Abstract
Background
Breast cancer outcomes are influenced by multiple factors including access to care, and payer status is a recognized barrier to treatment access. To further define the influence of payer status on outcome, the National Cancer Data Base data from 1998–2006 was analyzed.
Method
Data was analyzed from 976,178 female patients diagnosed with breast cancer registered in the National Cancer Data Base. Overall survival was the primary outcome variable while payer status was the primary predictor variable. Secondary predictor variables included stage, age, race, Charlson Comorbidity index, income, education, distance travelled, cancer program, diagnosing/treating facility, and treatment delay. Multivariate Cox regression was used to investigate the effect of payer status on overall survival while adjusting for secondary predictive factors.
Results
Uninsured (28.68%) and Medicaid (28.0%) patients had a higher percentage of patients presenting with stage III and stage IV cancer at diagnosis. In multivariate analysis, after adjusting for secondary predictor variables, payer status was a statistically significant predictor of survival. Patients with private, unknown, or Medicare status showed a decreased risk of dying compared to uninsured, with a decrease of 36%, 22%, and 15% respectively. However, Medicaid patients had an increased risk of 11% compared to uninsured. The direct adjusted median overall survival was 14.92, 14.76, 14.56, 13.64, and 12.84 years for payer status of private, unknown, Medicare, uninsured, and Medicaid respectively.
Conclusion
We observed that patients with no insurance or Medicaid were most likely to be diagnosed at stage III and IV. Payer status showed a statistically significant relationship with overall survival. This remained true after adjusting for other predictive factors. Patients with no insurance or Medicaid had higher mortality.
Similar content being viewed by others
Background
In 2014, there will be an estimated 232,670 new cases of breast cancer and approximately 40,000 deaths in the United States [1]. The estimated prevalence for women living with breast cancer in the United States was 3,131,440 [2]. The median age of diagnosis for breast cancer was 61 years [2]. The age-adjusted breast cancer incidence rate for women was 124.6 per 100,000 [3]. While the age-adjusted incidence rate was similar between white and black women, black women had higher mortality than white women [4].
Payer status, as well as income, education, age, and ethnicity, may affect access to health care and influence breast cancer stage at diagnosis [5] and patient survival [5-9]. Reduced access to healthcare has been linked to advanced stage of cancer [5,7] and worse survival [6,7]. Lower survival rates have been found in individuals with no insurance or Medicaid [6,7,10,11]. Lower education attained has been associated with large tumor size and advanced stage disease at breast cancer diagnosis [12], however, the association with patient survival has been mixed [13,14].
With the recent development of the Affordable Care Act [15], there may be a shift in health insurance coverage in the US. In the 2012 population, there were 50.90 million (16.4%) people enrolled in Medicaid, 48.88 million (15.7%) with Medicare, and 47.95 million (15.4%) with no insurance [16]. As the type and availability of insurance changes, it will be important to assess differential effects of payer status on the outcome of patient survival. This study used the large National Cancer Data Base (NCDB) data to evaluate how payer status, as well as secondary factors, impacts breast cancer survival.
Secondary factors, which may also reflect access to healthcare, include the following indicators: (1) The patient’s choice of treatment facility type (cancer program), (2) whether they are diagnosed and treated in the same facility (diagnosing/treating facility), (3) the distance a patient must travel to the facility (distance travelled), (4) the length of the delay to start treatment once diagnosed (treatment delay), and (5) their Charlson Comorbidity index.
