Abstract
Background
Type 2 diabetes mellitus (T2DM) has been suggested to increase the risk of cancers. The aim of this study was to investigate the risk of common cancers in Chinese patients with T2DM.
Methods
A population-based retrospective cohort study including 36,379 T2DM patients was conducted in Minhang District of Shanghai, China, during 2004 to 2010. All T2DM patients were enrolled from the standardized management system based on local electronic information system. Newly-diagnosed cancer cases were identified by record-linkage with the Shanghai Cancer Registry. Standardized incidence ratios (SIR) and 95 % confidence interval (CI) were used to estimate the risk of cancers among T2DM patients.
Results
Overall crude incidence rate (CIR) of cancers was 955.21 per 105 person-years in men and 829.57 per 105 person-years in women. Increased risk of cancer was found in both gender, with an SIR being 1.28 (95 % CI = 1.17–1.38) in men and 1.44 (95 % CI =1.32–1.55) in women. Increased risk of colon (SIR = 1.97; 95 % CI = 1.49 to 2.46), rectum (1.72; 1.23 to 2.21), prostate (2.87; 2.19 to 3.56), and bladder cancers (1.98, 1.28 to 2.68) were observed in men and elevated risk of colon (1.67; 1.25 to 2.08), breast (1.66; 1.38 to 1.95), and corpus uteri cancers (2.87; 2.03 to 3.71) were observed in women.
Conclusions
Our results indicate that Chinese patients with T2DM may have an increased risk of some cancers, and the increase may vary by sub-sites of cancers.
Similar content being viewed by others
Background
Type 2 diabetes mellitus (T2DM) is one of the major public health problems around the world. Over past decades, the prevalence of T2DM has been increasing rapidly in Asian populations [1]. In China, the prevalence of diabetes and pre-diabetes reached 9.7 and 15.5 %, respectively, in adults at age of 20 years or above in 2008, which means that 92 million Chinese adults were with diabetes and 148 million with pre-diabetes in the country [2].
Epidemiologic evidence is accumulating on the association between T2DM and cancers, particularly for cancers of colorectum [3–5], liver [6, 7], breast [8, 9], bladder [10], kidney [11], and prostate [12]. However, the results are not consistent [13–17], and the associations differ by race and ethnicity [18]. To elucidate the associations of T2DM with the risk of cancers is particularly important in China, because, with the huge population and high prevalence of T2DM, even a small increase in the cancer risk might have a significant public health impact at the population level for the country.
The present study was specifically designed to reveal the possible associations of T2DM with overall and sub-sites of specific cancers in Chinese adults by a population-based risk analysis.
Methods
Data sources
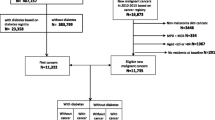
Based on the Chinese National Diabetes Prevention Guide, standardized management for diabetes patients, as a basic community health service, has been carried out since 2004 in Minhang district, an administrative division with 1,000,000 residents of Shanghai, China. The patients enrolled in the management system included: 1) part of prevalent T2DM patients; 2) newly-diagnosed T2DM patients from local comprehensive hospitals, 3) newly-diagnosed T2DM patients from regular health check-up programs for all local residents over 60 years provided by community health service centers (CHSC), and 4) newly-diagnosed T2DM patients from free screening for high risk group with specific symptoms or claims provided by CHSC. Due to that the T2DM patients diagnosed in hospitals out of Minhang district were not routinely enrolled for standardized management, most T2DM patients registered in the system were from local hospitals, which accounted for about 90 % of all newly-diagnosed patients in local hospitals each year.
