Abstract
Objective
The objective was to assess the efficacy and safety of low-dose aspirin for the prevention of preterm birth in nulliparous women.
Data sources
We searched PubMed, Embase and the Cochrane Central Register of Controlled Trials (CENTRAL) from inception to June 2022.
Study eligibility criteria
Randomized controlled trials that compared aspirin to placebo in nulliparous women were eligible.
Methods
This study was reported in accordance with the PRISMA 2020 checklist. The primary outcomes of this study were the rates of preterm birth at less than 37 weeks and less than 34 weeks of gestation. The secondary outcomes included postpartum hemorrhage, placental abruption, cesarean section, any hypertensive disorder of pregnancy and small for gestational age. Relative risks with their 95% confidence intervals were calculated for analysis. Heterogeneity was assessed by Cochran’s Q test and Higgins’s I2. A random-effects model was used when I2 was > 50% to generate the RR and 95% CI; otherwise, a fixed-effects model was used. The risk of publication bias was assessed by funnel plots. We performed sensitivity analysis by sequentially omitting each included study to confirm the robustness of the analysis.
Results
Seven studies with a total of 29,029 participants were included in this review. Six studies were assessed as having a low risk of bias or an unclear risk of bias, and one study was judged as having a high risk of bias. In nulliparous women, low-dose aspirin was associated with a significant reduction in the rate of preterm birth at less than 34 weeks of gestational age (RR 0.84,95% CI: 0.71–0.99; I2 = 0%; P = 0.04), but we did not observe a significant difference in the rate of preterm birth at less than 37 weeks of gestation (RR 0.96,95% CI: 0.90–1.02; I2 = 31%; P = 0.18). Low-dose aspirin was associated with a significant increase in the rates of postpartum hemorrhage (RR 1.32,95% CI: 1.14–1.54; I2 = 0%; P = 0.0003), placental abruption (RR 2.18,95% CI: 1.10–4.32; I2 = 16%; P = 0.02) and cesarean section (RR 1.053, 95% CI: 1.001–1.108; I2 = 0%; P = 0.05) in nulliparous women. We also did not observe a significant effect of low-dose aspirin on the rates of any hypertensive disorder of pregnancy (RR 1.05, 95% CI: 0.96–1.14; I2 = 9%; P = 0.28) or small for gestational age (RR 0.96, 95% CI: 0.91–1.02; I2 = 0%; P = 0.16) in nulliparous women. Funnel plots indicated that no significant publication bias existed in this meta-analysis. Except for preterm birth at less than 34 weeks of gestation, placental abruption and cesarean section, the sensitivity analysis showed similar results, which confirmed the robustness of this meta-analysis.
Conclusions
Low-dose aspirin might reduce the risk of preterm birth at less than 34 weeks of gestation in nulliparous women. The use of low-dose aspirin in nulliparous women increased the risk of postpartum hemorrhage and might increase the risk of placental abruption and cesarean section.
Similar content being viewed by others
Introduction
Preterm birth (PTB), associated with infant mortality and morbidity, is an important public health problem [1]. In 2015, PTB was the leading cause of under-5 and neonatal deaths in 194 WHO member states (1.055 million deaths and 0.944 million deaths, respectively) [2]. Due to the immaturity of several organ systems, surviving preterm infants are at increased risk for cerebral palsy, blindness, deafness, intraventricular hemorrhage, necrotizing enterocolitis, respiratory distress syndrome, jaundice, and intellectual disability [3, 4]. Additionally, preterm infants are more likely to develop adult-onset chronic diseases such as obesity, diabetes, and hypertension. The costs to the health care system for caring for preterm infants and their long-term effects are enormous. Moreover, PTB can cause psychological and physical distress and illness for the mother and the community [5]. There are a wide variety of treatments to prevent PTB, including cervical cerclage and progesterone [6,7,8,9]. However, two-thirds of patients with PTB have no apparent risk factors to explain its occurrence, which means that prevention of PTB may not be effective and it remains important to identify new preventive strategies for PTB [10].