Studies have demonstrated an improved prognosis for female breast cancer patients treated in large community hospitals compared with small community hospitals and Health Maintenance Organization (HMO) hospitals [17]. This is supported by evidence that shows better outcomes for high-risk surgery in high-volume hospitals [18]. Teaching hospitals, known for awareness of current treatment methods and higher medical research involvement, have also shown an advantage over nonteaching facilities [17,19,20]. Stage at diagnosis has been linked to distance travelled for healthcare [21]. Differences in survival rates [22] and timely mammography for breast cancer in women [23] have been found between urban and rural settings. A few studies have found that treatment delay has no significant relationship with breast cancer survival [24-26]. In contrast, one study found an 85% increased risk of breast cancer-specific mortality for low-income, late-stage breast cancer patients who waited >60 days to initiate treatment compared to those who waited <60 days [27]. More co-existing conditions or a higher Charlson Comorbidity index has also been found to be a predictor of late stage diagnosis in colon cancer [21] and to be associated with increased risk of breast cancer mortality [28]. This study investigated the effects of payer status on female breast cancer survival.
Method
This study examined 976,178 female breast cancer patients who were diagnosed between 1998 and 2006 and followed until December 31, 2011. The data used in this study was derived from a de-identified NCDB file. The NCDB captures approximately 70% of all newly diagnosed cases of cancer in the United States at the institutional level [29]. The International Classification of Disease for Oncology, third edition (ICD-O-3) codes (C500-C506, and C508, C509) associated with a diagnosis of breast cancer were used to select patients.
The primary outcome variable, survival time of breast cancer patients, was calculated from date of diagnosis to date of death, date of loss to follow-up, or date of study end (December 31, 2011). The primary predictor variable was payer status. Secondary predictor variables included tumor stage, age, race, Charlson Comorbidity score, income, education, distance travelled, cancer program, diagnosing/treating facility, and treatment delay.
Payer status was categorized as uninsured, private, Medicaid, Medicare (or other government insurance plan), or unknown. The American Joint Committee on Cancer (AJCC) stage was categorized as I, II, III, or IV for stage at diagnosis. Age was grouped as 18–49, 50–64, 65–74, or ≥75 years. Patient race was categorized as white, black, or other. The other race category included patients with Asian and Hispanic ethnicity. Charlson Comorbidity [28] is an index to reflect the overall health status of a patient. Charlson Comorbidity was categorized as 0, 1, ≥2, or unknown. Income, or median household income at zip code level, was grouped as < $30, $30-34, $35-45, or ≥ $46 k. Education, a measure of the percent of adults in the patient's zip code who did not graduate from high school, was grouped as ≥29%, 20-28%, 14-19%, and <14%. Education was determined using 2000 census data. Distance travelled, the distance from the patient’s residential zip code to a medical center, was grouped as <10, 10–24, 25–49, 50–99, or ≥100 miles. Cancer program was categorized as community, comprehensive, academic and research, or other (other services and clinics) cancer program. Diagnosing/treating facility was categorized as same or different. Treatment Delay was grouped as 0–5, 6–20, 21–30, or ≥31 days.
Chi-Square statistical tests were used to compare the distributions of stage by payer status and other categorical variables. Kaplan-Meier methods were used to estimate survival curves. Log rank tests were used to compare the survival distributions in univariate analysis. Šidák correction method was used for adjustment in Multiple Comparisons for the Log rank Test. Multivariate Cox regression was used to simultaneously estimate the hazard of death (Hazard Ratio) of payer status and adjusted other factors. Direct Adjusted Median Overall Survival (MOS) was calculated by using Multivariate Cox regression. Statistical Software SAS 9.4 (SAS Inc. Gary, NC) and STATA 13.1 (College Station, TX: Stata Corp LP) were used for data management, statistical analysis, and modeling. All p-values <0.05 were considered statistically significant.
Results
The mean age at diagnosis for all patients was 60 years, with mean ages of 60.5, 56.5, and 54.8 years for white, black, and other race respectively. The mean age at diagnosis was 61.5, 58.2, 58.1, and 61.8 years for stage I, II, III, and IV respectively.
