported by a research funding from Department of Medical Oncology, Cancer Institute Hospital.
Availability of data and materials
The datasets supporting conclusions of this article are included within the article.
Authors’ contributions
TK conceived the study, analyzed the data, and wrote the manuscript. JT, IF, TS, and NT acquired and analyzed the data, and participated in revising the manuscript. YI, TI, and, SO participated in designing the study and revising the manuscript. ST participated in the overall design and study coordination and finalized the draft of the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved and the need to obtain informed consent was waived by the Institutional Review Board of Cancer Institute Hospital (2015-1048).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kobayashi, T., Tomomatsu, J., Fukada, I. et al. Eribulin-induced liver dysfunction as a prognostic indicator of survival of metastatic breast cancer patients: a retrospective study. BMC Cancer 16, 404 (2016). https://doi.org/10.1186/s12885-016-2436-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-016-2436-5