Abstract
Background
The association between epidermal growth factor (EGF) gene +61A/G polymorphism (rs4444903) and hepatocellular carcinoma (HCC) susceptibility has been widely reported, but the results were inconsistent. To clarify the effect of this polymorphism on HCC risk, a meta-analysis was performed.
Methods
The PubMed, Embase, Cochrane Library, Web of Science, Chinese BioMedical Literature (CBM), Wanfang and Chinese National Knowledge Infrastructure (CNKI) databases were systematically searched to identify relevant studies published up to December 2013. Data were extracted independently by two authors. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated to assess the strength of association.
Results
A total of 16 studies including 2475 HCC cases and 5381 controls were included in this meta-analysis. Overall, a significantly increased HCC risk was observed under all genetic models (G vs. A: OR = 1.383, P < 0.001, 95% CI: 1.174-1.629; GG vs. GA + AA: OR = 1.484, P < 0.001, 95% CI: 1.198-1.838; GG + GA vs. AA: OR = 1.530, P < 0.001, 95% CI: 1.217-1.924; GG vs. AA: OR = 1.958, P < 0.001, 95% CI: 1.433-2.675; GA vs. AA: OR = 1.215, P = 0.013, 95% CI: 1.041-1.418). In the subgroup analyses by ethnicity, a significant association with HCC risk was found in Asian populations (G vs. A: OR = 1.151, P = 0.001, 95% CI: 1.056-1.255), European populations (G vs. A: OR = 1.594, P = 0.027, 95% CI: 1.053-2.413, and African populations (G vs. A: OR = 3.599, P < 0.001, 95% CI: 2.550-5.080), respectively.
Conclusions
Our study shows that EGF +61A/G polymorphism is significantly associated with the increased HCC risk, especially in Asian populations. Further large-scale and well-designed studies are required to confirm this conclusion.
Similar content being viewed by others
Background
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer-related death worldwide [1]. The estimated annual number of cases exceeds 500 000, with a mean annual incidence of around 3-4% [2]. Most cases of HCC (about 80%) occur in eastern Asia and sub-Saharan Africa, and China alone accounts for more than 50% of the total cases [3]. Despite advances in the diagnosis and treatment of HCC, it still has poor prognosis with a five-year survival rate of 5% in developing countries [4]. Carcinogenesis of HCC is a complex, multistep and multifactorial process. Major risk factors for development of HCC are chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV), liver cirrhosis, habitual alcohol abuse, high cigarette smoking, and exposure to aflatoxin B1 [3,5]. However, not all individuals with exposure to the risk factors develop HCC. Therefore, other causes, including genetic factors, might play important roles in the pathogenesis of HCC.
Epidermal growth factor (EGF) was first isolated in 1962 [6]. It stimulates proliferation, differentiation and tumorigenesis of epidermal and epithelial tissues by binding to its receptor (EGFR) and, hence, activating several signal pathways [7,8]. EGF is a mitogen for adult and fetal hepatocytes grown in culture, and its expression is up-regulated during liver regeneration [9]. Mounting evidence supports a role for EGF in malignant transformation, tumor growth and progression [10]. The EGF gene is located on chromosome 4q25-27 and contains 24 exons and 23 introns. The EGF +61A/G polymorphism (rs4444903) is a common single nucleotide polymorphism (SNP) in the 5′-untranslated region (5′-UTR) of the EGF gene, modulating the transcription of EGF gene and hence affecting serum levels of EGF [11]. For now, there are a number of studies conducted to examine the association between EGF +61A/G polymorphism and HCC susceptibility, but the results remain controversial and inconclusive [12-16]. These disparate findings may be due partly to insufficient power, false-positive results and ethnic diversity.
Meta-analysis offers a powerful means of overcoming the problems associated with small sample sizes, and particularly, of overcoming the inadequate statistical powers of genetic studies on complex traits [17]. Therefore, in this study, we performed a meta-analysis from all eligible studies to clarify the relationship between EGF +61A/G polymorphism and HCC risk.
Methods
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) criteria [18].
Literature searching strategy
We conducted a computerized literature search of PubMed, Embase, Cochrane Library, Web of Science, Chinese BioMedical Literature (CBM), Wanfang and Chinese National Knowledge Infrastructure (CNKI) databases to identify all potential studies published up to December 31, 2013. The following keywords and subject terms were included in searching: “EFG” or “Epidermal growth factor”, “liver cancer” or “hepatocellular carcinoma” or “HCC”, and “polymorphism” or “variant” or “allele”. References of retrieved articles and review articles were also screened.
