Abstract
Background
Lower socioeconomic position (SEP) associates with adverse pregnancy and perinatal outcomes and with less favourable metabolic profile in nonpregnant adults. Socioeconomic differences in pregnancy metabolic profile are unknown. We investigated association between a composite measure of SEP and pregnancy metabolic profile in White European (WE) and South Asian (SA) women.
Methods
We included 3,905 WE and 4,404 SA pregnant women from a population-based UK cohort. Latent class analysis was applied to nineteen individual, household, and area-based SEP indicators (collected by questionnaires or linkage to residential address) to derive a composite SEP latent variable. Targeted nuclear magnetic resonance spectroscopy was used to determine 148 metabolic traits from mid-pregnancy serum samples. Associations between SEP and metabolic traits were examined using linear regressions adjusted for gestational age and weighted by latent class probabilities.
Results
Five SEP sub-groups were identified and labelled ‘Highest SEP’ (48% WE and 52% SA), ‘High-Medium SEP’ (77% and 23%), ‘Medium SEP’ (56% and 44%) ‘Low-Medium SEP’ (21% and 79%), and ‘Lowest SEP’ (52% and 48%). Lower SEP was associated with more adverse levels of 113 metabolic traits, including lower high-density lipoprotein (HDL) and higher triglycerides and very low-density lipoprotein (VLDL) traits. For example, mean standardized difference (95%CI) in concentration of small VLDL particles (vs. Highest SEP) was 0.12 standard deviation (SD) units (0.05 to 0.20) for ‘Medium SEP’ and 0.25SD (0.18 to 0.32) for ‘Lowest SEP’. There was statistical evidence of ethnic differences in associations of SEP with 31 traits, primarily characterised by stronger associations in WE women e.g., mean difference in HDL cholesterol in WE and SA women respectively (vs. Highest-SEP) was -0.30SD (-0.41 to -0.20) and -0.16SD (-0.27 to -0.05) for ‘Medium SEP’, and -0.62SD (-0.72 to -0.52) and -0.29SD (-0.40 to -0.20) for ‘Lowest SEP’.
Conclusions
We found widespread socioeconomic differences in metabolic traits in pregnant WE and SA women residing in the UK. Further research is needed to understand whether the socioeconomic differences we observe here reflect pre-conception differences or differences in the metabolic pregnancy response. If replicated, it would be important to explore if these differences contribute to socioeconomic differences in pregnancy outcomes.
Similar content being viewed by others
Background
Extensive changes in maternal circulating metabolites occur during pregnancy, which are likely to be important for maternal health and normal fetal development [1,2,3], with some of these metabolites associating with adverse pregnancy and perinatal outcomes [4,5,6]. Studies indicate that lower socioeconomic position (SEP) associates with adverse pregnancy and perinatal outcomes, including gestational diabetes, preterm birth, and small-for-gestational-age [7,8,9]. Lower SEP has also been associated with worse metabolic profile in adolescents and adults [10] however, to the best of our knowledge, SEP differences in pregnancy metabolic profile have not been examined.
Besides SEP, ethnicity differences in pregnancy and perinatal outcomes [9, 11,12,13] and metabolic traits have been reported [14]. For example, evidence from the Born in Bradford (BiB) cohort shows that South Asian pregnant women had higher levels of amino acids, fatty acids, and glucose, and lower levels of cholesterol and lipoproteins than White Europeans [14]. Findings from BiB also show differences in SEP between White European and South Asian women [15], and studies report differences between ethnic groups in associations of SEP with pregnancy and perinatal outcomes [7, 8]. Understanding socioeconomic differences in pregnancy metabolic profiles, including across ethnic groups, may help inform public health interventions. Studies also often relate only one or a small number of indicators of SEP to an outcome, and so rarely acknowledge that SEP is multidimensional and reflects different but related factors including education, occupation, income, wealth, assets, and area deprivation [16, 17].
The aim of this study was to examine the associations between a composite measure of SEP, that should better reflect its multidimensional nature, and mid-pregnancy metabolic profiles in White European and South Asian women.
Methods
This study was done according to a pre-specified and publicly available analysis plan [18] and is reported in line with the STROBE guidelines.