The standardized management referred to regular following-up of patients carried out by General Practitioners. According to the Chinese National Diabetes Prevention Guide, the frequency of following-up and health care service provided to the patients were determined and standardized according to the patient’s clinical conditions. The patients with fasting blood glucose < 7.0 mmol/L were followed up every 3 months by measuring blood pressure and testing levels of blood glucose, glycosylated hemoglobin and urine protein, and the patients with fasting glucose > 7.0 mmol/L were followed up once per month. During the 7-year period from 2004 to 2010, a total of 36,379 diabetes patients were enrolled and followed-up. All these data was recorded as electronic health records (eHR) and transmitted into the eHR database. Using the unique identification card number of each individual, the incident cancer cases were identified through record-linkage of eHR system with Cancer Registry System in Minhang district, which is part of the Shanghai Cancer Registry system [19, 20]. The ICD-10 codes were assigned to each cancer case by the type of cancers such as Stomach (C16), Colon (C18), Rectum (C19-C20), Liver (C22), Pancreas (C25),Trachea / bronchus and lung (C33-C34), Brain/central nervous system (C70-72), Prostate (C61), Kidney (C64), Bladder (C67), Breast (C50), Corpus uteri (C53) and Thyroid (C73).
Datum of all subjects was acquired based on local basic community health service. Verbal informed consent was obtained from all these enrolled patients. Ethics approval was granted from the Institutional Review Board of Minhang Center for Disease Control and Prevention (NO: EC-P-2012-002).
Statistical analysis
Person-years (PY) of follow-up were calculated from the date that T2DM was first diagnosed to the date on which common cancer was diagnosed, or death date, or the December 31, 2010, whichever occurred first. Crude incidence rate (CIR) for cancers was calculated by the number of incident cancer cases divided by the number of observed person-years. The age-standardized rate (ASR [W]) was calculated using the World Standard Population.
The standardized incidence ratio (SIR) and its 95 % confident interval (95 % CI) were used to estimate the risk of common cancer in patients with T2DM. SIRs of cancers were determined by comparing the observed and expected number of cancer cases among T2DM patients, in which the latter was calculated by applying the age-specific incidence rates in the general population.
The 95 % CI of SIR was calculated using the following formula:
Where
U test based on Poisson distribution was applied to compare the CIRs of cancers between prevalent and newly-diagnosed T2DM patients by using the following formula:
X1 and X2 represent the number of cancer cases in prevalent and newly-diagnosed T2DM patients, and n1 and n2 represent person-years of observation for prevalent and newly-diagnosed T2DM patients, respectively.
All tests were two sided. P value less than 0.05 was considered as statistical significant.
Results
Between Jan 1, 2004 and Oct 31, 2010, 36,379 T2DM patients were enrolled and followed-up regularly through eHR system in Minhang district of Shanghai, China. The characteristics of the T2DM patients are shown in Table 1. Of the 36,379 T2DM patients, 16,166 (44.4 %) were men and 20,213 (55.6 %) were women, with mean age being 58.44 and 59.37 years, respectively. After 136,187 person-years of following-up, 1205 cancer cases were identified through record-linkage of eHR system with the Cancer Registry System. The overall crude incidence rate (CIR) of cancers was 955.21 per 105 person-years in men and 829.57 per 105 person-years in women, with ASR (W) being 289.69 and 246.22 per 105 person-years, respectively. The top ten cancer subtypes by CIR were Stomach, Colon, Rectum, Liver, Pancreas, Trachea, bronchus and lung, Brain, central nervous system, Prostate, Bladder, and Kidney cancer in men, and were Stomach, Colon, Rectum, Liver, Pancreas, Trachea/bronchus and lung, Brain, central nervous system, Breast, Corpus uteri, and Thyroid cancer in women, as shown in Table 2.
SIRs of common cancers in T2DM patients by gender and subtypes of cancers are shown in Table 3. An increased risk of overall cancer in T2DM patients were found in both men and women, with SIRs of 1.28 (95 % CI: 1.17–1.38) and 1.44 (95 % CI: 1.32–1.55), respectively. For subtypes of cancer, a significant increased risk of colon (1.97; 1.49 to 2.46), rectum (1.72; 1.23 to 2.21), prostate (2.87; 2.19 to 3.56), and bladder cancers (1.98, 1.28 to 2.68) was observed in men and elevated risk of colon (1.67; 1.25 to 2.08), breast (1.66; 1.38 to 1.95) and corpus uteri cancers (2.87; 2.03 to 3.71) was observed in women. We did not find a significant association between T2DM and Stomach, Liver, Pancreas, Brain, Kidney cancers.
