Abstract
Background
Non-invasive chromosome screening (NICS) and trophectoderm biopsy preimplantation genetic testing for aneuploidy (TE-PGT) were both applied for embryo ploidy detection, However, the cumulative live birth rates (CLBR) of NICS and TE-PGT in older age groups have yet to be reported. This study aimed to ascertain whether NICS and TE-PGT could enhance the cumulative live birth rates among patients of advanced maternal age.
Methods
A total of 384 couples aged 35–40 years were recruited. The patients were assigned to three groups: NICS, TE-PGT, and intracytoplasmic sperm injection (ICSI). All patients received frozen single blastocyst transfer. Patients in the NICS and TE-PGT groups underwent aneuploidy screening.
Results
When compared to the ICSI group, the CLBR was significantly higher in the NICS and TE-PGT groups (27.9% vs. 44.9% vs. 51.0%, p = 0.003 for NICS vs. ICSI, p < 0.001 for TE-PGT vs. ICSI). There were no significant differences in the clinical outcomes between the NICS and TE-PGT groups. Adjusting for confounding factors, the NICS and TE-PGT groups still showed a higher CLBR than the ICSI group (adjusted odds ratio (OR) 3.847, 95% confidence interval (CI) 1.939 to 7.634; adjusted OR 3.795, 95% CI 1.981 to 7.270). Additionally, the cumulative pregnancy loss rates of the NICS and TE-PGT groups were significantly lower than that of the ICSI group (adjusted OR 0.277, 95% CI 0.087 to 0.885; adjusted OR 0.182, 95% CI 0.048 to 0.693). There was no significant difference in the birth weights of the three groups (p = 0.108).
Conclusions
In women 35–40 years old, the CLBR can be increased by selecting euploid embryos using NICS and TE-PGT. For elderly women at high risk of embryonic aneuploidy, NICS, characterized by its safety and non-invasive nature, may emerge as an alternative option for preimplantation genetic testing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Embryo chromosomal aneuploidy is a major cause of implantation failure or miscarriage during in vitro fertilization (IVF) [1, 2]. Morphological evaluation is incapable of detecting the genetic characteristics of embryos [3]. Therefore, preimplantation genetic testing for aneuploidy (PGT-A) is commonly used to evaluate the ploidy of embryos in clinical practice. With advanced biopsy methods and genetic analysis strategies, preimplantation genetic testing (PGT) has rapidly developed in the past decade. The incidence of embryo aneuploidy increases with age, with 35 years being particularly noted as a turning point for higher aneuploidy rates [4]. Multiple studies have consistently demonstrated the potential of PGT-A to enhance clinical outcomes in patients aged 35–40 years [5,6,7].
PGT-A relies on biopsy, which is invasive and may harm embryo development. Since Stigliani et al. [8]. were the first to analyze the genomic DNA content in embryo medium in 2013; therefore, it is possible to use embryonic cell-free DNA (cfDNA) to identify blastocyst chromosomal ploidy [9,10,11]. Using genetic material sampled from spent culture medium (SCM), we conducted the first noninvasive chromosome screening (NICS) in 2016 [12]. In Huang et al.‘s study, using the whole embryo as the gold standard, NICS showed a concordance of 93.8% with the whole embryo, while trophectoderm biopsy preimplantation genetic testing for aneuploidy (TE-PGT) showed an 82.0% concordance [13]. In our previous research, the TE biopsy cells, Day 3-Day 5/Day 6 culture medium, and whole embryo from the same embryo were collected. Using the whole embryo as the gold standard, the concordance rates with the whole embryo were 85.2% for TE-PGT and 83.0% for NICS. These findings demonstrated that NICS is accurate and has a diagnostic performance close to that of TE-PGT [14]. In a retrospective cohort study, for patients aged 35–40, the live birth rate for those who transferred NICS euploid embryos was higher than for those who transferred non-euploid embryos (50.0% vs. 29.5%, p = 0.029) [15]. Li et al. conducted a prospective interventional clinical trial, where age-stratified results showed that for patients aged 35–40, NICS significantly improved the live birth rate (54.8% vs. 25.0%, p = 0.001) [16]. However, there are no reports on the cumulative clinical outcomes of NICS in females aged 35–40.