Cohort description
BiB is a population-based prospective pregnancy cohort that included 12,453 women who experienced 13,776 pregnancies between 2007 and 2011 [19]. Most women were recruited at approximately 26–28 weeks gestation at their oral glucose tolerance test, which is offered to all women booked for delivery at Bradford Royal Infirmary. As blood samples for metabolic profiling were those collected for the oral glucose tolerance test, all participants in this study were recruited at ~ 26–28 weeks. BiB has almost an equal split of White European and South Asian women, all residing in Bradford, UK, a city in the North of England with high levels of socioeconomic deprivation (the BiB study was started due to a high prevalence of poor child health in the city). Mothers, and their partners, recruited into the study provided detailed interview questionnaire data, measurements, and biological samples. The study website gives further information, including protocols, information on data access, and a list of all data (https://borninbradford.nhs.uk/research/documents-data/).
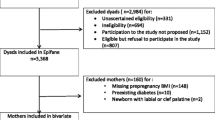
For this study, we included all first enrolled pregnancies to White European and South Asian women (the two main ethnic groups in BiB). After excluding other ethnicities, and those with missing data on SEP, metabolic traits, and gestational age at measurement of metabolic traits, our analysis sample comprised of 3,905 White Europeans and 4,404 South Asians (Fig. 1).
Ethnicity assessment and groups
Ethnicity was reported by the mother at the recruitment questionnaire interview or abstracted from medical records (for those missing questionnaire data) and defined according to the UK Office for National Statistics guidelines. For our main analysis, ethnicity groups were defined as White European or South Asian. White European ethnicity included women that indicated they were White British (n = 4,489) or other White European (n = 306). South Asian ethnicity included women that indicated they were Pakistani (n = 5,128), Indian (n = 439), Bangladeshi (n = 263) or other South Asian heritage (n = 63).
Indicators of SEP
A total of 19 individual-, household- and area-based indicators of SEP were used to derive a composite SEP latent variable (Table 1). All individual- and household-based indicators were reported by the mother at the recruitment questionnaire interview (at around 26–28 weeks of gestation) [20], and the area-based indicator was based on linkage to the mother’s residential address at the time of recruitment.
Area SEP was based on the English Index of Multiple Deprivation (IMD) Score in 2007 (i.e., at the time of pregnancy). IMD is a relative composite measure of multiple deprivation at small area level across England (mean population size in each area is 1500 residents). The domains used to derive IMD in 2007 were income deprivation; employment deprivation; health deprivation and disability; education deprivation; crime deprivation; barriers to housing and services deprivation; and living environment deprivation.
Individual-based indicators reflect educational attainment, occupation, need for financial benefits, and financial circumstances. The highest educational qualification obtained by the woman and the baby’s father was recorded along with the country it was obtained in. We equivalised the highest educational qualifications (based on qualification received and the country obtained) into one of seven categories using the UK National Academic Recognition Information Center. Those with equivalised education coded as other, foreign unknown, or do not know were excluded from each education variable. Because over 25% of women reported that they had never been employed, women’s employment was coded as currently employed, previously employed, or never employed. The baby’s father’s occupation was coded based on the National Statistics Socio-Economic Classification. Those coded as student or don’t know were excluded from this variable. Students (n = 110) were excluded because they do not fit into an occupational class, the SEP measure used in this study.
Women were coded as being in receipt of means tested benefits (i.e., benefits awarded to those that can demonstrate that their income and savings are below a certain level) if they reported receiving any of income support, income tested jobs seekers allowance, working families tax credit, or housing benefit. Women were asked how well they are managing financially with responses being either living comfortably, doing alright, just about getting by, or quite difficult or very difficult. Women were also asked how they are doing financially compared to a year ago, with responses coded as better off, worse off, about the same, or does not wish to answer. Those coded as does not wish to answer were excluded from this variable. Women also reported if they were able to have two pairs of all-weather shoes, money to make regular savings of £10 a month, and a small amount of money to spend each week on themselves.
Household-based indicators reflected questions about housing tenure, overcrowding, and ownership of material items and goods based on questions from the Households Below Average Income Survey. Housing tenure was reported as one of seven groups; owns outright, owns with a mortgage, lives rent free, owned by a private landlord, living in social housing, other and don’t know. Those coded as other, don’t know, or living rent free were excluded from this variable. Those coded as living rent free (n = 181 (4.7%) White European; n = 437 (10.2%) South Asian) were excluded because they likely comprise a heterogeneous group of women living in multigenerational homes related to cultural factors, and those living with parents because of financial or other difficulties. Responses to questions on numbers of household members and bedrooms were used to derive an indicator of overcrowding based on the person per room approach [21] by dividing the number of persons by the number of bedrooms in this household. Women were also asked whether they were up to date with household bills, if they had contents insurance, enough money to keep the home in a decent state of repair, money to replace any worn out furniture, money to replace or repair major electrical goods, and if they were able to keep their home warm enough in winter. For each of these variables, women that responded as don't want/need, doesn't wish to answer, or don't know were excluded.