Aspirin belongs to the family of nonsteroidal anti-inflammatory drugs (NSAIDs) and acts as an inhibitor of two cyclooxygenase isoenzymes (COX-1 and COX-2), thereby reducing the synthesis of prostaglandins and thromboxane [11]. Aspirin was first reported to prevent the recurrence of preeclampsia in 1978, and its effectiveness in preventing preeclampsia has been confirmed by multiple randomized trials and meta-analyses [12]. Currently, some health care organizations recommend that women with risk factors for preeclampsia use low-dose aspirin (LDA) to prevent or delay the onset of preeclampsia [13,14,15,16,17]. Because PTB partly resembles the pathophysiology of uteroplacental ischemic lesions in preeclampsia, LDA may also be used to prevent PTB [18, 19].
Evidence from randomized trials suggested that LDA was effective in preventing preeclampsia and associated PTB in women at high risk for preeclampsia [20, 21]. In 2017, a meta-analysis also reported that antiplatelet drugs reduced the rate of spontaneous PTB in pregnant women at risk of preeclampsia [22]. However, whether LDA can be used to prevent PTB in women not at risk of preeclampsia is unclear. On a population basis, nulliparity was strongly associated with PTB [10], which raised concerns about whether LDA can be used to prevent PTB in nulliparous women. In 2018 and 2020, a secondary analysis of a randomized trial of low-dose aspirin for the prevention of preeclampsia in nulliparous women and the ASPIRIN trial showed that LDA reduced the incidence of PTB in nulliparous women [23, 24], which might support the idea that LDA could be used to prevent PTB in nulliparous women.
The objective of this systematic review and meta-analysis was to assess the efficacy and safety of LDA for the prevention of PTB in nulliparous women.
Materials and methods
This was a systematic review and meta-analysis of randomized controlled trails that evaluated the impact of LDA on PTB in nulliparous women. The protocol was registered in PROSPERO (CRD42022343951).
Data sources and search strategy
PubMed, EMBASE and the Cochrane Central Register of Controlled Trials (CENTRAL) were searched from inception to June 2022 using MeSH terms and keywords related to aspirin and preterm birth in pregnant women (Shown in supplementary Table S1). The references of other systematic reviews and meta-analyses were evaluated to identify potentially relevant references. No language restriction was applied.
Eligibility criteria
Studies were included if they (1) were randomized controlled trials; (2) included one group of nulliparous women who received LDA at any dose that commenced at any time during pregnancy and another group that received placebo; and (3) reported data on the prevalence of preterm birth. Studies were excluded if they (1) included pregnant women who had the following issues: allergy or contraindication to aspirin, chronic kidney disease, diabetes, infections, and medical conditions for which LDA therapy is currently indicated (e.g., hypertension, systemic lupus erythematosus or antiphospholipid syndrome); (2) were review articles, editorials, case reports, conference abstracts, or non-placebo-controlled studies; and (3) were secondary analyses that used the same population data as other original studies.
Study selection and data extraction
All studies were independently selected by two reviewers [XY (Xin Yan) and JW] by initially screening abstracts and titles. Then, the full text of all potentially eligible studies was retrieved and read in detail by two reviewers [XY (Xin Yan) and JW] to assess inclusion eligibility. Relevant data were extracted for each study by two reviewers [XY (Xin Yan) and JW] independently, including the first author’s name, publication year, sample size, inclusion criteria and exclusion criteria, intervention, onset and duration, compliance, and outcomes. Microsoft Excel was used for data extraction. Any disagreements during study selection and data extraction were resolved by discussion among all authors [XY (Xin Yan), JW, XY (Xianxian Yuan), WZ, GL]. WZ and GL checked for accuracy.
Outcome measures
The primary outcomes of this study were the rates of preterm birth at less than 37 weeks and less than 34 weeks of gestation. Secondary outcomes included postpartum hemorrhage (> 500 ml) [25], placental abruption, cesarean section, any hypertensive disorder of pregnancy and small for gestational age (birthweight below the 10th centile by gestational age and sex) [26].