The risk of cancers was not observed to increase in patients at all age groups. In men, an increased risk was found in patients at ages of 70- and 80- years, with SIRs of 1. 54 (95 % CI: 1.34–1.73) and 2.79 (95 % CI: 2.17–3.42), respectively. In women, an increased risk was also observed in older patients, with SIRs being 1.48 (95 % CI: 1.28–1.69) in patients at ages of 60- years, 1.79 (95 % CI: 1.56–2.03) in patients at ages of 70- years, and 2.20 (95 % CI: 1.61–2.78) in patients at ages of 80- years, respectively (as shown in Table 4).
In this study, we did not find a significant difference in age distribution between prevalent and newly-diagnoseddiabetes patients. However, the risk of cancers was significantly higher in prevalent diabetes patients (CIR = 494.37 per 105 person-years) than in those newly-diagnosed (CIR = 991.04 per 105 person-years). The difference was significant in both men (U = 5.06, P < 0.05) and women (U = 8.75, P < 0.05) (as shown in Table 5).
Discussion
The association between T2DM and the risk of common cancers has been extensively investigated in western countries. In China, however, only a few studies have been focused on the associations, and the findings were not consistent [15, 16]. In this study, based on local population-based registry data, we observed an increased risk of overall cancer in Chinese with T2DM. Our results are consistent with Wideroff et al’s report [13], in which diabetic patients in Denmark was found having 10 % increased risk of all types of cancers (SIR = 1.1). However, no significant association was found between T2DM and an incidence of cancer (RR = 0.99, 95 % CI = 0.90–1.09) in a UK study [17]. Ethnic discrepancy on biologic effect of T2DM may partly explain the inconsistency [18]. Moreover, due to the fact that the risks of cancers in diabetes patients may vary by sub-sites of cancers and the ranks of cancers differ across populations, it is necessary to compare the associations of T2DM with the risk of site-specific cancers in different populations.
One of the main findings in previous studies is that diabetes patients have an increased risk of colorectal cancer [17, 21, 22]. However, the increases in risk of colon and rectum cancers were found much different. Ren, et al. [23] reported a significant increased risk of colon cancer in male T2DM patients and a borderline significant risk of colon cancer in females, and did not find a significant association between T2DM and risk of rectal cancer in both sexes. The results in this study are sometime inconsistent with Ren, et al’s report. Our patients, both male and female, were more likely to have colon cancer. The increase in the risk of rectum cancer was also observed in both sexes, but the association reached significant only in male patients.
Our finding of an elevated risk of prostate cancer in male T2DM patients is consistent with the results reported by Tseng, et al. [24] and Will, et al. [25], but is not in line with some other studies. Evidence has been available for the null [26] and inverse associations between T2DM and prostate cancer [27, 28]. The result from a meta-analysis indicates that diabetes was associated with an increased risk of prostate cancer in Asians but the RR was greatly attenuated by adjusting for some confounders (unadjusted RR = 2.82, adjusted RR = 1.31) [29]. The mechanism underlying the association is unclear. It is possible that the difference in exposures to common risk factors such as tobacco smoking, obesity, and some psychology factors [30] and difference in genetic background [31–33] may contribute to the inconsistency. Moreover, average level of PSA was significantly lower in diabetes patients than in non-diabetes, as were as frequency of PSA screening [34], which may partly explain the inverse association between T2DM and prostate cancer.
