Abstract
Background
Uveal melanoma is characterised by mutations in GNAQ and GNA11, resulting in Ras/Raf/MEK/ERK pathway activation. Treatment with selumetinib (AZD6244, ARRY-142886), a MEK1/2 inhibitor, results in antitumour effects in uveal melanoma pre-clinical models. A randomised phase II trial demonstrated improved progression-free survival (PFS) and response rate (RR) with selumetinib monotherapy versus chemotherapy with temozolomide or dacarbazine in patients with metastatic uveal melanoma. Pre-clinically, selumetinib in combination with alkylating agents enhanced antitumour activity compared with chemotherapy alone. We hypothesise that selumetinib in combination with dacarbazine will result in improved clinical outcomes in patients with metastatic uveal melanoma versus dacarbazine alone.
Methods/Design
SUMIT is a randomised, international, double-blind, placebo-controlled, phase III study assessing the efficacy and safety of selumetinib in combination with dacarbazine in patients with metastatic uveal melanoma who have not received prior systemic therapy. Primary endpoint is PFS. Secondary endpoints include objective RR, duration of response, change in tumour size at Week 6, overall survival, safety and tolerability. Exploratory endpoints include efficacy in tumours with GNAQ or GNA11 mutations. Eligible patients must have: ≥1 lesion that can be accurately measured at baseline, and is suitable for accurate repeated measurements; ECOG performance status 0–1; life expectancy >12 weeks. Mutation status for GNAQ/GNA11 will be assessed retrospectively.
An estimated 128 patients from approximately 50 sites globally will be randomised (3:1) to selumetinib 75 mg twice daily or placebo in combination with dacarbazine 1000 mg/m2 on Day 1 of every 21-day cycle until objective disease progression, intolerable toxicity or occurrence of another discontinuation criterion. Randomisation will be stratified by the presence/absence of liver metastases. Tumours will be evaluated by RECIST v1.1 every 6 weeks. All patients have the option of receiving selumetinib with or without dacarbazine at disease progression. Study enrolment began in April 2014 and is expected to complete in early 2015.
Discussion
Treatment of patients with metastatic uveal melanoma represents an area of high unmet medical need. This study evaluating selumetinib in combination with dacarbazine was designed with input from the US FDA, and is the first potential registration trial to be conducted in patients with metastatic uveal melanoma.
Trial registration
Clinicaltrials.gov (Date of registration, October 10, 2013)
Registration number: NCT01974752
Trial abbreviation: SUMIT
Similar content being viewed by others
Background
Uveal melanoma is the most common primary tumour of the eye [1]. Biologically distinct from cutaneous melanoma, it is a rare disease with an incidence per year of about 1200–1500 new cases in the US, accounting for around 5 % of all melanomas, and approximately 460 cases in Europe [2–4]. Metastasis is common, occurring in approximately 50 % of patients with posterior uveal melanoma within 15 years of the initial diagnosis and treatment [5], and prognosis is poor with a median overall survival (OS) of 4–15 months [6, 7].
Agents with regulatory approval for use in patients with advanced cutaneous melanoma have only a limited role in the treatment of advanced uveal melanoma, and there are no approved or effective therapies for the treatment of patients with this disease [6]. Although immunotherapy with ipilimumab has been demonstrated to improve survival in patients with metastatic cutaneous melanoma [8] (NCCN Practice Guidelines in Oncology melanoma version 4.2014 [http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#melanoma]), the efficacy of this agent in uveal melanoma is not well defined. Analysis of single- and multi-centre expanded access programmes indicates modest radiographic response rates in patients with metastatic uveal melanoma; however, any effect upon overall survival has yet to be demonstrated [9–14]. Some benefit has been observed with high dose interleukin-2, another immunological agent to be approved for the treatment of metastatic melanoma, in this patient population [15, 16]. Further prospective data are required to fully understand the potential value of immunotherapy, including pembrolizumab which was recently approved in the US, in this setting.
Vemurafenib and dabrafenib are small molecule inhibitors of BRAF approved for use in patients with advanced melanoma harbouring a V600 BRAF mutation (NCCN Practice Guidelines in Oncology melanoma version 4.2014 [www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf]). Antitumour efficacy is only observed in cells harbouring a BRAF mutation, with paradoxical activation of the Ras/Raf/MEK/ERK pathway observed in cells with wild-type BRAF [17–19]. Given that BRAF mutations are absent or rare in uveal melanoma [20–22], there is no utility for these agents in this disease.