Pregnancy metabolic traits
Full details of all metabolomic measurements undertaken in BiB have been published [22]. In this study we focus on maternal pregnancy nuclear magnetic resonance (NMR) metabolic traits. Women had a fasting mid-pregnancy serum sample taken by trained phlebotomists working in the antenatal clinic of Bradford Royal Infirmary (92% were obtained between 26–28 weeks gestation). Samples were processed within 2.5 h and placed in -80° freezers. There were no sample freeze–thaw events prior to their use for metabolomic profiling. In total, 227 metabolic traits were measured using a high-throughput targeted NMR platform (Nightingale Health©, Helsinki, Finland). The metabolic traits were quantified in absolute concentration units or ratios and included circulating lipoprotein lipids and subclasses, fatty acids, fatty acid compositions, amino acids, traits related to glycolysis, ketone bodies, fluid balance, and an inflammatory marker [23, 24]. In this study, derived measures and ratios were excluded, leaving 148 metabolic traits for analysis (Additional File 1: Data Set 1). Gestational age at serum sample collection was recorded.
Statistical analysis
Latent class analysis (LCA) was applied to all 19 SEP indicators to derive a composite SEP latent variable consisting of SEP sub-groups (latent classes). LCA is a finite mixture model that classifies individuals into unobserved sub-groups (called latent classes) based on their responses to two or more indicator variables, with the aim of identifying subgroups where individuals are more similar within groups than between groups [25]. LCA was done in White European and South Asian women (combined) with data on pregnancy NMR metabolic traits, gestational age at the metabolic traits’ sample collection, and at least one SEP indicator, with estimation by full information maximum likelihood (FIML). To avoid local maxima solutions (i.e., convergence to a likelihood value that is not the global (true) likelihood), we used 1500 random sets of starting values for the initial stage, 150 final stage optimizations, and 15 initial stage iterations. Models with two to six latent classes were compared and the optimal number of classes was identified based on a combination of BIC, entropy statistic, and Lo-Mendell-Rubin adjusted likelihood ratio test (Additional File 2: Table S1). Where these indicators disagreed, the more interpretable model was selected.
Linear regression models with robust standard errors were then used to examine associations between SEP latent class sub-groups (versus a reference SEP sub-group) and each metabolic trait. Models were weighted by the sum of latent class probabilities to allow for uncertainty in SEP latent class membership assignments and were adjusted for gestational age to control for gestational age-related differences in metabolic traits. Ethnic differences in the associations between SEP and metabolic traits were examined by including an interaction term between SEP and ethnicity (i.e., White European, or South Asian) in all models. All metabolic traits were standardised (to mean = 0, SD = 1) to aid comparison of results for different metabolic traits [26]. Standardisation was done separately by ethnicity because we have previously found differences in pregnancy metabolic traits between White European and South Asians in this cohort [14]. In a sensitivity analysis, we repeated LCA and regression modelling separately in White European and South Asian women and separately in the White British and Pakistani women (the two biggest groups within the White European and South Asian ethnic groups). This was done because of previously reported SEP differences between South Asian groups and between White European groups in this cohort [15].
To avoid overloading the main paper, here we present results for major groups of metabolic trait and sub-particles regardless of P-values, and provide all results with exact P-values and false discovery rate (FDR) corrected P-values [27] in additional files. All the results can also be viewed on the accompanying interactive app (https://aelhak.shinyapps.io/SEP_NMR_BiB/). LCA was done in Mplus version 6, and all other analyses were done in R version 4.2.2.
Missing data
All women with ≥ 1 SEP indicator were included in the LCA using FIML under the missing at random assumption (i.e., that the probability of a missing SEP indicator value can be entirely explained by other observed SEP indicators and so is not related to its value). For the regression analysis, women with missing data on metabolic traits and gestational age were excluded. To explore the potential impact of missing data, we compared characteristics of included women with those excluded due to missing data (Additional File 3: Table S2).
Deviations from pre-specified analysis plan
Following feedback on previous versions of this work presented at scientific conferences and scientific meetings, we decided to make the combined LCA analysis our focus instead of the ethnicity-specific analyses. We decided to analyse traits in SD units instead of perfuming log transformation because most pregnancy metabolic traits were normally distributed (Additional File 4: Fig. S1). No other changes were made to the analysis plan [18].