Inclusion criteria
Studies included in the meta-analysis had to meet all the following criteria: (1) evaluating the association between EGF +61A/G polymorphism and HCC risk, (2) using unrelated individuals, (3) providing sufficient data for estimating an odds ratio (OR) with its 95% confidence interval (CI), (4) using case–control, cohort or cross-sectional design, (5) published in English or Chinese. The corresponding authors were contacted to obtain missing information, and some studies were excluded if critical missing information was not obtained. Reviews, case reports, family-based studies, case-only studies, and studies without sufficient data were all excluded. When a study reported results on different subpopulations based on ethnicity or geographical region, we treated each subpopulation as a separate comparison. If more than one article was published using the same subjects, only the study with the largest sample size was selected.
Data extraction
All data were extracted independently by two investigators (Lifang Shao and Xiaobo Yu). Disagreement was resolved by discussion. The following data were extracted: authors, name of journal, year of publication, ethnicity and country of study population, inclusion and exclusion criteria, characteristics of cases and controls, numbers of HCC cases and controls, matching criteria, source of controls, HCC confirmation, study design, genotyping methods, genotype frequencies of cases and controls, and interactions between environment factors or genes.
Quality score assessment
Quality of studies was independently assessed by the same two investigators (Lifang Shao and Xiaobo Yu) according to a set of predetermined criteria (Additional file 1: Table S1), which was extracted and modified from previous studies [19,20]. These scores were based on traditional epidemiological considerations, as well as cancer genetic issues. Any disagreement was resolved by discussion between the two investigators. The total scores ranged from 0 (worst) to 24 (best). Studies scoring <16 were classified as “low quality”, and those scoring ≥16 as “high quality”.
Statistical analysis
The unadjusted OR with 95% CI was used to assess the strength of the association between EGF +61A/G polymorphism and HCC risk. The pooled ORs were performed under the allelic contrast (G versus A), co-dominant model (homozygote comparison: GG versus AA, heterozygote comparison: GA versus AA), dominant model (GG + GA versus AA), and recessive model (GG versus GA + AA), respectively. Between-study heterogeneity was measured using a Q-statistic test [21] and an I-square statistic [22]. P less than 0.10 (P < 0.10) was considered representative of significant statistical heterogeneity because of the low power of the statistic. I2 ranges between 0 and 100%, and represents the proportion of between-study variability that can be attributed to heterogeneity rather than chance. I2 values of 25%, 50%, and 75% were defined as low, moderate, and high estimates. If the significant Q-statistic indicated heterogeneity across studies, the random-effects model (DerSimonian and Laird method) was used, otherwise the fixed-effects model (Mantel-Haenszel method) was adopted [23]. The Z test was used to assess the significance of the pooled OR and a P-value less than 0.05 (P < 0.05) was considered significant.
Subgroup analyses were stratified by racial descent, study quality, source of controls, type of controls, and number of cases, respectively. Furthermore, meta-regression analysis [24] was performed to investigate five potential sources of heterogeneity including ethnicity (Asian populations versus not Asian populations), study quality (high quality studies versus low quality studies), source of controls (Hospital-based versus Population-based), type of controls (healthy controls versus controls with chronic liver diseases) and number of cases (<100 versus ≥100). Statistical significance was defined as a P-value less than 0.10 (P < 0.10) because of the relatively weak statistical power.
To evaluate the stability of the results, sensitivity analyses were performed by sequential omission of individual studies under various comparisons in overall and Asian populations, respectively. Publication bias was investigated by funnel plot. Funnel plot asymmetry was assessed by the method of Egger’s linear regression test [25]. Hardy-Weinberg equilibrium (HWE) was tested by the χ2 test. All P-values were two-sided. Data analyses were performed using the software Stata version 11.0 (StataCorp LP, College Station, TX, USA).
Results
Eligible studies
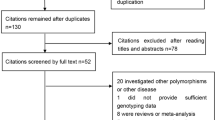
The present study met the PRISMA statement (Additional file 2: Checklist S1). A total of 413 potentially relevant records were initially obtained through searching the databases. After removing 127 duplications, 241 records were excluded because of obvious irrelevance to our study aim by browsing the titles and abstracts. According to the inclusion criteria, 32 of the remaining 45 records were further excluded by review of the full texts. The flow chart of the selection process was shown in Figure 1. In total, 13 articles were eligible, of which three provided the data in different populations [12,13,26]. We treated each population as a separate study. As a result, 16 studies (13 articles) including 2475 HCC cases and 5381 controls were identified and included in this meta-analysis [12-16,26-33].