The cumulative live birth rate (CLBR) is regarded as the most crucial patient-centered outcome when assessing an IVF program’s success [17]. In a recent randomized controlled trial, among females aged 20 to 37 years having ≥ 3 good-quality blastocysts, the CLBR in PGT-A was noninferior to the rate in conventional morphological evaluation [18]. However, in a meta-analysis study on preimplantation genetic testing for aneuploidy (PGT-A), the results demonstrated an improvement in the cumulative live birth rate among the included patients. But the study did not perform an age-stratified analysis [19]. Therefore, the impact of PGT-A on the cumulative live birth rate in the elderly population remains unexplored in the current literature. There is no standardized calculation method of cumulative live birth rate [20]. The probability of obtaining chromosomally normal oocytes decreases in advanced maternal age women, leading to a reduced number of embryos available for genetic testing and subsequent transplantation. Consequently, patients may undergo multiple oocyte retrieval cycles. Therefore, evaluating the effectiveness of PGT-A in terms of the cumulative live birth rate may be more objective when considering the number of oocyte retrieval cycles as the denominator, rather than the number of patients.
To evaluate the efficacy of genetic screening in advanced females aged 35–40 years, we designed a three-armed prospective cohort study. By comparing the clinical outcomes of patients in the NICS and TE-PGT groups, we investigated whether NICS can achieve similar clinical efficacy to TE-PGT. This study aimed to determine whether NICS and TE-PGT improve the CLBR in women aged 35–40 compared to embryo selection based on morphological grading.
Methods
Study design and patients
This was a prospective cohort study which included all couples with female patients between 35–40 years old attending the Reproductive Medicine Center of Nanjing Jinling Hospital (Nanjing, Jiangsu, People’s Republic of China) from April 2017 to May 2021. The study protocol was approved by the Ethics Committee of Nanjing Jinling Hospital (2016NZKY-028-02) and registered in the Chinese Clinical Trial Center www.clinicaltrails.gov (ChiCTR-DDD-17010376, date of first registration: 01/11/2017). Informed consents were obtained from all couples participated in this study.
First, all female patients underwent karyotyping before IVF according to routine clinical procedures. Inclusion criteria: the female patient was 35–40 years old, normal couple karyotype, underwent intracytoplasmic sperm injection (ICSI), with ≥ 1 blastocysts (morphology grading above BC or CB), agreed to culture all embryos to the blastocyst stage, and transferred a single thawed blastocyst.
Exclusion criteria: PGT for monogenic disorders (PGT-M) or PGT for chromosomal structural rearrangements (PGT-SR), uterine abnormality (e.g., untreated uterine septum, adenomyosis, or submucous myoma; uterine congenital malformation; endometrial polyps; or intrauterine adhesions), the presence of a contraindication to pregnancy, intended to undergo PGT-M or PGT-SR, or plan to use of donated oocytes or sperm.
Based on self-selection, the included patients meeting the inclusion and exclusion criteria were sorted into the genetic testing group and the control group. 187 couples chose to join the genetic testing group, then these 187 couples were randomized into the NICS group and TE-PGT group. A random data table was used for randomization.197 patients chose to enter the control group, undergoing ICSI only.
Oocyte retrieval and ICSI
Ovarian stimulation, follicular monitoring, and oocyte retrieval were all performed using the standard methods of our center. The dose of gonadotropin was adjusted according to the patient’s ovarian response, hormone level and follicle size, and when the follicle diameter and hormone level reached the standard of trigger. The female underwent oocyte retrieval under transvaginal ultrasound guidance 34–36 h after hCG injection, followed by the initiation of luteal support beginning on the day of retrieval. The oocytes were then assessed for maturity, and mature oocytes were subjected to ICSI. Prior to ICSI, cumulus cells surrounding the oocytes were thoroughly removed.
Embryo culture
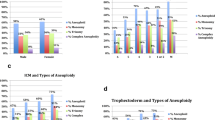
After ICSI, the embryos were cultured to cleavage stage (Day 3). The granulosa cells of the embryos were again removed. Subsequently, embryos were transferred into an individual drop of fresh blastocyst culture medium (SAGE ART-1029), with the culture medium volume being approximately 30 μL. Blastocysts at D5/6 were then assessed according to the morphological criteria developed by Gardner and Schoolcraft (1999) [21], which evaluated blastocyst expansion, the inner cell mass (ICM), and trophectoderm (TE) development.
Sample collection
Performed TE biopsies or collect culture medium from the embryos of patients in different groups. For the embryos from TE-PGT group, when the blastocysts developed to the standard of freezing, 3 to 5 TE cells were biopsied from each of the blastocysts before cryopreservation and placed into 5 μL of cell lysis buffer.