Quality evaluation
Preferred reporting items for systematic reviews and meta-analysis (PRISMA) tool were used to assess the quality of this review (Shown in supplementary Table S2) [27]. The risks of bias in each included study were independently assessed according to the Cochrane Handbook criteria by two reviewers [XY (Xin Yan) and WZ] [28].
Data synthesis and statistical analyses
RevMan 5.4 and STATA 16.0 were used for statistical analysis. For dichotomous outcomes, relative risks (RRs) with their 95% confidence intervals (CIs) were calculated for analysis [29]. Heterogeneity was assessed by Cochran’s Q test and Higgins’s I2 [30, 31]. A random-effects model was used when I2 was > 50% to generate the RR and 95% CI; otherwise, a fixed-effects model was used. We summarized the data using forest plots. The risk of publication bias was assessed by funnel plots. We performed sensitivity analysis by sequentially omitting each study to ensure the robustness of the analysis.
Results
Study selection
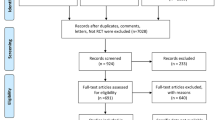
We retrieved 3575 citations from PubMed, Embase and the Cochrane Central Register of Controlled Trials up to June 2022. A total of 3568 citations were excluded, including duplicate publications, reviews and meta-analyses, irrelevant studies, case reports, secondary analyses, studies including the same population as another study, protocols, non-RCTs, editorials, commentaries, and studies for which PTB data were not available. We excluded 3 additional studies in which there was no placebo in the control group [32,33,34]. Finally, 7 studies with a total of 29,029 participants were included in this review [24, 35,36,37,38,39,40]. A flowchart of the study selection process is shown in Fig. 1.
Study characteristics
The characteristics of the included studies are shown in Table 1. Six out of the seven studies reported the effect of LDA on the rates of PTB at less than 37 weeks of gestation, and three out of the seven studies reported the effect of LDA on the rates of PTB at less than 34 weeks of gestation. The data for the rates of PTB at less than 37 weeks of gestation included the data for the rates of PTB at less than 34 weeks of gestation. There were two studies both reported the rates of PTB at less than 37 weeks of gestation and the rates of PTB at less than 34 weeks of gestation and these data are presented separately in each study [24, 36]. Regarding postpartum hemorrhage, placental abruption and cesarean section, four, three and six out of the seven studies reported the effect of aspirin, respectively. For any hypertensive disorder of pregnancy and small for gestational age, all studies and five out of the seven studies reported the effect of aspirin, respectively.
Risk of bias of included studies
The quality assessment of the included studies according to the Cochrane Handbook criteria is shown in Fig.2 and Fig.3; 6 studies were assessed as having a low risk of bias or an unclear risk of bias, and 1 study was judged as having a high risk of bias.
Synthesis of results
The synthesis of results for each of the assessed outcomes in the included studies is shown in Table 2.
In nulliparous women, LDA was associated with a significant reduction in the rate of PTB at less than 34 weeks of gestational age (RR 0.84, 95% CI: 0.71–0.99; I2 = 0%; P = 0.04; Fig. 4), but we did not observe a significant difference in the rate of PTB at less than 37 weeks of gestation (RR 0.96, 95% CI: 0.90–1.02; I2 = 31%; P = 0.18; Fig. 5). Given that the definition of PTB before 37 weeks of gestation encompasses PTB before 34 weeks of gestation, this study further examined the relationship between LDA and the incidence of PTB between 34 and 37 weeks of gestation. The analysis revealed that there was no statistically significant difference in the effect of LDA on the incidence of PTB between 34 and 37 weeks of gestation (RR 1.01, 95% CI: 0.78–1.32; I2 = 68%; P = 0.91; Supplementary Figure S1).
LDA was associated with a significant increase in the rates of postpartum hemorrhage (RR 1.32, 95% CI: 1.14–1.54; I2 = 0%; P = 0.0003; Fig. 6), placental abruption (RR 2.18, 95% CI: 1.10–4.32; I2 = 16%; P = 0.02; Fig. 7) and cesarean section (RR 1.053, 95% CI: 1.001–1.108; I2 = 0%; P = 0.05; Fig. 8) in nulliparous women. We did not observe a significant effect of LDA on the rates of any hypertensive disorder of pregnancy (RR 1.05, 95% CI: 0.96–1.14; I2 = 9%; P = 0.28; Fig. 9) or small for gestational age (RR 0.96, 95% CI: 0.91–1.02; I2 = 0%; P = 0.16; Fig. 10) in nulliparous women.