The patient’s payer status distribution by stage is shown in Table 1. For stage, 47.96%, 37.04%, 10.37%, and 4.62% of patients presented with stage I, II, III, and IV diseases, respectively. For payer status, 2.55%, 55.61%, 4.27%, 34.23%, and 3.34% of patients presented with uninsured, private, Medicaid, Medicare, and unknown payer status at diagnosis, respectively. Uninsured (28.68%) and Medicaid (28.0%) patients had a much higher proportion of advanced stage (stage III and IV) disease. Private (13.28%) and Medicare (12.79%) had a lower proportion of stage III and stage IV disease. A statistically significant difference in the presentation of advanced stage at diagnosis was found according to payer status (p < 0.05).
A statistically significant association was also found between stage at diagnosis and all secondary factors (data not shown). African American patients (28.15%) had the highest stage III and stage IV, and the percentages for white (14.06%) and other (14.47%) were much lower. Distinct patterns appeared in the stage distributions of Charlson Comorbidity, income, and education. As the Charlson Comorbidity increased, the percentage of stage II, III, and IV patients increased. As income and education level increased, the percentage of stage II, III, and IV patients decreased.
The results of univariate analysis can be seen in Table 2. For payer status, the MOS value for each level was statistically different from all other levels. Medicare payer status had the shortest MOS (MOS = 10.13 years), followed by Medicaid (13.08), unknown (14.56), uninsured (>14.89), and private (15.00).
Overall MOS was 14.75 years. With the exception of distance travelled and treatment delay, all secondary factors showed an MOS value for each level that was statistically different from all other levels. The largest differences were found for stage, age, and Charlson Comorbidity. MOS decreased as stage, age, and Charlson Comorbidity increased. Age ≥75 (7.14) and ≥2 Charlson Comorbidity (5.58) had the shortest survival for their groups. Stage III and IV (1.70) had much shorter survival compared to stage I and II. Education and income displayed a more subtle pattern. As the patient’s level of education and income increased, MOS also increased.
MOS was statistically inferior for distance travelled greater than 50 miles. Results for MOS according to treatment delay did not follow a clear pattern. Patients with treatment delay of 0–5 days and ≥31 days were not statistically different from each other but differed from the other delay groups (6–20 and 20–30 days).
Tables 1 and 2 demonstrate the need for multivariate regression to further investigate the effect of payer status. In these analyses, many factors are statistically related to survival.
Table 3 displays the results of hazard ratio (HR) of death from a multivariate cox regression analysis. After adjusting for secondary factors, payer status was a significant predicator for overall survival. Private, unknown, and Medicare payer status had a decreased risk of dying compared to uninsured, with decreases of 36% (HR = 0.64), 22% (0.78), and 15% (0.85) respectively. Patients with Medicaid insurance, however, had an 11% (1.11) increased risk of dying as compared to uninsured patients had.
Adjusting for other factors, age, race, Charlson Comorbidity index, and stage were also significant predictors of survival in Table 3. HR increased with increasing age. The HR was higher for age 50–64 (1.12), 65–74 (1.66), and ≥75 (4.0) compared with age 18–49. At age ≥75, patients were 4.0 times more likely to die than those age 18–49. Compared to white patients, black patients had a 31% (1.31) increase, and other race had a 22% (0.78) decrease. Patients with ≥2 (2.27) and 1 (1.43) Charlson Comorbidity were more likely to die than those with no comorbid conditions. Corresponding to the subtle pattern in Table 2, HR decreased as both income and education increased.
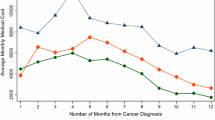
Figure 1 illustrates the Direct Adjusted MOS found for payer status only. The Direct Adjusted MOS was 14.92, 14.76, 14.56, 13.64, and 12.84 years for private, unknown, Medicare, uninsured, and Medicaid payer status respectively. Patients with private insurance had a 2.1 year longer survival compared to patients with Medicaid insurance.