For the embryos from NICS group, approximately 25 μL culture medium of the corresponding blastocysts was collected using different pipettes. The medium was put into the RNase/DNAase-free PCR tubes, which containing 5 μL of preservation solution. All collected samples were stored at -80 °C until use.
Whole genome amplification and next-generation sequencing
Whole genome amplification (WGA) was performed on the medium and TE cell biopsies as described previously [12, 22]. For culture medium and TE cells, whole genome amplification (WGA) and library preparation was performed using the NICSInst™ library preparation kit and ChromInst™ library preparation kit (Xukang Medical Technology (suzhou) Co., Ltd). Each step was performed according to the manufacturer’s protocol provided with the kit. Briefly, the amplification process began with annealing the DNA to a pool of random primers. This was followed by a quasi-linear pre-amplification step, after which the DNA was exponentially amplified to a final amount of up to 2 μg. To assess the amplification efficiency, Agarose gel electrophoresis and NanoDrop quantification were used. Then library construction was performed and sequenced on an Illumina platform, producing 2 million sequencing reads per sample.
Copy number variation (CNV) analysis
The human hg19 genome was used to map the high-quality reads after they were extracted. GC content and a reference dataset were used to normalize the reads after they had been stripped of duplicates and counted across the entire genome using a bin size of 1 Mb. The final copy number segments were computed using circular binary segmentation (CBS) algorithms. A measure of the amplification success was the coefficient of variation (CV), which is computed as the ratio of the read density standard deviation to its average. Successful amplification was defined as having a CV value of less than 0.2 or the median of the absolute values of all pairwise differences (MAPD) less than 0.25 [23]. The data were analyzed and visualized using the R program.
When the extent of mosaicism was above 30%, with a detection limit for segmental aneuploidy of ≥ 4 Mb were regarded as aneuploid in biopsied TE samples [24]. For culture medium, mosaicism ≥ 40% [25] and segmental aneuploidy of ≥ 10 Mb were set as the threshold to distinguish aneuploid from euploid. Our preliminary findings also validated the accuracy of the analysis standards [14]. The data were not sufficient for analysis because the amplification failure or non-informative results was defined as not available.
Embryo thawing and transfer
In order to prepare the endometrium for the frozen embryo transfer (FET) cycles, hormone replacement was used. Oral oestradiol pills were given for 10–18 days, and then progesterone was given when the endometrial thickness was 8 mm. The embryos were thawed using the commercial thawing kit (Kitazato, Japan). The patients in the NICS and TE-PGT groups received single euploid blastocyst transplantation. For the ICSI group, the embryos were selected based on morphological ratings, and were transferred with single blastocysts.
Outcomes
The primary outcome was the cumulative live birth rate which resulted from embryo transfers carried out within 1 year per retrieval cycle [26]. Live birth was defined as delivery at ≥ 28 weeks of gestation, and didn’t have any significant congenital defects. The secondary outcomes were the cumulative rates of biochemical, clinical pregnancy and pregnancy loss, and birth weight.
A serum level of 50 mIU per milliliter of human chorionic gonadotropin (β-hCG) at least 14 days after embryo transfer was considered biochemical pregnancy. Clinical pregnancy was examined by ultrasound at 28–30 days after transfer of a single blastocyst. Ongoing pregnancy was confirmed as a detectable fetal heart after 10–12 weeks of gestation. Pregnancy loss was defined as intrauterine pregnancy loss before 24 weeks of gestation.
Statistical analysis
The software SPSS version 25.0 (IBM, Armonk, NY, USA) was used for all analyses. Continuous variables were analyzed using the Kruskal-Wallis H test if they were nonnormally distributed, or one-way analysis of variance (ANOVA) if they were normally distributed. With the use of the chi-square test or Fisher’s exact test, categorical data—which are expressed as counts and percentages—were found to be statistically significant. The outcomes of the three groups were compared using multi-logistic regression analysis [27], which adjusted for covariables at p < 0.10 and clinically influential. The 95% confidence intervals (CI) and adjusted odds ratio (OR) were presented. A p-value that was < 0.05 on two sides was deemed statistically significant.