Characteristics of studies and subjects
The main characteristics of the 16 included studies were listed in Table 1. All articles were published in English except for one [26]. Of all the eligible studies, 9 were conducted in Asian populations, 2 in European populations, 2 in African populations, and 3 in mixed populations with more than 72% Caucasians. In all the studies, the cases were histologically confirmed (11 studies) or diagnosed by elevated α-fetoprotein and distinct iconography changes (abdominal ultrasound and Triphasic computed tomography). All the controls were free of cancer. Two studies used healthy populations, 4 studies used patients with chronic liver diseases (HBV infection, HCV infection, cirrhosis), and 10 studies included both healthy subjects and patients with chronic liver diseases as controls. Seven studies matched in age, 10 studies matched in gender, and 9 studies matched in hepatitis virus infection status. The sample size of the total participants ranged from 80 to 1774, with a mean of 491. The quality scores for the individual studies ranged from 11.5 to 21, with 9 out of the 16 studies classified as high quality. Fifteen studies used peripheral blood, and one study used either blood or liver tissue to extract genome DNA. Thirteen studies used the polymerase chain reaction-restriction fragment length polymorphism assay (PCR-RFLP), and three studies used Taqman method to genotype the EGF +61 A/G polymorphism. While genotyping, 10 studies repeated a portion of samples, and only 4 studies described use of blindness of the status of DNA samples. The genotype distribution in the controls of all studies was consistent with HWE.
Meta-analysis results
The frequency of +61G allele was 65% in Asian controls, 42% in European controls, and 30% in African controls. There were significant differences in terms of +61G allele frequency among the three major ethnicities (P < 0.001). Table 2 indicated the associations between EGF +61A/G polymorphism and HCC risk. Overall, the results of pooling all studies showed that the EGF +61A/G polymorphism was significantly associated with an increased HCC risk under all genetic models (G vs. A: OR = 1.383, P < 0.001, 95% CI: 1.174-1.629, I2 = 75.4%, Pheterogeneity < 0.001, Figure 2; GG vs. GA + AA: OR = 1.484, P < 0.001, 95% CI: 1.198-1.838, I2 = 69.3%, Pheterogeneity < 0.001; GG + GA vs. AA: OR = 1.530, P < 0.001, 95% CI: 1.217-1.924, I2 = 53.5%, Pheterogeneity = 0.006; GG vs. AA: OR = 1.958, P < 0.001, 95% CI: 1.433-2.675, I2 = 65.2%, Pheterogeneity < 0.001; GA vs. AA: OR = 1.215, P = 0.013, 95% CI: 1.041-1.418, I2 = 19.7%, Pheterogeneity = 0.229) (Table 2).
Forest plot for the association between EGF +61A/G polymorphism and HCC risk stratified according to different ethnicities (G vs. A). For each study, the estimate of OR and its 95% CI is plotted with a diamond (◆) and a horizontal line. The size of a box (gray square) is proportional to the weight that the study has in calculating the summary effect estimate (`). The center of the diamond indicates the OR and the ends of the diamond correspond to the 95% CI.