Importantly, 80–96 % of uveal melanomas harbour mutations in either the guanidine nucleotide binding protein (G protein), Q polypeptide 1 (GNAQ) or the G protein alpha 11 (GNA11) gene, in a mutually exclusive pattern [23–25]. Oncogenic mutations in GNAQ and GNA11 result in constitutive activation of these proteins and downstream signalling of pathways such as the YAP pathway [26, 27], the phosphoinositide-3 kinase/AKT [28] and the Ras/Raf/MEK/ERK pathway, thus playing a key role in the development and progression of uveal melanomas [23, 24, 29]. This biology suggests that inhibition of one or more of these signalling pathways may result in antitumour activity.
Selumetinib (AZD6244; ARRY-142886) is an orally available, potent and selective, non-ATP-competitive mitogen-activated protein kinase (MEK1/2) inhibitor [30]. In pre-clinical tumour models, selumetinib demonstrates single agent anti-cancer activity [31], including in models of uveal melanoma harbouring GNAQ or GNA11 mutations [32, 33].
In a hypothesis-generating phase II open-label study, patients with metastatic uveal melanoma, who were either temozolomide or dacarbazine treatment naïve, achieved an improved progression-free survival (PFS) with selumetinib versus chemotherapy alone with temozolomide or dacarbazine (15.9 vs 7 weeks; hazard ratio [HR] 0.46 [95 % confidence interval (CI) 0.30, 0.71]; p < 0.001). Tumour regression was observed in 49 % of patients treated with selumetinib. No Response Evaluation Criteria In Solid Tumors (RECIST) responses were observed in patients treated with chemotherapy [34].
Pre-clinical work has identified several promising strategies to improve the efficacy achieved with selumetinib alone, including the concurrent administration of chemotherapy with selumetinib, which results in increased expression of pro-apoptotic proteins such as BIM [35]. When evaluated in combination with chemotherapy, selumetinib enhanced antitumour efficacy compared with each agent alone, with particular sensitivity to BRAF/RAS-mutant tumours [31]. Selumetinib in combination with temozolomide, which has the same active metabolite as dacarbazine, enhanced tumour growth inhibition, DNA damage and apoptosis in a RAS-mutant tumour model versus temozolomide monotherapy [35]. A series of clinical trials assessing the efficacy of selumetinib in combination with chemotherapy have shown promise in patients with mutations associated with the KRAS and BRAF pathways [36, 37], including in combination with dacarbazine in patients with BRAF mutation-positive cutaneous or unknown melanoma [37].
This pre-clinical [31, 35] and clinical evidence [34, 36, 37] suggests that targeting the Ras/Raf/MEK/ERK pathway in combination with chemotherapy is an attractive therapy option to investigate in this disease setting. We therefore hypothesise that selumetinib in combination with dacarbazine, an alkylating agent approved for use in the treatment of advanced melanoma [38] (NCCN Practice Guidelines in Oncology melanoma version 4.2014 [www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf]), may offer improved clinical outcomes in patients with metastatic uveal melanoma versus dacarbazine alone.
Methods/Design
Study objectives
The primary objective is to assess the efficacy of selumetinib in combination with dacarbazine compared with placebo in combination with dacarbazine in terms of PFS in patients with metastatic uveal melanoma (Table 1). Assessment will be by blinded independent central review (BICR) of computed tomography (CT) or magnetic resonance imaging (MRI) scans according to RECIST v1.1.
Secondary objectives include further assessment of efficacy in terms of OS and objective response rate (ORR), duration of response (DoR), change in tumour size at Week 6, safety and tolerability. Exploratory objectives include assessment of mutations in GNAQ/GNA11, health-related quality of life (HRQoL) and biomarkers for response or development of cancer.
Trial design and treatment plan
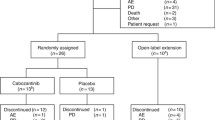
SUMIT (NCT01974752) is a randomised, international, double-blind, placebo-controlled phase III study assessing the efficacy and safety of selumetinib (75 mg, twice daily on a continuous oral administration) in combination with dacarbazine (1000 mg/m2, intravenously on Day 1 of every 21-day cycle) compared with matched placebo in combination with dacarbazine (same schedule) in patients who have not previously had a systemic therapy for metastatic uveal melanoma (Fig. 1).