Results
A total of 384 couples with female aged 35–40 years old were initially included in this cohort study. The flowchart is shown in Fig. 1. Table 1 listed basal characteristics, treatment indications for infertility, and stimulation results. The results revealed significant differences in the distribution of female age, female body mass index (BMI), basal luteinizing hormone (LH), basal estradiol (E2), basal testosterone (T), anti-Müllerian hormone (AMH), and number of mature oocytes between the three groups (all p values < 0.05). Both the NICS and TE-PGT groups had a significantly higher number of mature oocytes than the ICSI group (all p values < 0.05). The TE-PGT group also had a significantly higher detection success rate than the NICS group (98.7% vs. 92.9%, p < 0.001), but there was no significant difference in the euploid embryo rate (48.3% vs. 42.4%, p = 0.107).
Table 2 presented the results, which indicated significant variations in the cumulative biochemical pregnancy rate, cumulative clinical pregnancy rate, cumulative live birth rate, and cumulative pregnancy loss among the three groups (all p values < 0.05). The primary outcome of cumulative live birth occurred in 44 of 98 retrieval cycles (44.9%) in the NICS group, in 53 of 104 retrieval cycles (51.0%) in the TE-PGT group and 64 of 229 (27.9%) in the ICSI group (p < 0.001). In the NICS, ICSI and TE-PGT groups, there was a downwards trend in neonatal weight (3567.0 ± 403.6 vs. 3459.4 ± 476.1 vs. 3350 ± 611.3), but the difference was not statistically significant (p = 0.108).
Given that the study was a cohort study, the distribution of multiple baseline information was unbalanced across the three groups. Therefore, multiple logistic regression analysis was used to calculate an adjusted OR. The results are presented in Table 3 and showed that after adjusting for confounding factors (female age, female BMI, basal LH, basal estradiol, basal T, AMH, and No. of mature oocytes, No. of previous failed transfer cycles, No. of previous early spontaneous miscarriages, Indication for infertility treatment, Total motile sperm count (×10^6), Days of transferred embryos, Quality grade of transferred embryos). The cumulative live birth rate was significantly increased in both the NICS (adjusted OR 3.847, 95% CI 1.939 to 7.634) and TE-PGT (adjusted OR 3.795, 95% CI 1.981 to 7.270) groups compared to the ICSI group. The two PGT groups showed a trend towards a lower risk of miscarriages compared to the ICSI group (13.7% vs. 7.3% vs. 27.9%, p = 0.007). The miscarriage rates were lower in the NICS and TE-PGT groups than in the ICSI group (adjusted OR 0.277, 95% CI 0.087 to 0.885; adjusted OR 0.182, 95% CI 0.048 to 0.693). Outcomes were compared between the NICS and TE-PGT groups, with each euploid embryo transfer performed separately. There were no significant differences in the clinical outcomes between the NICS and TE-PGT groups. The NICS group showed a lower detection success rate than the TE-PGT group (adjusted OR 0.157, 95% CI 0.055–0.444), while the euploid embryo rate was not different (adjusted OR 0.776, 95% CI 0.571–1.054) (Table 3).
For all FET cycles, the proportion of patients who had a live birth, biochemical pregnancy, clinical pregnancy, and pregnancy loss were similar between the NICS and TE-PGT groups. The live birth rates per transfer cycle of NICS and TE-PGT groups were higher than in the ICSI group (51.8% vs. 53.5% vs. 32.2%, p < 0.001) (Table 2). Due to morphological grade of the blastocysts, there is an impact on the clinical outcome of the patients, and in this study there was a significant difference between the three groups. Consequently, we stratified the morphological grade of the transferred embryos. Regardless of embryo quality (good, fair, poor), there was a trend towards higher live birth rates in the NICS and TE-PGT groups compared to the ICSI group. For good embryos (AA/BA/AB), the TE-PGT group had a significantly higher live birth rate (67.6% vs. 40.4%, p = 0.013) and significantly lower pregnancy loss rate (0% vs. 34.5%, p = 0.001) than the ICSI group. For fair embryos (BB), the NICS group showed a significantly higher clinical pregnancy rate (63.4% vs.42.7%, p = 0.022) and live birth rate (56.1% vs.31.5%, p = 0.005) compared to the ICSI group. For poor embryos (CA/BC/CB), due to the small number of cycles, the differences among the three groups were not statistically significant (Supplementary Table I).
Discussion
The non-invasive chromosomal screening technique based on embryo culture media was established in 2016 and has subsequently been validated by numerous studies worldwide. The Huang’s study demonstrated the higher accuracy and reliability of PGT testing using embryo culture media compared to TE cell biopsy, through a comparison of 52 culture media samples, TE biopsy samples, and true ploidy results obtained from whole embryo genetic sequencing [13]. Yeung et al. compared the results of 116 TE biopsy samples and culture media samples, and achieved favorable clinical outcomes in 15 cases following euploid embryo transfer [24]. Fang et al. utilized this technique for screening euploid embryos in 43 couples, resulting in an overall clinical pregnancy rate of 58% [10]. However, there are currently no clinical studies reporting the application of this technique in older women ages 35–40 years, nor are there reports on cumulative live-birth rates.