Results
Participant characteristics
A total of 3,905 White European and 4,404 South Asian pregnant women with at least one SEP indicator and data on metabolic traits and gestational age at collection of serum samples for the assessment of metabolic traits were included in the study (Fig. 1). Mean gestational age was 26.6 weeks (SD = 1.8) in White Europeans and 26.7 weeks (SD = 1.9) in South Asians, and mean age was 26.6 (SD = 6.0) and 27.9 (SD = 5.2) years respectively. When compared with included women, those excluded due to missing data on metabolic traits and gestational age (n = 1,407) had higher proportion of South Asian ethnicity (63% versus 53%) and broadly similar socioeconomic circumstances as indicated by similar levels across most SEP indicators (Additional File 3: Table S2).
SEP sub-groups
LCA (in the combined sample of White Europeans and South Asians) identified five SEP sub-groups which we have labelled ‘Highest SEP’, ‘High-Medium SEP’, ‘Medium SEP’, ‘Low-Medium SEP’, and ‘Lowest SEP’. The proportions of White Europeans and South Asians in the Lowest SEP, Medium SEP, and the Highest SEP groups were broadly similar, but there were fewer White Europeans than South Asians in the Low-Medium SEP group (29% vs. 79%) and more in the High-Medium SEP group (77% vs. 23%) (Fig. 2). The differentiation into SEP sub-groups was driven largely by five SEP indicators (mother’s educational level and employment status, partner’s educational level and occupational class, and means tested benefits). There was little difference between SEP sub-groups in whether women reported being able to keep the home warm enough in winter and being able to afford to afford two pairs of all-weather shoes. The remaining 12 indicators each contributed with modest differences. When compared with High-Medium SEP subgroup, the Low-Medium SEP sub-group was more likely to own a house outright without a mortgage (Fig. 2).
Association of SEP sub-groups with pregnancy metabolic traits
Lower SEP was associated with (mostly) less favourable levels of 113 metabolic traits at the FDR corrected P < 0.05 threshold (Additional File 5: Data Set 2, Additional File 6: Data Set 3). This included associations between lower SEP and higher VLDL cholesterol, total triglycerides (Fig. 3), glycoprotein acetyls, and VLDL concentration, lower levels of cholines (Fig. 4), and higher VLDL and lower HDL in cholesterol and phospholipids (Fig. 5). Conversely, there was less evidence of associations with LDL particles and no differences in albumin, glycine, histidine, or lactate (Additional File 6: Data Set 3).
Mean difference in cholesterol, fatty acids, triglycerides, glycolysis-related metabolites, ketone bodies, and fluid balance traits by SEP sub-groups in the combined sample of White European and South Asian women, shown for traits without statistical evidence of SEP by ethnicity interaction (reference: Highest SEP sub-group)
Mean difference in lipoprotein particle concentration, amino acids, other lipids, inflammation, and apolipoproteins by SEP sub-groups in the combined sample of White European and South Asian women, shown for traits without statistical evidence of SEP by ethnicity interaction (reference: Highest SEP sub-group)
There was a statistical interaction between SEP and ethnicity (at the FDR corrected P < 0.1 threshold) for 31 metabolic traits (Additional File 5: Data Set 2). For most of these traits, differences by SEP group were larger and showed a clearer gradient (across SEP categories) in White European than South Asian women (Additional File 7: Data Set 4). This included stronger association with omega − 3 fatty acids, cholesterol and triglycerides in large HDL, docosahexaenoic acid, and degree of unsaturation in White Europeans (Fig. 6). Differences in HDL and VLDL particle size were larger in White Europeans but the difference in LDL particle size was larger in South Asians (Additional File 7: Data Set 4).
Association of ethnicity-specific SEP sub-groups with pregnancy metabolic traits
Ethnicity-specific LCA identified five sub-groups in White Europeans and three in South Asians. SEP sub-groups in White Europeans were labelled ‘Highest SEP’, ‘High-Medium SEP’, ‘Medium SEP’, ‘Low-Medium SEP’, and ‘Lowest SEP’, and SEP sub-groups in South Asians were labelled ‘Highest SEP’, ‘Medium SEP’, ‘and ‘Lowest SEP’ (Fig. 7). As seen for the combined SEP sub-groups, differentiation into sub-groups was driven by a few SEP indicators and there was little difference between sub-groups in whether being able to keep the home warm enough in winter or able to afford two pairs of all-weather shoes (Fig. 7).