Patients will be randomised in a 3:1 ratio to receive selumetinib in combination with dacarbazine or placebo in combination with dacarbazine, and stratified by the presence/ absence of liver metastases (yes/no) at randomisation. Following confirmation of objective disease progression by BICR, all patients have the option of receiving open-label selumetinib with or without dacarbazine or an alternative treatment approach.
All randomised patients will be assessed by CT or MRI at screening, Week 6 and every 6 weeks thereafter, relative to the date of randomisation until objective disease progression regardless of whether or not they are on study treatment. Up to the data cut-off for the primary analysis, RECIST v1.1, defined by BICR, will be used to assess each patient’s tumour response to treatment and allow calculations of PFS, ORR, duration of response and tumour size at Week 6. For patients receiving open-label selumetinib with or without dacarbazine, tumour assessments will be performed in accordance with local practice at the investigational site and will not be sent for BICR.
Adverse events (AEs) will be collected from the time of informed consent, coded using the Medical Dictionary for Regulatory Activities (MedDRA), and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE). AEs will continue to be collected for patients who opt to receive open-label selumetinib (either alone or in combination with dacarbazine) as post-progression therapy.
Ophthalmologic examinations and echocardiogram/multi-gated acquisitions will be performed at randomisation and then every 6 and 12 weeks, respectively, thereafter or as clinically indicated. For both, a 30-day follow-up assessment will be required if an on-treatment assessment was abnormal at the time of discontinuation of selumetinib/placebo, to confirm reversibility of the abnormality.
The European Organization for Research and Treatment of Cancer 30-item core quality of life questionnaire version 3 will be used to assess HRQoL at baseline and thereafter every 3 weeks following randomisation until objective disease progression or death.
Archival tumour samples will be collected for all randomised patients for the assessment of GNAQ/GNA11 mutation status. In addition, patients will provide plasma samples for analysis of circulating free tumour DNA. Correlation between tumour and plasma-based mutation analysis will be assessed.
All patients are required to provide written informed consent. The study will be performed in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice. The protocol was approved by the Institutional Review Board at each study site (approximately 50 sites, Table 2) and complied with local country regulations.
Study population
Patients will be eligible for inclusion if they are ≥18 years of age with a clinical diagnosis of metastatic uveal melanoma (histologically or cytologically confirmed), and have ≥1 lesion that can be accurately measured at baseline, and is suitable for accurate repeated measurements. Patients must have an Eastern Cooperative Oncology Group (ECOG) performance status 0–1, a life expectancy >12 weeks and be able to provide informed consent.
Patients will be excluded from the study if they have received previous treatment with a systemic anticancer therapy, or have symptomatic brain metastases or spinal cord compression. Full patient selection criteria are presented in Table 3.
Statistical methods
An estimated 128 patients with metastatic uveal melanoma will be randomised 3:1 to the selumetinib plus dacarbazine group (96 patients) or placebo plus dacarbazine group (32 patients), to obtain approximately 93 PFS events. The sample size is driven by the number of required events. Assuming a true PFS HR of 0.46 [34], this number of events will provide 90 % power to demonstrate a statistically significant difference for PFS at a 5 % 2-sided significance level. OS will be analysed at the time of PFS analysis and updated at 65 % maturity (approximately 83 events). Assuming a true OS HR of 0.49, with 83 deaths, the trial has 80 % power to demonstrate a statistically significant difference for OS with a 1-sided type-1 error of 2.43 %. The type-1 error has been adjusted to allow for a single interim analysis based on approximately 45 death events.
Efficacy analyses will be performed on the efficacy analysis set on an intent-to-treat (ITT) basis according to randomised treatment. PFS, based on BICR, and OS will be analysed by a stratified log-rank test, with the presence of liver metastases at randomisation included as a stratification factor. The effect of treatment will be estimated by the HR together with its corresponding 2-sided CI and p-value. Kaplan-Meier plots of PFS and OS will also be presented. ORR (based on BICR) will be analysed using a logistic regression adjusted for the stratification factor presence/absence of liver metastases.