For other types of cancers, we observed higher risks of breast and Corpus uteri cancers in females and bladder cancer in males, which is consistent with previous studies [15, 16]. A significant inverse association between diabetes and lung cancer was observed in men, which is also consistent with previous studies [16]. However, we did not find a significant association of T2DM with the risk of pancreatic and kidney cancers, which is not consistent with the positive association reported in several studies [35, 36]. Inadequate statistical power due to small sample size may explain the results on rare cancers. Moreover, we did not take some potential confounders into consideration such as sedentary lifestyle, tobacco smoking and obesity, the shared risk factors of T2DM and certain cancers [37]. More studies are needed to distinguish the effect of diabetes from those of shared risk factors and diabetes management.
Many biological mechanisms may be involved in the elevated risks of overall or certain sub-sites cancers among T2DM patients. Usually, diabetic patients have hyperinsulinemia and are associated with reduced insulin sensitivity and compensatory hyperinsulinemia as well as an increased insulin-like growth factor (IGF)-1 level, which may stimulate cell proliferation in pancreas, colon, breast, and other organs. Insulin itself exerts a mitogenic effect on various tissues including breast cancer cell lines, which are usually oestrogen receptor positive [38]. In breast cancer, insulin induces aromatase activity and reduces sex hormone binding globulin (SHBG), leading to increased free oestrogen levels, which in turn increases mitogenicity [39]. Interestingly, breast cancer cells appear to have high levels of insulin receptors, compared to normal breast tissue [40]. Insulin may exert a mitogenic effect through insulin-like growth factor-1 (IGF-1) receptors. Prospective studies have shown that people with circulating IGF-1 have an increased risk of common epithelial cancers such as breast, colon and prostate cancer [41]. A recent large population based survey showed that women with high blood concentrations of IGF-1 are more likely to develop breast cancer, women with the highest concentration of IGF-1 were found to have a 28 % higher risk of developing breast cancer than women with the lowest concentration [OR 1.28 (95 % CI 1.14–1.44)] [42]. The direct link between diabetes and cancer through hyperinsulinemia, hyperglycemia and inflammation may explain the high risk of cancers in prevalent T2DM patients than in those newly-diagnosed. It has been suggested that duration of diabetes and use of exogenous insulin were associated with the cancer risk [30]. It seems that long-term exposure to insulin has direct relevance to the cancer risk.
Strengths and limitations
The strengths of this study included its prospective study design, representative and relative large sample size of diabetes patients, confirmation system of diagnosis and high quality cancer registry system, which provide us a good opportunity to evaluate the possible effect of T2DM on cancer risk in Chinese population. Several limitations of the study should be mentioned. First, the small number of some site-specific cancers may lead to a low statistical power to detect a significant association. Therefore, we only focused on the top ten subtypes of cancers to ensure statistical power. Second, the average following-up time was rather short for many patients, which also may influence the statistical power. Moreover, due to the number of patients screened varied year by year, it is possible that the patients recruited in certain years were detected earlier while those in other years were not, which may lead to heterogeneity of the patient population. Third, although age and gender were controlled in calculation of SIR, we did not obtain and adjust other risk factors such as smoking, alcohol consumption, body mass index (BMI), physical activity and use of antidiabetic medicines, in which use of antidiabetic drug metformin has been associated with reduced risk of cancers [36] and BMI was reported to be associated with increased risk of both of T2DM and cancers [37]. Finally, diabetes is an under-diagnosed disease and some T2DM patients remained undiagnosed, which may lead to misclassification bias.
Conclusion
In summary, the present study suggested that type 2 diabetes may increase the risks of overall cancers, particularly in older patients and prevalent patients. Diabetes is associated with an increased risk some cancers (colon, rectum, prostate, and bladder cancers in men and colon, breast, and corpus uteri cancers in women), and related with a reduced risk of lung cancer in men. For some other cancer sites, there appears to be no association possibly due to inadequate statistics power.
References
World Health Organization (WHO): The World Health Report 2004: Changing History. 2004. Available: http://www.who.int/whr/2004/en/
Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of diabetes among Men and women in China. N Engl J Med. 2010;362:1090–101.