Sensitivity analyses
Sensitivity analysis was performed by omitting studies sequentially (Table 3). For preterm birth at less than 37 weeks of gestation, the pooled RR ranged from 0.94 (95% CI: 0.88, 1.01) to 1.02 (95% CI: 0.93, 1.12). For preterm birth at less than 34 weeks of gestation, the pooled RR ranged from 0.82 (95% CI: 0.68, 0.99) to 0.91 (95% CI: 0.64, 1.30), and when the study by Hoffman was omitted, the estimated effect became nonsignificant (RR 0.91, 95% CI: 0.64, 1.30). For postpartum hemorrhage, the pooled RR ranged from 1.26 (95% CI: 1.00, 1.58) to 1.34 (95% CI: 1.13, 1.57). For placental abruption, the pooled RR ranged from 1.51 (95% CI: 0.68, 3.35) to 4.40 (95% CI: 1.26, 15.41), and when the study by Sibai et al. was omitted, the estimated effect became nonsignificant (RR 1.51, 95% CI: 0.68, 3.35). The pooled RR for cesarean section ranged from 1.04 (95% CI: 0.99, 1.10) to 1.06 (95% CI: 1.01–1.12), which indicated heterogeneity, and when the study by Golding or by Hoffman et al. was omitted, the estimated effect became nonsignificant (RR 1.04, 95% CI: 0.99, 1.10 and RR 1.05, 95% CI: 0.96, 1.15; independently). For any hypertensive disorder of pregnancy, the pooled RR ranged from 1.03 (95% CI: 0.93, 1.15) to 1.08 (95% CI: 0.98, 1.18). For small for gestational age, the pooled RR ranged from 0.95 (95% CI: 0.90, 1.01) to 0.97 (95% CI: 0.92–1.03).
Publication bias
Funnel plots for each outcome in this review were created to qualitatively evaluate publication bias (Supplementary Figures S2-8). All funnel plots seem to be symmetric, which indicates that no significant publication bias existed in this meta-analysis.
Comment
Principal findings
This meta-analysis showed that low-dose aspirin might reduce the risk of PTB at less than 34 weeks of gestation in nulliparous women. However, its impact on reducing the overall risk of PTB at less than 37 weeks of gestation is not statistically significant. Further analysis reveals that there is no statistically significant difference in the effect of LDA on the incidence of PTB between 34 and 37 weeks of gestation. The use of LDA in nulliparous women increased the risk of postpartum hemorrhage and might increase the risk of placental abruption and cesarean section. We also did not observe a significant effect of LDA on the incidence of any hypertensive disorder of pregnancy or small for gestational age in nulliparous women.
PTB is a syndrome with multiple etiological risk factors and phenotypic characteristics [41]. Research on preventive interventions for PTB would be meaningless and inexplicable without a deep understanding of this. Generally, PTB can be divided into early PTB and late PTB according to gestational age. PTB at less than 34 weeks of gestation may have different pathophysiological mechanisms compared to those between 34 and 37 weeks, potentially leading to differential effects of LDA. Research has indicated that early PTB is more likely to be associated with inflammation [18, 42]. In this meta-analysis, the ability of low-dose aspirin to prevent PTB at less than 34 weeks of gestation might be due to its anti-inflammatory properties. In 2018, Andrikopoulou et al. reported similar results that low-dose aspirin reduced the risk of spontaneous PTB at less than 34 weeks of gestation but not 37 weeks of gestation in healthy nulliparous women [23]. In addition, it is important to emphasize that only considering gestational age to classify preterm births also has limitations. The clinical phenotype, such as one or more features of maternal, fetal, placental, and delivery manifestations, should also be considered [43]. However, all studies included in our meta-analysis simply classified early and late PTB only by gestational age, which might lead to heterogeneity among studies. This might also partly explain the unstable results of preterm birth at less than 34 weeks of gestation when performing sensitivity analysis in our meta-analysis. Furthermore, it was observed that the results of the meta-analysis examining the effect of LDA on the incidence of PTB between 34 and 37 weeks of gestation demonstrated a high level of heterogeneity. This heterogeneity may be attributable to variations in the study populations, the timing of the studies conducted, and the dosages of aspirin administered. Therefore, to elucidate the impact of LDA on the incidence of PTB within this specific gestational age, additional research is warranted for a more comprehensive understanding.