To describe the nature of benefits of selumetinib treatment, PFS, ORR and OS will be tested at a 2-sided significance level of 5 (PFS and ORR based on BICR). In order to strongly control the type-1 error at 2.5 % 1-sided, a multiple testing procedure with an alpha-exhaustive recycling strategy [39] will also be employed across the primary endpoint PFS and secondary endpoints ORR and OS. No formal statistical testing will be performed on the safety data. AEs will be summarised by preferred term and system organ class (using MedDRA). Summaries of AEs by causality and National Cancer Institute CTCAE grade will also be presented.
Data for exploratory patient-reported outcome endpoints will be analysed descriptively for the efficacy (ITT) analysis set. Data will be presented in terms of minimum, maximum, mean, standard deviation and median scores together with 95 % CIs at each visit as well as change from baseline to each scheduled visit (including end of treatment).
Discussion
We hypothesise that selumetinib in combination with dacarbazine will provide improved clinical outcomes versus dacarbazine alone in patients with metastatic uveal melanoma. This is founded on the encouraging results from a prior phase II study, which reported a statistically significant improvement in PFS for patients with metastatic uveal melanoma receiving selumetinib compared with those receiving chemotherapy (HR 0.46; 95 % CI 0.30, 0.71; p < 0.001) [34]. A comparable PFS improvement to that in the overall population was observed in patients with tumours harbouring a mutation in GNAQ or GNA11 [34].
This phase III trial will build on pre-clinical and clinical evidence assessing the efficacy of selumetinib in combination with chemotherapy. Pre-clinically, the combination of selumetinib with chemotherapy has been shown to increase the cytotoxicity of chemotherapy alone [31], including in RAS-mutant tumour models, i.e. cells dependent on the Ras/Raf/MEK/ERK pathway [35]. Clinically, two phase II trials have demonstrated the efficacy of selumetinib in combination with chemotherapy in patients with Ras/Raf/MEK/ERK-pathway-dependent cancer [36, 37]. As noted, tumours dependent on the Ras/Raf/MEK/ERK pathway include uveal melanomas harbouring oncogenic GNAQ/GNA11 mutations [24]. Thus, the combination of selumetinib with chemotherapy in this disease setting may pose a favourable treatment approach.
Dacarbazine was selected as the therapy for use in combination with selumetinib in this study based on a number of factors. Selumetinib in combination with temozolomide (which has the same active metabolite as dacarbazine but does not require liver metabolism for activation [40]) enhanced the antitumour effect of temozolomide monotherapy [35]. Although this study utilised a human colorectal tumour xenograft model, it provides positive evidence for the efficacy of selumetinib in combination with temozolomide/dacarbazine in a RAS-mutant model. The combination of selumetinib and dacarbazine has demonstrated clinical activity in a phase II trial, with significant improvements in PFS observed in patients with BRAF mutation-positive advanced cutaneous or unknown melanoma receiving the combination versus dacarbazine alone [37]. Clinically, dacarbazine is the only chemotherapy approved for use in the treatment of melanoma (NCCN Practice Guidelines in Oncology melanoma version 4.2014 [www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf]) and is the most commonly prescribed chemotherapy for both metastatic cutaneous and uveal melanoma. Taken together, these data provide the rationale for selecting dacarbazine as the combination agent for selumetinib in this study.
To address concerns that dacarbazine may be a less optimal therapy than selumetinib for patients with uveal melanoma, based on the encouraging clinical efficacy with selumetinib in this patient population [34], an unequal randomisation ratio (3:1) will be used in this study for selumetinib and dacarbazine. The evaluation of response at Week 6 and every 6 weeks thereafter will enable the identification of early progressors on chemotherapy and permit rapid crossover of patients to selumetinib, if required. At the point of objective disease progression, patients will have the option of receiving open-label selumetinib with or without dacarbazine. In the phase II study, 86 % of patients with metastatic uveal melanoma were clinically sufficiently fit to receive selumetinib treatment after experiencing disease progression with temozolomide or dacarbazine. In these patients, efficacy with selumetinib was lower with a median PFS of 8 weeks (95 % CI 8, 12 weeks) compared with 15.9 weeks (95 % CI 8.4, 21.1 weeks) when selumetinib was given initially [34]. However, these findings need to be interpreted with caution as this was a post-hoc analysis, and the reasons behind these findings are not clear.