In the subgroup analyses based upon ethnicity, a significantly elevated association between EGF +61A/G polymorphism and HCC risk was observed in Asian populations (G vs. A: OR = 1.151, P = 0.001, 95% CI: 1.056-1.255, I2 = 4.6%, Pheterogeneity = 0.397), European populations (G vs. A: OR = 1.594, P = 0.027, 95% CI: 1.053-2.413, I2 = 0.0%, Pheterogeneity = 0.582), and African populations (G vs. A: OR = 3.599, P < 0.001, 95% CI: 2.550-5.080, I2 = 57.6%, Pheterogeneity = 0.125), respectively (Figure 2). When stratifying by study quality, the results showed that EGF +61A/G polymorphism was associated with an increased HCC risk both in high-quality studies (G vs. A: OR = 1.178, P < 0.001, 95% CI: 1.077-1.289, I2 = 0.0%, Pheterogeneity = 0.539) and in low-quality studies (G vs. A: OR = 1.740, P = 0.010, 95% CI: 1.144-2.648, I2 = 87.1%, Pheterogeneity < 0.001). In the subgroup analyses by source of controls, the results showed that EGF +61A/G polymorphism was significantly associated with HCC risk in hospital-based studies (G vs. A: OR = 1.439, P < 0.001, 95% CI: 1.205-1.719, I2 = 75.8%, Pheterogeneity < 0.001), but not in population-based studies (G vs. A: OR = 1.087, P = 0.202, 95% CI: 0.956-1.236, I2 = 0.0%, Pheterogeneity = 0.815). Furthermore, according to chronic liver disease status in Asian controls, a significant association between EGF +61A/G polymorphism and HCC risk was obtained in patients with chronic liver diseases (G vs. A: OR = 1.165, P = 0.017, 95% CI: 1.028-1.321, I2 = 23.3%, Pheterogeneity = 0.266), and in healthy controls (G vs. A: OR = 1.142, P = 0.043, 95% CI: 1.004-1.299, I2 = 4.2%, Pheterogeneity = 0.383) (Table 2).
Heterogeneity analysis
Q-statistic indicated statistically significant heterogeneity among all studies under all genetic models except for heterozygote comparison (Table 2). However, in the subgroup analyses by ethnicity, the between-study heterogeneity was not observed in Asian populations, European populations or African populations. Moreover, meta-regression indicated that both ethnicity and study quality significantly contributed to the heterogeneity for EGF +61A/G polymorphism (Table 3).
Sensitivity analysis and publication bias
Sensitivity analysis was performed by sequential omission of individual studies. The pooled ORs were consistently significant in overall populations or Asian populations by omitting one study at a time under the allelic contrast, recessive model and homozygote comparison, suggesting robustness of our results. Funnel plots and Egger’s test were performed to assess publication bias. The results showed that bias may exist in overall populations (G vs. A: t = 2.62, P = 0.020; GG vs. GA + AA: t = 2.70, P = 0.017), but not in Asian populations (G vs. A: t = 1.71, P = 0.130; GG vs. GA + AA: t = 1.25, P = 0.250) (Figure 3).
Begg’s funnel plot of the Egger’s test for publication bias of EGF +61A/G polymorphism and HCC risk (G vs. A). A: Overall populations; B: Asian populations. The horizontal line in the funnel plot indicates summary estimate, whereas the sloping lines indicate the expected 95% confidence intervals for a given standard error.
Discussion
This article investigated the relationship between EGF +61A/G polymorphism and HCC susceptibility. A total of 16 studies from 13 articles (2475 cases and 5381 controls) were finally included in this meta-analysis. Overall, the EGF +61A/G polymorphism was significantly associated with an increased HCC risk under all genetic models. However, considerable heterogeneity was detected across studies. Meta-regression showed that both ethnicity and study quality significantly contributed to the heterogeneity for EGF +61A/G polymorphism. Nevertheless, in the subgroup analyses by ethnicity and study quality, this significant association still existed in each subgroup, and the between-study heterogeneity became insignificant in Asian, European or African populations. Moreover, sensitivity analysis further strengthened the validity of the positive association in overall populations, and in Asian populations, indicating robustness of our results.
It is possible that the effects of genetic factors related to cancer are different across various ethnic populations. In this study, ethnicity was identified as a potential source of between-study heterogeneity by meta-regression and subgroup analyses. Although the reason for these discrepancies was not well known, some possibilities should be considered. First, there were significant differences in terms of +61G allele frequency among the three major ethnicities. The frequency of EGF +61G allele was greatest in Asian populations (65%), intermediate in European populations (42%), and lowest in African populations (30%). The higher HCC prevalence among Asian populations may be partly ascribed to the higher prevalence of EGF +61G allele. The frequency of EGF +61G among the controls of all studies was consistent with that in 1000 Genome Project, except for two studies [13,28]. The omission of these two studies did not substantially alter the results, indicating reliability of our results. Second, different linkage disequilibrium patterns may contribute to the discrepancy. The EGF +61A/G polymorphism may be in close linkage with nearby causal variant in one ethnic population, but not in another. Third, clinical heterogeneity such as age, gender ratio, life style and disease severity may also explain the discrepancy. The discrepancy might be due to genetic background and environmental exposure differences. Last but not least, owing to the limited number of studies in European and African populations included in this meta-analysis, the ethnic discrepancy was likely to be caused by chance. Therefore, further studies were needed to investigate the reason for this discrepancy.