Ethnicity-specific SEP was associated with 115 and 98 metabolic traits at the FDR corrected P < 0.05 threshold in White Europeans and South Asians, respectively (Additional File 8: Data Set 5). Results were consistent with those in the combined SEP analysis and included associations in both White Europeans and South Asians between lower SEP and lower HDL-cholesterol and cholines, and higher triglycerides (Fig. 8). Analyses in White British and Pakistani women identified similar SEP groups, and similar differences in metabolic traits to those found in White Europeans and South Asians, respectively (Additional File 8: Data Set 5, Additional File 9: Fig. S2).
Discussion
We examined the association between a composite measure of SEP that should better reflect its multidimensional nature and 148 serum metabolic traits in pregnant White European and South Asian women. We found widespread socioeconomic differences across most metabolic traits characterized by more adverse levels of traits in lower SEP subgroups. These included SEP differences across most medium, large, and very large HDL lipoprotein subclasses, and small, medium, large, and very large VLDL subclasses. There was statistical evidence that associations with some traits were larger in White Europeans than South Asians, including omega − 3 fatty acids, cholesterol and triglycerides in large HDL, docosahexaenoic acid, pyruvate, apolipoprotein A1, degree of unsaturation, and HDL and VLDL particle size.
To the best of our knowledge, this is the first study to explore SEP differences in multiple metabolic traits in pregnancy. Comparing our results with those seen in women outside of pregnancy has some value in beginning to understand the extent to which any differences we observe are likely to be pregnancy specific or reflect SEP differences that were likely present in these women before conception. Our findings are consistent with results from 30 000 adults and 4000 children across 10 UK and Finnish cohort studies which found associations between lower educational attainment and occupational class and more adverse (NMR-derived) metabolic traits including lower HDL traits [10]. We found that lower SEP associated with higher levels of the inflammatory marker glycoprotein acetyls, which corroborates findings from a study of 605 women showing that higher educational level was associated with lower inflammatory biomarkers in pregnancy [28]. Given that SEP and NMR metabolic traits can inform on adverse pregnancy and perinatal outcomes [4,5,6,7,8,9], our findings suggest SEP might influence adverse pregnancy and perinatal outcomes via effects on metabolic traits.
There are complex and multi-factorial factors contributing to socioeconomic differences in health outcomes [29,30,31]. Individual-level attributes including diet, physical activity, BMI, and smoking are strongly socially patterned and can influence metabolic profiles and therefore are likely to explain some of the socioeconomic differences observed [32,33,34,35]. For example, BMI, smoking, HDL-cholesterol and blood pressure have been shown to explain a considerable amount of the association between SEP and adverse pregnancy and perinatal outcomes [36]. Features of the built environment might also contribute to socioeconomic differences in pregnancy metabolic profiles [37,38,39,40]. Socioeconomic differences in metabolic traits might also be attributable to differential developmental trajectories shaped by early life experiences and cumulative allostatic load over the life course [41].
We found statistical evidence to suggest that associations between SEP and 31 metabolic traits were mostly stronger and had clearer socioeconomic gradient in White Europeans than South Asians. Given that distributions of most pregnancy metabolic traits differed between White European and South Asians [14], it is possible that SEP contributes to differences in pregnancy metabolic traits between White Europeans and South Asians. Less variation in risk factors, including health behaviours, between SEP sub-groups in South Asian women might be one explanation for the stronger socioeconomic differences in White Europeans found in our study. For example, South Asian women in the lower SEP groups might have healthier dietary habits, e.g., higher home-prepared food consumption and lower snack consumption [42,43,44], and lower smoking rates [45] than lower SEP White European women.
Our ethnicity specific LCA identified fewer SEP sub-groups in South Asians indicating lesser variability in SEP in South Asians, which might also contribute to ethnic differences in our associations. One reason for the lower variability in SEP in South Asians might be because they are mostly first-generation immigrants. New immigrants from the same geographic area tend to be more homogenous in their socioeconomic background and would have not yet established the inequality patterns and socioeconomic gradients of the local population since these require time and acculturation before they emerge in subsequent generations [46]. Finally, difference in perceived adversities and the way to face social adversities could also contribute to explaining ethnic group difference in how SEP influences metabolic traits [47].