The study is the first to report the cumulative live birth rate of NICS, which has been proven to be as effective as TE-PGT. Both different chromosomal analysis techniques for PGT-A increased the cumulative live birth rate compared with that of the ICSI group. NICS revealed equivalent results in terms of cumulative rates of biochemical, clinical pregnancy and live birth rates and cumulative pregnancy loss rates compared with TE-PGT. In clinical in vitro fertilization, the most popular genetic test for identifying aneuploidies in embryos is PGT-A with TE biopsy. However, it is highly challenging to quantify the possible harm that could compromise implantation potential as well as potential issues with long-term effects on the offspring. In addition, TE biopsy requires expert embryologists to undertake embryo manipulation and specific equipment, raising the price of executing PGT-A. The noninvasive approach not only offers important benefits such as avoiding invasive biopsy and lowering cost, but also shows high concordance with TE biopsy results for prioritizing embryo euploidy, which may make it more accessible to a larger patient population.
Our results reported that the primary outcome of cumulative live birth occurred in 44 of 98 retrieval cycles (44.9%) in the NICS group, 53 of 104 retrieval cycles (51.0%) in the TE-PGT group and 64 of 229 (27.9%) in the ICSI group (p < 0.001) (Table 2). Compared to the ICSI group, both the NICS and TE-PGT groups showed a significant increase in cumulative live birth (adjusted OR 3.847, 95% CI 1.939 to 7.634; adjusted OR 3.795, 95% CI 1.981 to 7.270) (Table 3). The ICSI group is morphologically preferred. If the patient has transferable embryos and the endometrium is normal, a pregnancy will eventually be successful. However, the reality is that most elderly patients will not continue to transfer remaining embryos or have a strong demand for the transfer of 2 blastocysts after the first relatively high-quality blastocyst transfer, which also led to 13 patients dropping out in the ICSI group. The main reason is that these elderly patients cannot accept the possibility of transfer failure temporarily due to the unknown chromosome status of the embryo. However, patients who have undergone NICS or PGT-A testing and confirmed euploid embryos will complete the second or third attempt as soon as possible within one year, so the cumulative pregnancy rate obtained by PGT-A and NICS will be significantly higher than that of the ICSI group. If the patient has enough confidence and time to insist on transplantation one by one, the pregnancy outcome of the ICSI group will be even better than that of the PGT group. However, the choice of elderly patients is often more cautious, and more indicators besides morphological preference are needed to choose the most suitable embryos, reducing the time to live birth. Compared to previous studies, the cumulative live birth rates in the NICS group (44.9%) and TE-PGT group (51.0%) were not high in our study. This may be related to the age of the patients included in the study, as older patients have lower live birth rates per transplantation, and the cumulative live birth rates after multiple transplantations are lower in older patients than in younger patients.
When comparing NICS with the TE-PGT results, the clinical outcomes were similar. The NICS and PGT-A groups each included 98 and 104 retrieval cycles, respectively. However, compared with 87 retrieval cycles in the PGT-A group, only 77 retrieval cycles in the NICS group had euploid embryos for transfer. This may be related to the lower detection success rate (92.9% vs. 98.7%) and euploid rate (42.4% vs. 48.3%) in the NICS group (Table 1). After adjusting for confounding factors, the informative rate was significantly different between the two PGT groups (p < 0.001), but there was no significant difference in the euploid embryo rate (p = 0.105). Only euploid embryos were transplanted to exclude the influence of embryo mosaicism. More patients in the NICS group had no euploid embryos to transfer, so the cumulative live birth rate was lower in the NICS group than in the PGT-A group, although there was no significant difference. In this study, 27 (7.1%, 27/381) embryos with SCM failed amplification in the NICS group, but only 5 embryos (1.3%, 5/386) with SCM failed amplification in the PGT-A group (Table 1). The DNA for PGT-A comes from the biopsy cells, whereas the free DNA in culture medium originates from the embryo and apoptotic cells, making its quantity uncertain. However, sampling culture medium is non-invasive compared to TE biopsy. Some studies have reported this phenomenon, where the amount of DNA in culture medium was larger in embryos with poor-quality cleavage than in good-grade embryos [8]. A transfer of 53 TE-euploid blastocysts with blastocyst fluid (BF)-failed amplification resulted in a 77% clinical pregnancy rate in the group [28]. To avoid embryo wastage, we developed a noninvasive embryo grading system utilizing the random forest machine learning algorithm. Blastocysts were categorized into three grades A, B, C based on the NICS results, with euploid probability. Grade A- and B- embryos accounted for 78.8% of the total embryos and were recommended for transfer due to their better clinical outcomes [29]. The failed amplification embryos will also be analyzed in the grading system, thus greatly avoiding embryo wastage. There were 27 embryos with SCM that failed amplification in the NICS group in our study without transplantation. The next step is to use the machine learning-guided embryo grading system to reselect these 27 embryos and avoid wasting potential embryos [29].