Discussion
Payer status had a statistically significant relationship to stage distribution and overall survival for female breast cancer patients. Patients with no insurance or Medicaid had the highest proportion at stage III and stage IV when diagnosed (Table 1), a finding which supports the results of another study [5]. In multivariate analysis, adjusting for other factors including stage, payer status was a significant predictor of overall survival. Patients with private and unknown payer status were less likely to die than uninsured and Medicaid patients. Private patients had a Direct Adjusted MOS over 1.3 and 2.1 years longer than uninsured and Medicaid patients respectively (Figure 1). Our findings support other studies that show higher stage at diagnosis [5] and worse survival for patients with no insurance or Medicaid [6,7,10,11]. Higher stage at diagnosis and lower survival in these populations might be explained by lower access to preventive screening and high-quality care. Further research is needed to investigate the barriers for these populations and to develop targeted interventions.
In the multivariate analysis, the secondary significant predictors of survival were age, race, Charlson Comorbidity index, and stage. Patient’s age ≥75 were 4.00 times more likely to die than patients 18–49. As expected, older patients have a higher risk because of the aging process. African American patients had the highest mortality when compared to white patients. This was consistent with literature demonstrating lower survival in African American patients [9,30,31]. Patients with ≥2 Charlson Comorbidity were 2.27 times more likely to die than those with no comorbid conditions. Another study indicated an association of one unit of change of Charlson Index with a 2.3-fold increase in the 10-year mortality in breast cancer patients [28]. As a measure of overall health status, a higher risk of dying is expected with a higher Charlson Index.
In this study, the HR estimation for various factors was more reliable, with a narrow 95% confidence interval, because so many patients were studied. However, because of this, the reader must differentiate between statistical and clinical significance when interpreting the results. Although all categories in the multivariate analysis were statistically significant, not all HR changes would be clinically important. For example, with an HR of 0.96, some factors were statistically significant even though there was only a risk reduction of 4%.
This study investigated how a patient’s access to health care can impact survival. The level of patient adherence to National Comprehensive Cancer Network treatment guidelines was not studied here, but could also be an important factor. Addressing patient adherence in future research might provide a more complete understanding of the influence of treatment characteristics.
Another issue was the information collected from the NCDB. The database did not collect Charlson Comorbidity information consistently before 2003. The reference group (0 Charlson Comorbidity) for 2003–2006 was used to estimate the Charlson Comorbidity effect for patients diagnosed before 2003 (coded as unknown Charlson Comorbidity). This estimate may only represent an average of all Charlson Comorbidity conditions in the earlier group. The NCDB also did not collect cause-specific death information. We assessed the effect of payer status on overall survival instead of cause-specific survival. Measuring the effect on cause-specific survival may produce different results. Additionally, education and income by zip-code was collected instead of individual education and income. Using individual education and income level would strengthen the analysis of these factors. The NCDB, a large retrospective national database, may also be sensitive to bias in patient selection and variation in institution reporting [32].
Conclusion
We observed that uninsured and Medicaid patients were most likely to be diagnosed at stage III and stage IV. Payer status, our primary focus, showed a statistically significant relationship with overall survival. This remained true after adjusting for secondary predictive factors. Patients with no insurance or Medicaid had higher mortality than private, Medicare, unknown insurance. Further research is needed to investigate patient treatment adherence and cause-specific survival.
Ethics statement
With the support from the Chair of Louisiana State University Hospital in Shreveport (currently University Health Shreveport) Cancer program, the corresponding author has applied and has been awarded the National Cancer Data Base (NCDB) Participant Use Data File (PUF) for 1998 to 2011 from the Commission on Cancer (CoC). The PUF is a Health Insurance Portability and Accountability Act (HIPAA) compliant data file containing cases submitted to the Commission on Cancer’s (CoC) National Cancer Data Base (NCDB). The PUF contains de-identified patient level data that do not identify hospitals, healthcare providers, or patients as agreed to in the Business Associate Agreement that each CoC-accredited program has signed with the American College of Surgeons. The PUFs are designed to provide investigators associated with CoC-accredited cancer programs with a data resource they can use to review and advance the quality of care delivered to cancer patients through analyses of cases reported to the NCDB. NCDB PUFs are only available through an application process to investigators associated with CoC-accredited cancer programs.