As the treatment of patients with metastatic uveal melanoma represents an area of high unmet medical need, the results of the described phase II studies, coupled with pre-clinical and clinical evidence, provide the rationale for assessing selumetinib in combination with dacarbazine in this patient population. This study is the first potential registration trial to be conducted in patients with metastatic uveal melanoma and was designed with input from the US Food and Drug Administration (FDA). Study enrolment began in April 2014 and the study is expected to complete in early 2015.
Abbreviations
- AE:
-
Adverse event
- BICR:
-
Blinded independent central review
- BID:
-
Twice daily
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- DoR:
-
Duration of response
- ECOG:
-
Eastern Cooperative Oncology Group
- EORTC-QLQC30 v3:
-
European Organisation for Research and Treatment of Cancer 30-item core quality of life questionnaire version 3
- FDA:
-
Food and Drug Administration
- G protein:
-
Guanidine nucleotide binding protein
- GNA11 :
-
Guanidine nucleotide binding protein alpha 11
- GNAQ :
-
Guanidine nucleotide binding protein, Q polypeptide 1
- HR:
-
Hazard ratio
- HRQoL:
-
Health-related quality of life
- ITT:
-
Intent-to-treat
- iv:
-
Intravenous
- MedDRA:
-
Medical Dictionary for Regulatory Activities
- MEK:
-
Mitogen-activated protein kinase
- MRI:
-
Magnetic resonance imaging
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- RECIST:
-
Response Evaluation Criteria In Solid Tumors
References
Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83:1664–78.
Egan KM, Seddon JM, Glynn RJ, Gragoudas ES, Albert DM. Epidemiologic aspects of uveal melanoma. Surv Ophthalmol. 1988;32:239–51.
Ramaiya KJ, Harbour JW. Current management of uveal melanoma. Exp Rev Ophthalmol. 2007;2:939–46.
Virgili G, Gatta G, Ciccolallo L, Capocaccia R, Biggeri A, Crocetti E, Lutz JM, Paci E. Incidence of uveal melanoma in Europe. Ophthalmology. 2007;114:2309–15.
Kujala E, Makitie T, Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44:4651–9.
Augsburger JJ, Correa ZM, Shaikh AH. Effectiveness of treatments for metastatic uveal melanoma. Am J Ophthalmol. 2009;148:119–27.
Postow MA, Kuk D, Bogatch K, Carvajal RD: Assessment of overall survival from time of metastasis in mucosal, uveal, and cutaneous melanoma [abstract]. J Clin Oncol 2014, 15(Suppl):Abstract 9074.
Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23.
Alexander M, Mellor JD, McArthur G, Kee D. Ipilimumab in pretreated patients with unresectable or metastatic cutaneous, uveal and mucosal melanoma. Med J Aust. 2014;201:49–53.
Danielli R, Ridolfi R, Chiarion-Sileni V, Queirolo P, Testori A, Plummer R, Boitano M, Calabro L, Rossi CD, Giacomo AM, Ferrucci PF, Ridolfi L, Altomonte M, Miracco C, Balestrazzi A, Maio M. Ipilimumab in pretreated patients with metastatic uveal melanoma: safety and clinical efficacy. Cancer Immunol Immunother. 2012;61:41–8.
Kelderman S, van der Kooij MK, van den Eertwegh AJ, Soetekouw PM, Jansen RL, van den Brom RR, Hospers GA, Haanen JB, Kapiteijn E, Blank CU. Ipilimumab in pretreated metastastic uveal melanoma patients. Results of the Dutch Working group on Immunotherapy of Oncology (WIN-O). Acta Oncol. 2013;52:1786–8.
Khattak MA, Fisher R, Hughes P, Gore M, Larkin J. Ipilimumab activity in advanced uveal melanoma. Melanoma Res. 2013;23:79–81.
Luke JJ, Callahan MK, Postow MA, Romano E, Ramaiya N, Bluth M, Giobbie-Hurder A, Lawrence DP, Ibrahim N, Ott PA, Flaherty KT, Sullivan RJ, Harding JJ, D'Angelo S, Dickson M, Schwartz GK, Chapman PB, Wolchok JD, Hodi FS, Carvajal RD. Clinical activity of ipilimumab for metastatic uveal melanoma: a retrospective review of the Dana-Farber Cancer Institute, Massachusetts General Hospital, Memorial Sloan-Kettering Cancer Center, and University Hospital of Lausanne experience. Cancer. 2013;119:3687–95.