Study design is an area of concern and can influence the interpretation of the results of meta-analysis. Among the eligible studies, there were 15 hospital-based studies, but only 5 population-based studies. Our results showed that EGF +61A/G polymorphism was significantly associated with HCC risk in hospital-based studies, but not in population-based studies. Therefore, the results should be treated with caution, because controls from hospital-based studies may not represent the general population. Larger population-based studies were required to further confirm the association between EGF +61A/G polymorphism and HCC susceptibility. Furthermore, according to chronic liver disease status in Asian controls, a significant association between EGF +61A/G polymorphism and HCC risk was obtained both in controls with chronic liver diseases, and in healthy controls, indicating reliability of the pooled results in Asian populations. Besides, all Asian studies were based on Chinese populations except for one Japanese study [14]. The frequency of EGF +61G allele was a little lower in Chinese populations than that in Japanese populations, according to 1000 Genome Project and our results. Geographical discrepancy should be considered in the analyses. The pooled results of these Chinese studies were consistent with those from Asian studies. Therefore, EGF +61A/G polymorphism may be associated with HCC risk in Asian populations, especially in Chinese populations. In addition, study quality was also identified as a potential source of heterogeneity by meta-regression. In this meta-analysis, 9 of the 16 studies were classified as high quality. Studies with low-quality design usually did not exclude those possible factors that may bias the estimate of the real effects and may result in incorrect conclusions. However, the association between EGF +61A/G polymorphism and HCC risk was significant in both high-quality and low-quality studies, suggesting that this bias cannot affect the final results.
Epidermal growth factor is a mitogen for hepatocytes, and plays a critical role in liver tissue regeneration, malignant transformation, tumor growth and progression [34]. Transgenic mice with liver-targeted overexpression of the secreted EGF fusion protein develop hepatocellular carcinoma, and blockade of EGF receptor activity halt the development and progression of HCC [35-37]. Thus, overexpression of EGF might be an important step toward development of liver cancer. For EGF +61A/G polymorphism, several studies have demonstrated that GG or GA genotype was associated with significantly higher EGF production both in normal peripheral blood mononuclear cell cultures and in serum and liver tissues of individuals [11,12,29]. It was thought that EGF +61A/G polymorphism might be correlated to HCC. Our results showed that EGF +61G allele was significantly associated with an increased HCC risk, which was consistent with the hypothesis. However, the molecular mechanism of the association between EGF +61A/G polymorphism and HCC risk remains relatively unclear.
To our knowledge, this present meta-analysis is the most comprehensive one related to the relationship between EGF +61A/G polymorphism and HCC risk. Compared with the previous meta-analysis [38], another eight studies were included in this meta-analysis. The sample size of total participants in our study (2475 cases and 5381 controls) was much larger than that in the previous one (1304 cases and 2613 controls). Thus, the pooled results were more reliable and robust in our study. Furthermore, the quality of the included studies was evaluated in our study, but not in the previous one. Meta-regression was performed to explore the sources of heterogeneity among studies, which allowed a more thorough examination and appropriate qualification of our results.
Despite our efforts in performing a comprehensive analysis, several limitations should be considered. Firstly, obvious publication bias was detected in overall populations. Bias may result from our exclusion of unpublished data, as well as studies published in languages other than English and Chinese. Secondly, the controls were not uniformly defined. Some studies were population-based, while others were hospital-based. Considering the overwhelming impact of chronic liver diseases on HCC development, controls were divided into healthy controls and controls with chronic liver diseases. The subgroup analyses showed that the significant association between EGF +61A/G and HCC was present both in healthy controls and in patients with chronic liver diseases, indicating the role of EGF +61A/G in the risk of HCC, regardless of type of controls. Moreover, the pooled ORs for individuals with chronic liver diseases were higher than those for healthy controls under all genetic models. Therefore, the chronic liver diseases may change the environment in vivo and mediate the ability of genetic factors to contribute to HCC. More studies should be designed to investigate the role of EGF polymorphisms in combination with chronic liver diseases in HCC pathogenesis. Thirdly, our meta-analysis was based on unadjusted estimates. If individual data were available, adjusted estimates by confounding factors could be obtained to conduct a more precise analysis. Fourthly, gene-gene and gene-environment interactions were not addressed in our meta-analysis due to lack of sufficient data. Aside from genetic factors, other factors such as exposure to aflatoxin B1, high cigarette smoking, and habitual alcohol abuse might also play vital roles in the development of HCC. However, we could not perform subgroup analyses based on environmental exposure owing to the limited reported information on such associations in those included studies. Finally, the number of studies included in the meta-analysis for European populations and African populations was relatively small, which may lead to low statistical power and generate fluctuation in estimation.