Limitations
Our study only included White Europeans and South Asians and therefore findings may not generalise to other ethnic groups. The participants were from a high-income country and so findings might not generalise to Whites and South Asians in low-income countries. Women with incomplete data on all SEP indicators were included in LCA using FIML which gives unbiased results under the missing at random assumption. However, if this assumption does not hold, this can produce bias and make the model selection criteria less reliable. We found that only a few SEP indicators explained most of the variation between SEP latent classes therefore, future studies might want to compare LCA to the conventional approach of using one SEP indicator. The Nightingale NMR platform used here primarily covers lipoproteins and therefore we have not assessed other classes of metabolites in detail.
Conclusions
We found widespread socioeconomic differences in metabolic traits in pregnant White European and South Asian women characterized by more adverse levels of metabolic traits in lower SEP subgroups, with statistical evidence of stronger associations for some of the metabolic traits in White European than South Asian women. Further research is needed to understand whether the socioeconomic differences we observe here reflect pre-conception differences or differences in the metabolic pregnancy response. If replicated, it would be important to explore if these differences contribute to the socioeconomic differences in pregnancy and perinatal outcomes.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BiB:
-
Born in Bradford
- IMD:
-
Index of Multiple Deprivation
- LCA:
-
Latent class analysis
- NMR:
-
Nuclear Magnetic Resonance
- SEP:
-
Socioeconomic position
References
Mills HL, Patel N, White SL, et al. The effect of a lifestyle intervention in obese pregnant women on gestational metabolic profiles: findings from the UK Pregnancies Better Eating and Activity Trial (UPBEAT) randomised controlled trial. BMC Med. 2019;17(1):15.
Liang L, Rasmussen M-LH, Piening B, et al. Metabolic Dynamics and Prediction of Gestational Age and Time to Delivery in Pregnant Women. Cell. 2020;181(7):1680–92.e15.
Wang Q, Würtz P, Auro K, et al. Metabolic profiling of pregnancy: cross-sectional and longitudinal evidence. BMC medicine. 2016;14(1):205.
Sovio U, Clayton GL, Cook E, et al. Metabolomic Identification of a Novel, Externally Validated Predictive Test for Gestational Diabetes Mellitus. J Clin Endocrinol Metab. 2022;107(8):e3479–86.
McBride N, Yousefi P, Sovio U, et al. Do Mass Spectrometry-Derived Metabolomics Improve the Prediction of Pregnancy-Related Disorders? Findings from a UK Birth Cohort with Independent Validation. Metabolites. 2021;11(8):530.
McBride N, Yousefi P, White SL, et al. Do nuclear magnetic resonance (NMR)-based metabolomics improve the prediction of pregnancy-related disorders? Findings from a UK birth cohort with independent validation. BMC Med. 2020;18(1):366.
Joseph KS, Liston RM, Dodds L, Dahlgren L, Allen AC. Socioeconomic status and perinatal outcomes in a setting with universal access to essential health care services. CMAJ. 2007;177(6):583–90.
Blumenshine P, Egerter S, Barclay CJ, Cubbin C, Braveman PA. Socioeconomic Disparities in Adverse Birth Outcomes: A Systematic Review. Am J Prev Med. 2010;39(3):263–72.
Jardine J, Walker K, Gurol-Urganci I, et al. Adverse pregnancy outcomes attributable to socioeconomic and ethnic inequalities in England: a national cohort study. Lancet. 2021;398(10314):1905–12.
Robinson O, Carter AR, Ala-Korpela M, et al. Metabolic profiles of socio-economic position: a multi-cohort analysis. Int J Epidemiol. 2021;50(3):768–82.
Bryant AS, Worjoloh A, Caughey AB, Washington AE. Racial/ethnic disparities in obstetric outcomes and care: prevalence and determinants. Am J Obstet Gynecol. 2010;202(4):335–43.
Farrar D, Fairley L, Santorelli G, et al. Association between hyperglycaemia and adverse perinatal outcomes in south Asian and white British women: analysis of data from the Born in Bradford cohort. Lancet Diabetes Endocrinol. 2015;3(10):795–804.
Farrar D, Santorelli G, Lawlor DA, et al. Blood pressure change across pregnancy in white British and Pakistani women: analysis of data from the Born in Bradford cohort. Sci Rep. 2019;9(1):13199.
Taylor K, Ferreira DLS, West J, Yang T, Caputo M, Lawlor DA. Differences in Pregnancy Metabolic Profiles and Their Determinants between White European and South Asian Women: Findings from the Born in Bradford Cohort. Metabolites. 2019;9(9):190.