In addition, the cumulative pregnancy loss rates of the NICS and TE-PGT group were both significantly lower than that of the ICSI group (13.7% vs. 27.3%, adjusted OR 0.277, 95% CI 0.087 to 0.885; 7.3% vs. 27.3%, adjusted OR 0.182, 95% CI 0.048 to 0.693) (Table 3). Several studies have shown that biopsy has an impact on neonatal outcomes, but no studies have focused on the impact of NICS on neonatal outcomes. Our study indicated a decreasing trend in neonatal weight (3567.0 ± 403.6 vs. 3459.4 ± 476.1 vs. 3350.0 ± 611.3) among the NICS, ICSI and TE-PGT groups, but no significant differences were observed among these groups (p = 0.108, Table 2). This may be due to the invasiveness of TE biopsy on embryos, which affects embryonic development and subsequently impacts neonatal weight. Nevertheless, these observations imply the need for further investigation.
The primary limitation of the study is its single-center design and the fact that enrolled patients were restricted to those seeking treatment at the reproductive center. As such, the level of evidence available is not as high as that of randomized controlled trials. Larger prospective studies are necessary for reaching definite conclusions regarding the accuracy of NICS in representing the genetic composition of the whole embryo, as well as randomized clinical trials to report the benefits of NICS for improving pregnancy outcomes.
Conclusions
Overall, compared with morphological assessment, both NICS and TE-PGT for preimplantation chromosome screening can increase the cumulative live birth rate in females aged 35–40 years old, and reduce the cumulative pregnancy loss. There was no significant difference in cumulative live birth rates between the NICS and TE-PGT groups. These findings suggest that NICS could be a noninvasive alternative to TE biopsy for PGT-A in the future.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AMH:
-
Anti-Müllerian hormone
- BF:
-
Blastocyst fluid
- BMI:
-
Body mass index
- cfDNA:
-
Cell-free DNA
- CLBR:
-
Cumulative live birth rate
- Basal E2:
-
Basal estradiol
- ICSI:
-
Intracytoplasmic sperm injection
- IVF:
-
In vitro fertilization
- LH:
-
Basal luteinizing hormone
- NICS:
-
Non-invasive chromosome screening
- PGT-A:
-
Preimplantation genetic testing for aneuploidy
- SCM:
-
Spent culture medium
- Basal T:
-
Basal testosterone
- TE-PGT:
-
Trophectoderm biopsy preimplantation genetic testing for aneuploidy
References
Scott RT, Ferry K, Su J, Tao X, Scott K, Treff NR. Comprehensive chromosome screening is highly predictive of the reproductive potential of human embryos: a prospective, blinded, nonselection study. Fertil Steril. 2012;97:870–5. https://doi.org/10.1016/j.fertnstert.2012.01.104
Sahoo T, Dzidic N, Strecker MN, Commander S, Travis MK, Doherty C, et al. Comprehensive genetic analysis of pregnancy loss by chromosomal microarrays: outcomes, benefits, and challenges. Genet Medicine: Official J Am Coll Med Genet. 2017;19:83–9. https://doi.org/10.1038/gim.2016.69
Alfarawati S, Fragouli E, Colls P, Stevens J, Gutiérrez-Mateo C, Schoolcraft WB, et al. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. 2011;95:520–4. https://doi.org/10.1016/j.fertnstert.2010.04.003
Dang TT, Phung TM, Le H, Nguyen TB, Nguyen TS, Nguyen TL, et al. Preimplantation genetic testing of aneuploidy by next generation sequencing: association of maternal age and chromosomal abnormalities of blastocyst. Open Access Macedonian J Med Sci. 2019;7:4427–31. https://doi.org/10.3889/oamjms.2019.875
Munné S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071–e10791077. https://doi.org/10.1016/j.fertnstert.2019.07.1346