LDA is recommended for women with risk factors for preeclampsia to prevent or delay the onset of preeclampsia. In recent years, several cost-effectiveness analyses have suggested that the routine use of aspirin to prevent preeclampsia in all women was associated with lower costs and greater health benefits compared with the use of aspirin only in women with risk factors after screening [44,45,46]. A cost-effectiveness analysis indicated that routine aspirin use to prevent preeclampsia in low-risk nulliparous women was also a preferred strategy [47]. In contrast, our meta-analysis showed that low-dose aspirin was ineffective in preventing preeclampsia in nulliparous women. The mechanism behind the effect of aspirin on preeclampsia in low-risk nulliparous women is unclear. Hence, the universal use of low-dose aspirin to prevent preeclampsia in women without risk factors is not acceptable [48]. Abuse of low-dose aspirin in nulliparous women might pose additional risks. Further research is needed to address these issues.
In our meta-analysis, the use of LDA in nulliparous women increased the risk of postpartum hemorrhage and potentially increased the risk of placental abruption and cesarean section. In contrast, a meta-analysis revealed that there was no increased risk of postpartum hemorrhage in nulliparous women who took low-dose aspirin, which might be due to the lack of several studies in this meta-analysis [49]. A recent population-based cohort study from Sweden also showed that aspirin use during pregnancy was associated with an increased risk for postpartum hemorrhage [50]. Andrikopoulou et al. found that placental abruption was more common in nulliparous women receiving low-dose aspirin than in those receiving placebo (OR 10.0; 95% CI: 1.16–100.0) [23]. A secondary analysis of the effects of aspirin in gestation and reproduction trials by Eubanks et al. showed that preconception-initiated LDA was not associated with the risk of cesarean section (RR 1.02; 95% CI: 0.98–1.07) compared with placebo [51]. Overall, the safety of aspirin use in healthy nulliparous women has not been proven, and further studies are needed.
Comparison with the existing literature
In 2017, van Vliet et al. conducted a meta-analysis suggesting that antiplatelet drugs reduced the rate of spontaneous PTB in pregnant women at risk of preeclampsia [22]. However, with further subgroup analysis, this meta-analysis revealed that aspirin was not effective in preventing spontaneous preterm birth at less than 37 and 34 weeks of gestation in nulliparous women who were at risk of preeclampsia. In addition, this meta-analysis did not demonstrate the effectiveness of aspirin in preventing PTB in nulliparous women who were not at risk of preeclampsia.
Man et al. performed a meta-analysis in healthy nulliparous women with singleton pregnancies and found that taking aspirin reduced the relative risk of preterm birth less than 34 weeks of gestation by 50% but there were no significant differences in preterm birth less than 37 weeks of gestation, which was similar to our study [49]. It is worth noting that the meta-analysis by Man et al. and our study differ in terms of search strategies and primary outcomes. As a result, two studies included in our meta-analysis were not captured in their analysis [39, 40]. Moreover, the meta-analysis conducted by Man et al. included two secondary analyses that shared the same population with an original study. Interestingly, they excluded the original study from their analysis. This approach differs from our study, where we included the original study and excluded the secondary analyses to prevent duplication of data [23, 36, 52]. Furthermore, it should be noted that the meta-analysis by Man et al. included three studies that had no treatment in the control group. However, we made a different decision in our meta-analysis and chose to exclude these studies from our analysis due to concerns related to the study design. The three studies had small sample sizes, and we believed that the absence of a placebo in the control group may have introduced performance and detection bias. Therefore, by excluding these studies, we aimed to minimize potential bias and ensure the robustness of our findings. Additionally, it is important to mention that the meta-analysis conducted by Man et al. did not report on the effects of aspirin on outcomes such as placental abruption and cesarean section. In contrast, our meta-analysis specifically examined these outcomes and included relevant studies to provide a comprehensive analysis of the effects of aspirin. By including these outcomes, we aimed to assess the broader impact of aspirin on maternal and fetal health.