Conclusions
In summary, this meta-analysis suggests that EGF gene +61A/G polymorphism is significantly associated with the increased risk of HCC, particularly in Asian populations. Further well-designed and large-scale studies with the consideration of gene-gene and gene-environment interactions should be conducted to investigate the association in different ethnic populations.
Abbreviations
- EGF:
-
Epidermal growth factor
- HCC:
-
Hepatocellular carcinoma
- CBM:
-
Chinese BioMedical Literature
- CNKI:
-
Chinese National Knowledge Infrastructure
- OR:
-
Odds ratio
- 95% CI:
-
95% confidence interval
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- SNP:
-
Single nucleotide polymorphism
- 5′-UTR:
-
5′-untranslated region
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- HWE:
-
Hardy-Weinberg equilibrium
- PCR-RFLP:
-
Polymerase chain reaction-restriction fragment length polymorphism assay
References
Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–6.
Michielsen PP, Francque SM, van Dongen JL. Viral hepatitis and hepatocellular carcinoma. World J Surg Oncol. 2005;3:27.
El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Dragani TA. Risk of HCC: genetic heterogeneity and complex genetics. J Hepatol. 2010;52:252–7.
Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962;237:1555–62.
Carpenter G, Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216.
Fisher DA, Lakshmanan J. Metabolism and effects of epidermal growth factor and related growth factors in mammals. Endocr Rev. 1990;11:418–42.
Mullhaupt B, Feren A, Fodor E, Jones A. Liver expression of epidermal growth factor RNA. Rapid increases in immediate-early phase of liver regeneration. J Biol Chem. 1994;269:19667–70.
Singletary SE, Baker FL, Spitzer G, Tucker SL, Tomasovic B, Brock WA, et al. Biological effect of epidermal growth factor on the in vitro growth of human tumors. Cancer Res. 1987;47:403–6.
Shahbazi M, Pravica V, Nasreen N, Fakhoury H, Fryer AA, Strange RC, et al. Association between functional polymorphism in EGF gene and malignant melanoma. Lancet. 2002;359:397–401.
Tanabe KK, Lemoine A, Finkelstein DM, Kawasaki H, Fujii T, Chung RT, et al. Epidermal growth factor gene functional polymorphism and the risk of hepatocellular carcinoma in patients with cirrhosis. JAMA. 2008;299:53–60.
Yuan JM, Fan Y, Ognjanovic S, Wang R, Van Den Berg D, Govindarajan S, et al. Genetic polymorphisms of epidermal growth factor in relation to risk of hepatocellular carcinoma: two case–control studies. BMC Gastroenterol. 2013;13:32.
Suenaga M, Yamada S, Fujii T, Fuchs BC, Okumura N, Kanda M, et al. A functional polymorphism in the epidermal growth factor gene predicts hepatocellular carcinoma risk in Japanese hepatitis C patients. Onco Targets Ther. 2013;6:1805–12.
Wu J, Zhang W, Xu A, Zhang L, Yan T, Li Z, et al. Association of epidermal growth factor and epidermal growth factor receptor polymorphisms with the risk of hepatitis B virus-related hepatocellular carcinoma in the population of North China. Genet Test Mol Biomarkers. 2013;17:595–600.
Qi P, Wang H, Chen YM, Sun XJ, Liu Y, Gao CF. No association of EGF 5′UTR variant A61G and hepatocellular carcinoma in Chinese patients with chronic hepatitis B virus infection. Pathology. 2009;41:555–60.
Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315:1533–7.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Chen Z, Xu L, Ye X, Shen S, Li Z, Niu X, et al. Polymorphisms of microRNA sequences or binding sites and lung cancer: a meta-analysis and systematic review. PLoS One. 2013;8:e61008.
Wang F, Sun G, Zou Y, Fan L, Song B. Lack of association of miR-146a rs2910164 polymorphism with gastrointestinal cancers: evidence from 10206 subjects. PLoS One. 2012;7:e39623.
Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–6.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Petitti DB, editor. Meta-Analysis, Decision Analysis, and Cost-Effectiveness Analysis. New York: Oxford University Press; 1994.
Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18:2693–708.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Wang HX. Epidermal growth factor gene polymorphism associated with susceptibility to hepatocellular carcinoma. Master Dissertation: Guanxi Medical University; 2009.
Abbas E, Shaker O, Abd El Aziz G, Ramadan H, Esmat G. Epidermal growth factor gene polymorphism 61A/G in patients with chronic liver disease for early detection of hepatocellular carcinoma: a pilot study. Eur J Gastroenterol Hepatol. 2012;24:458–63.
Shi HZ, Ren P, Lu QJ, Niedrgethmnn M, Wu GY. Association between EGF, TGF-beta1 and TNF-alpha gene polymorphisms and hepatocellular carcinoma. Asian Pac J Cancer Prev. 2012;13:6217–20.
Abu Dayyeh BK, Yang M, Fuchs BC, Karl DL, Yamada S, Sninsky JJ, et al. A functional polymorphism in the epidermal growth factor gene is associated with risk for hepatocellular carcinoma. Gastroenterology. 2011;141:141–9.
Chen K, Wei Y, Yang H, Li B. Epidermal growth factor +61 G/A polymorphism and the risk of hepatocellular carcinoma in a Chinese population. Genet Test Mol Biomarkers. 2011;15:251–5.
Li Y, Xie Q, Lu F, Zhao J, Mao P, Li Z, et al. Association between epidermal growth factor 61A/G polymorphism and hepatocellular carcinoma susceptibility in Chinese patients. Liver Int. 2010;30:112–8.
El-Bendary M, Neamatallah M, El-Maksoud MA, El-Gendy A, El-Wehedy A, Amin M. 640 Epidermal growth factor genetic polymorphism predicts risk of hepatocellular carcinoma in egyptian patients with HCV (Genotype 4)-related cirrhosis. J Hepatol. 2013;58(1):S261.
Cmet S, Fabris C, Fattovich G, Falleti E, Bitetto D, Cussigh A, et al. Carriage of the EGF rs4444903 A > G functional polymorphism associates with disease progression in chronic HBV infection. Clin Exp Immunol. 2012;167:296–302.
Stoscheck CM, King Jr LE. Role of epidermal growth factor in carcinogenesis. Cancer Res. 1986;46:1030–7.
Tonjes RR, Lohler J, O’Sullivan JF, Kay GF, Schmidt GH, Dalemans W, et al. Autocrine mitogen IgEGF cooperates with c-myc or with the Hcs locus during hepatocarcinogenesis in transgenic mice. Oncogene. 1995;10:765–8.
Okamoto K, Neureiter D, Alinger B, Meissnitzer M, Sass G, Schmitz V, et al. The dual EGF/VEGF receptor tyrosine kinase inhibitor AEE788 inhibits growth of human hepatocellular carcinoma xenografts in nude mice. Int J Oncol. 2008;33:733–42.
Schiffer E, Housset C, Cacheux W, Wendum D, Desbois-Mouthon C, Rey C, et al. Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology. 2005;41:307–14.
Zhong JH, You XM, Gong WF, Ma L, Zhang Y, Mo QG, et al. Epidermal growth factor gene polymorphism and risk of hepatocellular carcinoma: a meta-analysis. PLoS One. 2012;7:e32159.
Acknowledgements
This work was supported in part by grants from the National Natural Science Foundation of China (81172830), the National Key Sci-Tech Special Project of China (2013ZX10002011-006), and Education Department of Zhejiang Province (Y200909848).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
GJ, KY, and SZ contributed to the conception and design of the study, the analysis and interpretation of data, the revision of the article as well as final approval of the version to be submitted. CH and PQ participated in the design of the study, searched and selected the trials. LS and XY performed data acquisition and quality score assessment. HX, JL, and JZ performed statistical analysis and drafted the article. All authors read and approved the final version of the manuscript.
Additional files
Additional file 1: Table S1.
Scale for Quality Assessment.
Additional file 2:
Checklist S1. PRISMA checklist.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Jiang, G., Yu, K., Shao, L. et al. Association between epidermal growth factor gene +61A/G polymorphism and the risk of hepatocellular carcinoma: a meta-analysis based on 16 studies. BMC Cancer 15, 314 (2015). https://doi.org/10.1186/s12885-015-1318-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-015-1318-6