Sanders KD, Silvestri G, Gordon T, Griffin DK. Analysis of IVF live birth outcomes with and without preimplantation genetic testing for aneuploidy (PGT-A): UK human fertilisation and embryology authority data collection 2016–2018. J Assist Reprod Genet. 2021;38:3277–85. https://doi.org/10.1007/s10815-021-02349-0
Sarkar P, New EP, Sprague RG, Stillman R, Widra E, Jahandideh S, et al. Live birth per embryo transfer with next generation sequencing preimplantation genetic testing: an analysis of 26,107 cycles. Syst Biology Reproductive Med. 2023;69:379–86. https://doi.org/10.1080/19396368.2023.2208253
Stigliani S, Anserini P, Venturini PL, Scaruffi P. Mitochondrial DNA content in embryo culture medium is significantly associated with human embryo fragmentation. Hum Reprod. 2013;28:2652–60. https://doi.org/10.1093/humrep/det314
Vera-Rodriguez M, Diez-Juan A, Jimenez-Almazan J, Martinez S, Navarro R, Peinado V, et al. Origin and composition of cell-free DNA in spent medium from human embryo culture during preimplantation development. Hum Reprod. 2018;33:745–56. https://doi.org/10.1093/humrep/dey028
Fang R, Yang W, Zhao X, Xiong F, Guo C, Xiao J, et al. Chromosome screening using culture medium of embryos fertilised in vitro: a pilot clinical study. J Translational Med. 2019;17:73. https://doi.org/10.1186/s12967-019-1827-1
Rubio C, Navarro-Sanchez L, Garcia-Pascual CM, Ocali O, Cimadomo D, Venier W, et al. Multicenter prospective study of concordance between embryonic cell-free DNA and trophectoderm biopsies from 1301 human blastocysts. Am J Obstet Gynecol. 2020;223. https://doi.org/10.1016/j.ajog.2020.04.035. :751 e751-751 e713.
Xu J, Fang R, Chen L, Chen D, Xiao JP, Yang W, et al. Noninvasive chromosome screening of human embryos by genome sequencing of embryo culture medium for in vitro fertilization. Proc Natl Acad Sci USA. 2016;113:11907–12. https://doi.org/10.1073/pnas.1613294113
Huang L, Bogale B, Tang Y, Lu S, Xie XS, Racowsky C. Noninvasive preimplantation genetic testing for aneuploidy in spent medium may be more reliable than trophectoderm biopsy. Proc Natl Acad Sci USA. 2019;116:14105–12. https://doi.org/10.1073/pnas.1907472116
Chen L, Sun Q, Xu J, Fu H, Liu Y, Yao Y, et al. A non-invasive chromosome screening strategy for prioritizing in vitro fertilization embryos for implantation. Front cell Dev Biology. 2021;9:708322. https://doi.org/10.3389/fcell.2021.708322
Chen R, Tang N, Du H, Yao Y, Zou Y, Wang J, et al. Clinical application of noninvasive chromosomal screening for elective single-blastocyst transfer in frozen-thawed cycles. J Translational Med. 2022;20:553. https://doi.org/10.1186/s12967-022-03640-z
Li X, Yao Y, Zhao D, Chang X, Li Y, Lin H, et al. Clinical outcomes of single blastocyst transfer with machine learning guided noninvasive chromosome screening grading system in infertile patients. Reproductive Biology Endocrinology: RB&E. 2024;22:61. https://doi.org/10.1186/s12958-024-01231-9
Wilkinson J, Roberts SA, Vail A. Developments in IVF warrant the adoption of new performance indicators for ART clinics, but do not justify the abandonment of patient-centred measures. Hum Reprod. 2017;32:1155–9. https://doi.org/10.1093/humrep/dex063
Yan J, Qin Y, Zhao H, Sun Y, Gong F, Li R, et al. Live birth with or without preimplantation genetic testing for aneuploidy. N Engl J Med. 2021;385:2047–58. https://doi.org/10.1056/NEJMoa2103613
Simopoulou M, Sfakianoudis K, Maziotis E, Tsioulou P, Grigoriadis S, Rapani A, et al. PGT-A: who and when? Α systematic review and network meta-analysis of RCTs. J Assist Reprod Genet. 2021;38:1939–57. https://doi.org/10.1007/s10815-021-02227-9
Maheshwari A, McLernon D, Bhattacharya S. Cumulative live birth rate: time for a consensus? Hum Reprod. 2015;30:2703–7. https://doi.org/10.1093/humrep/dev263