Limitations of the study
To our knowledge, this is the first meta-analysis in which the primary outcome was the effect of aspirin on the prevention of PTB in nulliparous women. The most obvious limitation of this meta-analysis was that not all included studies reported the incidence of PTB, and only 3 studies reported the incidence of PTB at less than 34 weeks of gestation [24, 36, 37]. Due to the small number of studies included in this meta-analysis, the effect of onset timing and the dose of LDA on the rate of PTB was not evaluated. Furthermore, the study did not differentiate between spontaneous and iatrogenic preterm births due to a lack of adequate studies. Sensitivity analysis demonstrated that the results of this meta-analysis were robust, except for PTB at less than 34 weeks of gestation, placental abruption and cesarean section. Two of the seven studies did not distinguish between singleton and multiple pregnancies, which caused unclear bias for this meta-analysis, but considering the very small number of women with multiple pregnancies, we decided to include these two studies in our meta-analysis [36, 40]. Finally, most studies (6/7) had a low or an unclear risk of bias, with one study having a high risk of bias due to poor compliance [38].
Conclusions and implications
The results of this meta-analysis suggested that prophylactic LDA might reduce the risk of PTB at less than 34 weeks of gestation in nulliparous women. LDA might be beneficial for specific phenotypes of PTB, but given the phenotypic heterogeneity of PTB, how to identify women who would benefit from prophylactic LDA remains to be elucidated. Future work will require large prospective randomized controlled trials to evaluate the preventive effect of LDA on specific PTB phenotypes. Moreover, we found that LDA increased the risk of postpartum hemorrhage and might increase the risk of placental abruption and cesarean section in nulliparous women, which implied that prophylactic use of LDA to prevent PTB in all nulliparous women must be done with great caution.
Given the wide range of enrollment periods during pregnancy, extra caution is warranted when interpreting the conclusions of this meta-analysis. Currently, there is a scarcity of studies on the administration of aspirin for the prevention of preterm birth in nulliparous women. Further research is needed to examine the potential benefits of aspirin in this population. Specifically, future studies should investigate the optimal timing, dosage, and discontinuation of aspirin, as well as its effectiveness in preventing various subtypes of preterm birth.
In summary, although the meta-analysis indicated that prophylactic use of LDA might prevent preterm birth at less than 34 weeks of gestation in nulliparous women, much work remains to be done to determine the impact of LDA on PTB [53]. Potential risks and benefits need to be considered when using low-dose aspirin to prevent PTB in nulliparous women.
Data availability
All data generated or analysed during this study are included in this published article and are available from the corresponding author on reasonable request.
Abbreviations
- PTB:
-
preterm birth
- LDA:
-
low dose aspirin
- RR:
-
relative risks
- CI:
-
confidence intervals
References
Goodfellow L, Care A, Alfirevic Z. Controversies in the prevention of spontaneous preterm birth in asymptomatic women: an evidence summary and expert opinion. BJOG. 2021;128(2):177–94.
Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet (London England). 2016;388(10063):3027–35.
The global. Burden of preterm birth. Lancet. 2009;374(9697):1214.
Rubens CE, Sadovsky Y, Muglia L, Gravett MG, Lackritz E, Gravett C. Prevention of preterm birth: harnessing science to address the global epidemic. Sci Transl Med. 2014;6(262):262sr5.
Harrison M, Goldenberg R. Global burden of prematurity. Semin Fetal Neonatal Med. 2016;21(2):74–9.
Breslin N, Gyamfi-Bannerman C. Current Preterm Birth Prevention Strategies. Clin Perinatol. 2020;47(4):705–17.
Matei A, Saccone G, Vogel JP, Armson AB. Primary and secondary prevention of preterm birth: a review of systematic reviews and ongoing randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2019;236:224–39.