Larsson SC, Giovannucci E, Wolk A. Diabetes and colorectal cancer incidence in the cohort of Swedish men. Diabetes Care. 2005;28:1805–7.
Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–87.
Elwing JE, Gao F, Davidson NO, Early DS. Type 2 diabetes mellitus: the impact on colorectal adenoma risk in women. Am J Gastroenterol. 2006;101:1866–71.
Polesel J, Zucchetto A, Montella M, Dal Maso L, Crispo A, La Vecchia C, et al. The impact of obesity and diabetes mellitus on the risk of hepatocellular carcinoma. Ann Oncol. 2008;20:353–7.
Donadon V. Association between hepatocellular carcinoma and type 2 diabetes mellitus in Italy: potential role of insulin. World J Gastroenterol. 2008;14:5695.
Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121:856–62.
Michels KB, Solomon CG, Hu FB, Rosner BA, Hankinson SE, Colditz GA, et al. Nurses’ health study. T2DM and subsequent incidence of breast cancer in the Nurses’ health study. Diabetes Care. 2003;26:1752–8.
Larsson SC, Andersson SO, Johansson JE, Wolk A. Diabetes mellitus, body size and bladder cancer risk in a prospective study of Swedish men. Eur J Cancer. 2008;44:2655–60.
Larsson SC, Wolk A. Diabetes mellitus and incidence of kidney cancer: a meta-analysis of cohort studies. Diabetologia. 2011;54:1013–8.
Waters KM, Henderson BE, Stram DO, Wan P, Kolonel LN, Haiman CA. Association of diabetes with prostate cancer risk in the multiethnic cohort. Am J Epidemiol. 2009;169:937–45.
Wideroff L, Gridley G, Mellemkjaer L, Chow WH, Linet M, Keehn S, et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst. 1997;89:1360–5.
Oba S, Nagata C, Nakamura K, Takatsuka N, Shimizu H. Self-reported diabetes mellitus and risk of mortality from all causes, cardiovascular disease, and cancer in Takayama: a population-based prospective cohort study in Japan. J Epidemiol. 2008;18:197–203.
Zhang P, Chen Z, Lv D, Xu YY, Gu WL, Zhang XH, et al. Increased risk of cancer in patients with type 2 diabetes mellitus: A retrospective cohort study in China. BMC Public Health. 2012;12:567.
Cheng Chieh L, Jen Huai C, Chia Ing L, et al. Cancer risks among patients with type 2 diabetes: a 10-year follow-up study of a nationwide population-based cohort in Taiwan. BMC Cancer. 2014;14:381.
Ogunleye AA, Ogston SA, Morris AD, Evans JM. A cohort study of the risk of cancer associated with type 2 diabetes. Br J Cancer. 2009;101:1199–201.
Woodward M, Zhang X, Barzi F, Pan W, Ueshima H, Rodgers A, et al. The effects of diabetes on the risks of major cardiovascular diseases and death in the Asia-Pacific region. Diabetes Care. 2003;26:360–6.
Xiang YB, Jin F, Gao YT. Cancer survival in Shanghai, China, 1992–1995. IARC Sci Publ. 2011;162:55–68.
Shao Hua X, Juan C, Bo Z, Feng W, Shan Shan L, Chang Hui X, et al. Time trends and age-period-cohort analyses on incidence rates of thyroid cancer in Shanghai and Hong Kong. BMC Cancer. 2014;14:975.
Leitzmann MF, Ahn J, Albanes D, Hsing AW, Schatzkin A, Chang S-C, et al. Diabetes mellitus and prostate cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Causes Control. 2008;19:1267–76.
Limburg PJ, Vierkant RA, Fredericksen ZS, Leibson CL, Rizza RA, Gupta AK, et al. Clinically confirmed T2DM mellitus and colorectal cancer risk: a population-based, retrospective cohort study. Am J Gastroenterol. 2006;101:1872–9.