Schoolcraft WB, Gardner DK, Lane M, Schlenker T, Hamilton F, Meldrum DR. Blastocyst culture and transfer: analysis of results and parameters affecting outcome in two in vitro fertilization programs. Fertil Steril. 1999;72:604–9. https://doi.org/10.1016/s0015-0282(99)00311-8
Lu S, Zong C, Fan W, Yang M, Li J, Chapman AR, et al. Probing meiotic recombination and aneuploidy of single sperm cells by whole-genome sequencing. Sci (New York NY). 2012;338:1627–30. https://doi.org/10.1126/science.1229112
Osman EK, Neal SA, Tiegs AW, Hanson BM, Kim JG, Franasiak JM, et al. Consistency in rates of diagnosis of embryonic mosaicism, segmental abnormalities, and no call results among experienced embryologists performing preimplantation genetic testing for aneuploidy. F&S Rep. 2020;1:119–24. https://doi.org/10.1016/j.xfre.2020.05.005
Yeung QSY, Zhang YX, Chung JPW, Lui WT, Kwok YKY, Gui B, et al. A prospective study of non-invasive preimplantation genetic testing for aneuploidies (NiPGT-A) using next-generation sequencing (NGS) on spent culture media (SCM). J Assist Reprod Genet. 2019;36:1609–21. https://doi.org/10.1007/s10815-019-01517-7
Jiao J, Shi B, Sagnelli M, Yang D, Yao Y, Li W, et al. Minimally invasive preimplantation genetic testing using blastocyst culture medium. Hum Reprod. 2019;34:1369–79. https://doi.org/10.1093/humrep/dez075
Toftager M, Bogstad J, Lossl K, Praetorius L, Zedeler A, Bryndorf T, et al. Cumulative live birth rates after one ART cycle including all subsequent frozen-thaw cycles in 1050 women: secondary outcome of an RCT comparing GnRH-antagonist and GnRH-agonist protocols. Hum Reprod. 2017;32:556–67. https://doi.org/10.1093/humrep/dew358
Boulet SL, Kirby RS, Reefhuis J, Zhang Y, Sunderam S, Cohen B, et al. Assisted reproductive technology and birth defects among liveborn infants in Florida, Massachusetts, and Michigan, 2000–2010. JAMA Pediatr. 2016;170:e154934. https://doi.org/10.1001/jamapediatrics.2015.4934
Magli MC, Albanese C, Crippa A, Tabanelli C, Ferraretti AP, Gianaroli L. Deoxyribonucleic acid detection in blastocoelic fluid: a new predictor of embryo ploidy and viable pregnancy. Fertil Steril. 2019;111:77–85. https://doi.org/10.1016/j.fertnstert.2018.09.016
Chen L, Li W, Liu Y, Peng Z, Cai L, Zhang N, et al. Non-invasive embryo selection strategy for clinical IVF to avoid wastage of potentially competent embryos. Reprod Biomed Online. 2022;45:26–34. https://doi.org/10.1016/j.rbmo.2022.03.006
Acknowledgements
We want to thank all the patients who agreed to participate in this study and medical staff for their assistance.
Funding
The study was supported by the Key Medical Research Project of Jiangsu Provincial Health Commission (ZD2022004), National Natural Science Foundation of China (82274651), and Key Research and Development Program of Jiangsu Province (BE2022712).
Author information
Authors and Affiliations
Contributions
SQ, XJJ, YYX and HX designed the experiments and drafted the manuscript; XJJ and HX contributed to the clinical samples; SQ and ZDM conducted the experiments and the data analysis; and LSJ, YB and CL reviewed and revised the manuscript. The work was finalized by all the authors.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Nanjing Jinling Hospital (2016NZKY-028-02) and registered in the Chinese Clinical Trial Center (ChiCTR-DDD-17010376), and all included couples signed written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, Q., Xu, J., Yao, Y. et al. Efficacy of non-invasive chromosome screening, preimplantation genetic testing for aneuploidy, and morphological grading in selecting embryos of patients with advanced maternal age: a three-armed prospective cohort study. BMC Pregnancy Childbirth 24, 545 (2024). https://doi.org/10.1186/s12884-024-06736-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-024-06736-0