Abbreviations
- HMO:
-
Health Maintenance Organization
- NCDB:
-
National Cancer Data Base
- AJCC:
-
American Joint Committee on Cancer
- MOS:
-
Median overall survival
- HR:
-
Hazard ratio
References
Breast Cancer. [http://seer.cancer.gov/statfacts/html/breast.html]
Society. AC. Cancer Treatment and Survivorship Facts & Figures 2014–2015. Atlanta, Georgia: American Cancer Society; 2014. p. 1. Figure 1.
SEER Cancer Statistics Review 1975–2011 [http://seer.cancer.gov/csr/1975_2011/browse_csr.php?sectionSEL=4&pageSEL=sect_04_table.05.html]
SEER Cancer Statistics Review 1975–2011 [http://seer.cancer.gov/csr/1975_2011/browse_csr.php?sectionSEL=4&pageSEL=sect_04_table.12.html]
Farkas DT, Greenbaum A, Singhal V, Cosgrove JM. Effect of Insurance Status on the Stage of Breast and Colorectal Cancers in a Safety-Net Hospital. J Oncol Pract. 2012;8(3S):16s–21s.
Shi R, Mills G, McLarty J, Burton G, Shi Z, Glass J. Commercial insurance triples chances of breast cancer survival in a public hospital. Breast J. 2013;19(6):664–7.
Ward E, Halpern M, Schrag N, Cokkinides V, DeSantis C, Bandi P, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58(1):9–31.
Halpern MT, Bian J, Ward EM, Schrag NM, Chen AY. Insurance status and stage of cancer at diagnosis among women with breast cancer. Cancer. 2007;110(2):403–11.
Newman LA, Mason J, Cote D, Vin Y, Carolin K, Bouwman D, et al. African-American ethnicity, socioeconomic status, and breast cancer survival: a meta-analysis of 14 studies involving over 10,000 African-American and 40,000 White American patients with carcinoma of the breast. Cancer. 2002;94(11):2844–54.
Sabik LM, Bradley CJ. Differences in mortality for surgical cancer patients by insurance and hospital safety net status. Med Care Res Rev. 2013;70(1):84–97.
Niu X, Roche LM, Pawlish KS, Henry KA. Cancer survival disparities by health insurance status. Cancer Med. 2013;2(3):403–11.
DeSantis C, Jemal A, Ward E. Disparities in breast cancer prognostic factors by race, insurance status, and education. Cancer Cause Control. 2010;21(9):1445–50.
Albano JD, Ward E, Jemal A, Anderson R, Cokkinides VE, Murray T, et al. Cancer mortality in the united states by education level and race. J Natl Cancer Inst. 2007;99(18):1384–94.
Shariff-Marco S, Yang J, John EM, Sangaramoorthy M, Hertz A, Koo J, et al. Impact of Neighborhood and Individual Socioeconomic Status on Survival after Breast Cancer Varies by Race/Ethnicity: The Neighborhood and Breast Cancer Study. Cancer Epidem Biomar. 2014;23(5):793–811.
Patient Protection and Affordable Care Act, 42 U.S.C. § 18001 et seq. (2010). http://housedocs.house.gov/energycommerce/ppacacon.pdf
DeNavas-Walt, Carmen, Bernadette D. Proctor, and Jessica C. Smith, U.S. Census Bureau, Current Population Reports, P60-245, Income, Poverty, and Health Insurance Coverage in the United States: 2012, U.S. Government Printing Office, Washington, DC, 2013. https://www.census.gov/prod/2013pubs/p60-245.pdf
Lee-Feldstein A, Anton-Culver H, Feldstein PJ. Treatment differences and other prognostic factors related to breast cancer survival. Delivery systems and medical outcomes. JAMA. 1994;271(15):1163–8.