Ren X, Zhang X, Zhang X, Gu W, Chen K, Le Y, et al. T2DM mellitus associated with increased risk for colorectal cancer: evidence from an international ecological study and population-based risk analysis in China. Public Health. 2009;123:540–4.
Chin Hsiao T. Diabetes and risk of prostate cancer, a study using the national health insurance. Diabetes Care. 2011;34(3):616–21.
Will JC, Vinicor F, Calle EE. Is diabetes mellitus associated with prostate cancer incidence and survival. Epidemiology. 1999;10(3):313–8.
Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2056–62.
Pierce BL, Plymate S, Ostrander EA, Stanford JL. Diabetes mellitus and prostate cancer risk. Prostate. 2008;68:1126–32.
Smith MR, Bae K, Efstathiou JA, Hanks GE, Pilepich MV, Sandler HM, et al. Diabetes and mortality in Men with locally advanced prostate cancer: RTOG 92–02. J Clin Oncol. 2008;26:4333–9.
Long X-J, Lin S, Sun Y-N. Diabetes mellitus and prostate cancer risk in Asian countries:a meta-analysis. Asian Pacific J Cancer Prevent. 2012;13:4097–100.
Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60:207–21.
Gudmundsson J, Sulem P, Steinthorsdottir V. Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet. 2007;39(8):977–83.
Frayling TM, Colhoun H, Florez JC. A genetic link between type 2 diabetes and prostate cancer. Diabetologia. 2008;51(10):1757–60.
Meyer TE, Boerwinkle E, Morrison AC, Volcik KA, Sanderson M, Coker AL, et al. Diabetes genes and prostate cancer in the atherosclerosis risk in communities study. Cancer Epidemiol Biomarkers Prev. 2010;19(2):558–65.
Waters KM, Henderson BE, Stram DO. Association of diabetes with prostate cancer risk in the multiethnic cohort. Am J Epidemiol. 2009;169:937–45.
Hemminki K, Li X, Sundquist J, Sundquist K. Risk of cancer following hospitalization for T2DM. Oncologist. 2010;15:548–55.
Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–8.
Zheng W, McLerran DF, Rolland B. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med. 2011;364:719–29.
Rose DP, Komninou D, Stephenson GD. Obesity, adipocytokines and insulin resistance in breast cancer. Obes Rev. 2004;5:153–65.
McTernan PG, Anwar A, Eggo MC, Barnett AH, Stewart PM, Kumar S. Gender differences in the regulation of P450 aromatase expression and acitivity in human adipose tissue. Int J Obes Relat Metab Disord. 2000;24:875–81.
Papa V, Pezzino V, Costantino A, Belfiore A, Giuffrida D, Frittitta L. Elevated insulin receptor content in human breast cancer. J Clin Invest. 1990;86:1503–10.
Pollack M, Schernhammer ES, Hankinsin SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–18.
The Endogenous Hormones and Breast Cancer Collaborative Group. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2011;11(6):530–42.
Acknowledgements
This study was supported by Research Fund from Shanghai Municipal Health Bureau (NO: 20124013 and 20124y179), Characteristic Program of Chronic Disease from Minhang District Health Bureau (NO:2013MWTX01) and Talent Development Fund and Young Doctor Fund of Minhang district, Shanghai.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HLX, HF and YPZ were involved in study concept and design. JZ, YJY, LYZ, BDY, YNL, FZ, WXL, JLZ and LQL involved in data acquisition. Analysis and interpretation of data was done by HLX, WHX, NQZ, BDY, GYQ and NW. Manuscript writing was made by HLX, HF, WHX and YPZ. Manuscript review and final approval was done by all authors.
Hui-Lin Xu and Hong Fang contributed equally to this work.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Xu, HL., Fang, H., Xu, WH. et al. Cancer incidence in patients with type 2 diabetes mellitus: a population-based cohort study in Shanghai. BMC Cancer 15, 852 (2015). https://doi.org/10.1186/s12885-015-1887-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-015-1887-4