Maio M, Danielli R, Chiarion-Sileni V, Pigozzo J, Parmiani G, Ridolfi R, De Rosa F, Del Vecchio M, Di Guardo L, Queirolo P, Picasso V, Marchetti P, De Galitiis F, Mandala M, Guida M, Simeone E, Ascierto PA. Efficacy and safety of ipilimumab in patients with pre-treated, uveal melanoma. Ann Oncol. 2013;24:2911–5.
Becker JC, Terheyden P, Kampgen E, Wagner S, Neumann C, Schadendorf D, Steinmann A, Wittenberg G, Lieb W, Brocker EB. Treatment of disseminated ocular melanoma with sequential fotemustine, interferon alpha, and interleukin 2. Br J Cancer. 2002;87:840–5.
Soni S, Lee DS, DiVito Jr J, Bui AH, DeRaffele G, Radel E, Kaufman HL. Treatment of pediatric ocular melanoma with high-dose interleukin-2 and thalidomide. J Pediatr Hematol Oncol. 2002;24:488–91.
Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, Ludlam MJ, Stokoe D, Gloor SL, Vigers G, Morales T, Aliagas I, Liu B, Sideris S, Hoeflich KP, Jaiswal BS, Seshagiri S, Koeppen H, Belvin M, Friedman LS, Malek S. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–5.
Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, Hussain J, Reis-Filho JS, Springer CJ, Pritchard C, Marais R. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–21.
Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–30.
Malaponte G, Libra M, Gangemi P, Bevelacqua V, Mangano K, D'Amico F, Mazzarino MC, Stivala F, McCubrey JA, Travali S. Detection of BRAF gene mutation in primary choroidal melanoma tissue. Cancer Biol Ther. 2006;5:225–7.
Cruz III F, Rubin BP, Wilson D, Town A, Schroeder A, Haley A, Bainbridge T, Heinrich MC, Corless CL. Absence of BRAF and NRAS mutations in uveal melanoma. Cancer Res. 2003;63:5761–6.
Zuidervaart W, van Nieuwpoort F, Stark M, Dijkman R, Packer L, Borgstein AM, Pavey S, van der Velden P, Out C, Jager MJ, Hayward NK, Gruis NA. Activation of the MAPK pathway is a common event in uveal melanomas although it rarely occurs through mutation of BRAF or RAS. Br J Cancer. 2005;92:2032–8.
Onken MD, Worley LA, Long MD, Duan S, Council ML, Bowcock AM, Harbour JW. Oncogenic mutations in GNAQ occur early in uveal melanoma. Invest Ophthalmol Vis Sci. 2008;49:5230–4.
Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, Obenauf AC, Wackernagel W, Green G, Bouvier N, Sozen MM, Baimukanova G, Roy R, Heguy A, Dolgalev I, Khanin R, Busam K, Speicher MR, O'Brien J, Bastian BC. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363:2191–9.
Piperno-Neumann S, Kapiteijn E, Larkin JMG, Carvajal RD, Luke JJ, Seifert H, Roozen I, Zoubir M, Ramkumar T, Emery C, Derti A, Yerramilli-Rao P, Hodi FS, Schwartz GK: Landscape of genetic alterations in patients with metastatic uveal melanoma [abstract]. J Clin Oncol 2014, 32(Suppl 5):Abstract 9043.
Feng X, Degese MS, Iglesias-Bartolome R, Vaque JP, Molinolo AA, Rodrigues M, Zaidi MR, Ksander BR, Merlino G, Sodhi A, Chen Q, Gutkind JS. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated Rho GTPase signaling circuitry. Cancer Cell. 2014;25:831–45.
Yu FX, Luo J, Mo JS, Liu G, Kim YC, Meng Z, Zhao L, Peyman G, Ouyang H, Jiang W, Zhao J, Chen X, Zhang L, Wang CY, Bastian BC, Zhang K, Guan KL. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell. 2014;25:822–30.
Ambrosini G, Musi E, Ho AL, de Stanchina E, Schwartz GK. Inhibition of mutant GNAQ signaling in uveal melanoma induces AMPK-dependent autophagic cell death. Mol Cancer Ther. 2013;12:768–76.
Bauer J, Kilic E, Vaarwater J, Bastian BC, Garbe C, de Klein A. Oncogenic GNAQ mutations are not correlated with disease-free survival in uveal melanoma. Br J Cancer. 2009;101:813–5.