Fairley L, Cabieses B, Small N, et al. Using latent class analysis to develop a model of the relationship between socioeconomic position and ethnicity: cross-sectional analyses from a multi-ethnic birth cohort study. BMC Public Health. 2014;14(1):835.
Galobardes B, Shaw M, Lawlor DA, Lynch JW, Davey SG. Indicators of socioeconomic position (part 1). J Epidemiol Community Health. 2006;60(1):7–12.
Galobardes B, Shaw M, Lawlor DA, Lynch JW, Davey SG. Indicators of socioeconomic position (part 2). J Epidemiol Community Health. 2006;60(2):95–101.
Elhakeem A. Socioeconomic position and metabolic profile in pregnant South Asian and White European women: findings from the Born in Bradford cohort. 20 February 2023 2023. https://osf.io/xrf8s/.
Wright J, Small N, Raynor P, et al. Cohort Profile: the Born in Bradford multi-ethnic family cohort study. Int J Epidemiol. 2013;42(4):978–91.
Born in Bradford Study. Born in Bradford Mothers’ Baseline Questionnaire. 2007. https://borninbradford.nhs.uk/wp-content/uploads/Mothers-Q-V41-14-09-2007.pdf (accessed 17 April 2024).
Cable N, Sacker A. Validating overcrowding measures using the UK Household Longitudinal Study. SSM Popul Health. 2019;8:100439.
Taylor K, McBride N, J Goulding N, et al. Metabolomics datasets in the Born in Bradford cohort [version 2; peer review: 1 approved, 1 approved with reservations]. Wellcome open research 2021;5(264).
Soininen P, Kangas AJ, Würtz P, Suna T, Ala-Korpela M. Quantitative Serum Nuclear Magnetic Resonance Metabolomics in Cardiovascular Epidemiology and Genetics. Circ Cardiovasc Genet. 2015;8(1):192–206.
Ussher JR, Elmariah S, Gerszten RE, Dyck JR. The Emerging Role of Metabolomics in the Diagnosis and Prognosis of Cardiovascular Disease. J Am Coll Cardiol. 2016;68(25):2850–70.
McLachlan GJ, Lee SX, Rathnayake SI. Finite Mixture Models. Annual Review of Statistics and Its Application. 2019;6(1):355–78.
Elhakeem A, Ronkainen J, Mansell T, et al. Effect of common pregnancy and perinatal complications on offspring metabolic traits across the life course: a multi-cohort study. BMC Med. 2023;21(1):23.
Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc: Ser B (Methodol). 1995;57(1):289–300.
Keenan-Devlin LS, Smart BP, Grobman W, et al. The intersection of race and socioeconomic status is associated with inflammation patterns during pregnancy and adverse pregnancy outcomes. Am J Reprod Immunol. 2022;87(3): e13489.
Marmot M. Social determinants of health inequalities. Lancet. 2005;365(9464):1099–104.
Braveman P, Gottlieb L. The social determinants of health: it’s time to consider the causes of the causes. Public Health Rep. 2014;129(Suppl 2):19–31.
Bann D, Wright L, Hughes A, Chaturvedi N. Socioeconomic inequalities in cardiovascular disease: a causal perspective. Nat Rev Cardiol. 2024;21(4):238–49. https://doi.org/10.1038/s41569-023-00941-8.
Stringhini S, Carmeli C, Jokela M, et al. Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1·7 million men and women. Lancet. 2017;389(10075):1229–37.
Bann D, Johnson W, Li L, Kuh D, Hardy R. Socioeconomic Inequalities in Body Mass Index across Adulthood: Coordinated Analyses of Individual Participant Data from Three British Birth Cohort Studies Initiated in 1946, 1958 and 1970. PLoS medicine. 2017;14(1):e1002214-e.
Kramer MS, Séguin L, Lydon J, Goulet L. Socio-economic disparities in pregnancy outcome: why do the poor fare so poorly? Paediatr Perinat Epidemiol. 2000;14(3):194–210.
Pampel FCKP, Denney JT. Socioeconomic Disparities in Health Behaviors. Annu Rev Sociol. 2010;36:349–70.
Rogne T, Gill D, Liew Z, et al. Mediating Factors in the Association of Maternal Educational Level With Pregnancy Outcomes: A Mendelian Randomization Study. JAMA Network Open. 2024;7(1):e2351166-e.
Robinson O, Tamayo I, de Castro M, et al. The Urban Exposome during Pregnancy and Its Socioeconomic Determinants. Environ Health Perspect. 2018;126(7): 077005.