Council. IoMaNR. Ensuring Quality Cancer Care. Washington, DC: The National Academies Press; 1999.
Chaudhry R, Goel V, Sawka C. Breast cancer survival by teaching status of the initial treating hospital. CMAJ. 2001;164(2):183–8.
Hartz AJ, Krakauer H, Kuhn EM, Young M, Jacobsen SJ, Gay G, et al. Hospital characteristics and mortality rates. N Engl J Med. 1989;321(25):1720–5.
Massarweh NN, Chiang YJ, Xing Y, Chang GJ, Haynes AB, You YN, et al. Association Between Travel Distance and Metastatic Disease at Diagnosis Among Patients With Colon Cancer. J Clin Oncol. 2014;32(9):942 − +.
Klein J, Ji M, Rea NK, Stoodt G. Differences in male breast cancer stage, tumor size at diagnosis, and survival rate between metropolitan and nonmetropolitan regions. Am J Men's Health. 2011;5(5):430–7.
Doescher MP, Jackson JE. Trends in cervical and breast cancer screening practices among women in rural and urban areas of the United States. J Public Health Manage Pract. 2009;15(3):200–9.
Delgado DJ, Lin WY, Coffey M. The role of Hispanic race/ethnicity and poverty in breast cancer survival. P R Health Sci J. 1995;14(2):103–16.
Redondo M, Rodrigo I, Pereda T, Funez R, Acebal M, Perea-Milla E, et al. Prognostic implications of emergency admission and delays in patients with breast cancer. Support Care Cancer. 2009;17(5):595–9.
Smith ER, Adams SA, Das IP, Bottai M, Fulton J, Hebert JR. Breast cancer survival among economically disadvantaged women: The influences of delayed diagnosis and treatment on mortality. Cancer Epidem Biomar. 2008;17(10):2882–90.
McLaughlin JM, Anderson RT, Ferketich AK, Seiber EE, Balkrishnan R, Paskett ED. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol. 2012;30(36):4493–500.
Charlson ME, Pompei P, Ales KL, Mackenzie CR. A NEW METHOD OF CLASSIFYING PROGNOSTIC CO-MORBIDITY IN LONGITUDINAL-STUDIES - DEVELOPMENT AND VALIDATION. J Chronic Dis. 1987;40(5):373–83.
National Cancer Data Base [http://www.facs.org/cancer/ncdb/index.html]
Simon MS, Banerjee M, Crossley-May H, Vigneau FD, Noone AM, Schwartz K. Racial differences in breast cancer survival in the Detroit Metropolitan area. Breast Cancer Res Treat. 2006;97(2):149–55.
Albain KS, Unger JM, Crowley JJ, Coltman Jr CA, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101(14):984–92.
Rueth NM, Lin HY, Bedrosian I, Shaitelman SF, Ueno NT, Shen Y, et al. Underuse of trimodality treatment affects survival for patients with inflammatory breast cancer: an analysis of treatment and survival trends from the national cancer database. J Clin Oncol. 2014;32(19):2018–24.
Acknowledgements
The authors wish to acknowledge the Commission on Cancer of the American College of Surgeons and the American Cancer Society for making public data available through the NCDB. The data used in this study were derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed or the conclusions drawn from these data by the investigator. The authors also wish to thank Mrs. Thu Vu for her assistance in the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RS designed the study, obtained the dataset, performed all data management, carried out the statistical data analysis, and drafted the manuscript. HT assisted with drafting the manuscript. JM, LL, GM, and GB participated in the design of the study and drafted the manuscript. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Shi, R., Taylor, H., McLarty, J. et al. Effects of payer status on breast cancer survival: a retrospective study. BMC Cancer 15, 211 (2015). https://doi.org/10.1186/s12885-015-1228-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-015-1228-7