Yeh TC, Marsh V, Bernat BA, Ballard J, Colwell H, Evans RJ, Parry J, Smith D, Brandhuber BJ, Gross S, Marlow A, Hurley B, Lyssikatos J, Lee PA, Winkler JD, Koch K, Wallace E. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007;13:1576–83.
Davies BR, Logie A, McKay JS, Martin P, Steele S, Jenkins R, Cockerill M, Cartlidge S, Smith PD. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther. 2007;6:2209–19.
Ambrosini G, Pratilas CA, Qin LX, Tadi M, Surriga O, Carvajal RD, Schwartz GK. Identification of unique MEK-dependent genes in GNAQ mutant uveal melanoma involved in cell growth, tumor cell invasion, and MEK resistance. Clin Cancer Res. 2012;18:3552–61.
Mitsiades N, Chew SA, He B, Riechardt AI, Karadedou T, Kotoula V, Poulaki V. Genotype-dependent sensitivity of uveal melanoma cell lines to inhibition of B-Raf, MEK, and Akt kinases: rationale for personalized therapy. Invest Ophthalmol Vis Sci. 2011;52:7248–55.
Carvajal RD, Sosman JA, Quevedo JF, Milhem MM, Joshua AM, Kudchadkar RR, Linette GP, Gajewski TF, Lutzky J, Lawson DH, Lao CD, Flynn PJ, Albertini MR, Sato T, Lewis K, Doyle A, Ancell K, Panageas KS, Bluth M, Hedvat C, Erinjieri J, Ambrosini G, Marr B, Abramson DH, Dickson MA, Wolchok JD, Chapman PB, Schwartz GK. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA. 2014;311:2397–405.
Holt SV, Logie A, Odedra R, Heier A, Heaton SP, Alferez D, Davies BR, Wilkinson RW, Smith PD. The MEK1/2 inhibitor, selumetinib (AZD6244; ARRY-142886), enhances anti-tumour efficacy when combined with conventional chemotherapeutic agents in human tumour xenograft models. Br J Cancer. 2012;106:858–66.
Janne PA, Shaw AT, Pereira JR, Jeannin G, Vansteenkiste J, Barrios C, Franke FA, Grinsted L, Zazulina V, Smith P, Smith I, Crino L. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol. 2013;14:38–47.
Robert C, Dummer R, Gutzmer R, Lorigan P, Kim KB, Nyakas M, Arance A, Liszkay G, Schadendorf D, Cantarini M, Spencer S, Middleton MR. Selumetinib plus dacarbazine versus placebo plus dacarbazine as first-line treatment for BRAF-mutant metastatic melanoma: a phase 2 double-blind randomised study. Lancet Oncol. 2013;14:733–40.
Dummer R, Hauschild A, Guggenheim M, Keilholz U, Pentheroudakis G: Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012, 23(Suppl 7):vii86-vii91.
Burman CF, Sonesson C, Guilbaud O. A recycling framework for the construction of Bonferroni-based multiple tests. Stat Med. 2009;28:739–61.
Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000;6:2585–97.
Acknowledgements
The study is funded by AstraZeneca. Medical writing services were provided by Sandra Brave of iMed Comms and were funded by AstraZeneca. The authors thank in advance all of the patients, investigators and institutions that will be involved in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
RC is a consultant for AstraZeneca. GS has received honoraria from AstraZeneca and served on its recent advisory board for the selumetinib drug programme. PN received fees from AstraZeneca for an advisory board. HM is an employee of AstraZeneca and IS is a former employee of AstraZeneca.
Authors’ contributions
RC, GS, PN and IS participated in the design of the study. HM designed the statistical analysis plan. All authors contributed to the implementation of the study, were involved in revising the manuscript critically, and gave their final approval of the version to be published.
Authors’ information
Ian Smith is no longer an AstraZeneca employee.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Carvajal, R.D., Schwartz, G.K., Mann, H. et al. Study design and rationale for a randomised, placebo-controlled, double-blind study to assess the efficacy of selumetinib (AZD6244; ARRY-142886) in combination with dacarbazine in patients with metastatic uveal melanoma (SUMIT). BMC Cancer 15, 467 (2015). https://doi.org/10.1186/s12885-015-1470-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-015-1470-z