Torres Toda M, Avraam D, James Cadman T, et al. Exposure to natural environments during pregnancy and birth outcomes in 11 European birth cohorts. Environ Int. 2022;170: 107648.
Dadvand P, Wright J, Martinez D, et al. Inequality, green spaces, and pregnant women: Roles of ethnicity and individual and neighbourhood socioeconomic status. Environ Int. 2014;71:101–8.
Maitre L, Bustamante M, Hernández-Ferrer C, et al. Multi-omics signatures of the human early life exposome. Nat Commun. 2022;13(1):7024.
Lu MC, Halfon N. Racial and Ethnic Disparities in Birth Outcomes: A Life-Course Perspective. Matern Child Health J. 2003;7(1):13–30.
Chowbey P, Harrop D. Healthy eating in UK minority ethnic households: influences and way forward. A Race Equality Foundation Briefing Paper. Race Equality Foundation; 2016. Available at: https://raceequalityfoundation.org.uk/wp-content/uploads/2022/10/Better-Health-42-Healthy-Eating-final.pdf. Accessed 26 Apr 2024.
LeCroy MN, Stevens J. Dietary intake and habits of South Asian immigrants living in Western countries. Nutr Rev. 2017;75(6):391–404.
Clifford Astbury C, Penney TL, Adams J. Home-prepared food, dietary quality and socio-demographic factors: a cross-sectional analysis of the UK National Diet and nutrition survey 2008–16. Int J Behav Nutr Phys Act. 2019;16(1):82.
Mathur R, Schofield P, Smith D, Gilkes A, White P, Hull S. Is individual smoking behaviour influenced by area-level ethnic density? A cross-sectional electronic health database study of inner south-east London. ERJ Open Res. 2017;3(1):00130–2016.
Bhopal R, Hayes L, White M, et al. Ethnic and socio-economic inequalities in coronary heart disease, diabetes and risk factors in Europeans and South Asians. J Public Health. 2002;24(2):95–105.
Bhopal RS. Migration, ethnicity, race, and health in multicultural societies. 2nd ed. Oxford University Press; 2014.
Acknowledgements
We are grateful to everyone involved in the Born in Bradford study. This includes the families who kindly participated, as well as the practitioners and researchers all of whom made Born in Bradford happen. Sample processing and NMR analysis were carried out at the Bristol Bioresource Laboratory and the NMR Metabolomics facility at University of Bristol.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreements No. 874583 (ATHLETE), and No. 874739 (LongITools) and the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 101021566 (ART-HEALTH), which contribute to part of AE and DAL’s salary. AE, GLC, AGS, KT, and DAL work in a unit supported by the University of Bristol and UK Medical Research Council (MC_UU_00032/05 & MC_UU_00032/02) and DAL’s contribution to this research is further supported by the British Heart Foundation (CH/F/20/90003 & AA/18/1/34219). BiB has received funding from the Wellcome Trust (101597), a joint grant from the UK Medical Research Council and UK Economic and Social Science Research Council (MR/N024391/1), and a British Heart Foundation Clinical Study grant (CS/16/4/32482). ISGlobal acknowledges support from the grant CEX2018-000806-S funded by MCIN/AEI/ https://doi.org/10.13039/501100011033, and support from the Generalitat de Catalunya through the CERCA Program. The funders had no role in the design and conduct of the study; management, analysis, and interpretation of data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
AE developed the idea for this study with initial input from MV and LM. AE developed the analysis plan with input from all authors. AE undertook all analysis and wrote the first draft of the manuscript. GLC, AGS, KT, LM, GS, NJT, JW, DAL, and MV provided feedback on the draft and approved the final manuscript for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
BiB had ethical approval from Bradford Research Ethics Committee (07/H1302/112). All BiB participants provided informed consent or assent to participate in the study and secondary data analyses.
Consent for publication
Not Applicable.
Competing interests
DAL reported grants from national and international government and charity funders, Roche Diagnostics, and Medtronic Ltd for work unrelated to this publication. DAL also declares that she is an editor for BMC Medicine. The other authors report no conflicts.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Elhakeem, A., Clayton, G.L., Soares, A.G. et al. Social inequalities in pregnancy metabolic profile: findings from the multi-ethnic Born in Bradford cohort study. BMC Pregnancy Childbirth 24, 333 (2024). https://doi.org/10.1186/s12884-024-06538-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-024-06538-4