Medley N, Poljak B, Mammarella S, Alfirevic Z. Clinical guidelines for prevention and management of preterm birth: a systematic review. BJOG. 2018;125(11):1361–9.
Shennan AH. Prediction and prevention of preterm birth: a quagmire of evidence. Ultrasound Obstet Gynecol. 2018;51(5):569–70.
Martin J, DʼAlton M, Jacobsson B, Norman J. Pursuit of Progress toward Effective Preterm Birth reduction. Obstet Gynecol. 2017;129(4):715–9.
ACOG Committee Opinion No. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132(1):e44–52.
Rolnik DL, Nicolaides KH, Poon LC. Prevention of preeclampsia with aspirin. Am J Obstet Gynecol. 2022;226(2s):S1108–19.
LeFevre ML. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(11):819–26.
Hypertension in pregnancy. Report of the American College of Obstetricians and gynecologists’ Task Force on hypertension in pregnancy. Obstet Gynecol. 2013;122(5):1122–31.
Organization WH. WHO Recommendations for Prevention and Treatment of Pre-eclampsia and Eclampsia. Clin Endocrinol. 2011;53(2):260–1.
Redman CWG. Hypertension in pregnancy: the NICE guidelines. Heart. 2011;97(23):1967.
Combs CA, Montgomery DM. Society for maternal-fetal Medicine Special Statement: checklists for preeclampsia risk-factor screening to guide recommendations for prophylactic low-dose aspirin. Am J Obstet Gynecol. 2020;223(3):B7–11.
Landman A, de Boer MA, Visser L, Nijman TAJ, Hemels MAC, Naaktgeboren CN, et al. Evaluation of low-dose aspirin in the prevention of recurrent spontaneous preterm labour (the APRIL study): a multicentre, randomised, double-blinded, placebo-controlled trial. PLoS Med. 2022;19(2):e1003892.
Berger R, Kyvernitakis I, Maul H. Spontaneous Preterm Birth: is Prevention with aspirin possible? Geburtshilfe Frauenheilkd. 2021;81(3):304–10.
Davidson K, Barry M, Mangione C, Cabana M, Caughey A, Davis E, et al. Aspirin use to prevent Preeclampsia and related morbidity and mortality: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326(12):1186–91.
Allshouse AA, Jessel RH, Heyborne KD. The impact of low-dose aspirin on preterm birth: secondary analysis of a randomized controlled trial. J Perinatol. 2016;36(6):427–31.
van Vliet EOG, Askie LA, Mol BWJ, Oudijk MA. Antiplatelet agents and the Prevention of spontaneous Preterm Birth: a systematic review and Meta-analysis. Obstet Gynecol. 2017;129(2):327–36.
Andrikopoulou M, Purisch SE, Handal-Orefice R, Gyamfi-Bannerman C. Low-dose aspirin is associated with reduced spontaneous preterm birth in nulliparous women. Am J Obstet Gynecol. 2018;219(4):399. e1-e6.
Hoffman MK, Goudar SS, Kodkany BS, Metgud M, Somannavar M, Okitawutshu J, et al. Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): a randomised, double-blind, placebo-controlled trial. Lancet (London England). 2020;395(10220):285–93.
WHO Guidelines Approved by the Guidelines Review Committee. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. Geneva: World Health Organization Copyright © 2012, World Health Organization.; 2012.
Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857–68.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester (UK): Wiley; 2019.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Higgins J, Thompson S, Deeks J, Altman D. Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J Health Serv Res Policy. 2002;7(1):51–61.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Mone F, Mulcahy C, McParland P, Breathnach F, Downey P, McCormack D, et al. Trial of feasibility and acceptability of routine low-dose aspirin versus early screening test indicated aspirin for pre-eclampsia prevention (TEST study): a multicentre randomised controlled trial. BMJ open. 2018;8(7):e022056.
Bakhti A, Vaiman D. Prevention of gravidic endothelial hypertension by aspirin treatment administered from the 8th week of gestation. Hypertens Res. 2011;34(10):1116–20.
Taherian A-A, Taherian A, Shirvani A. Prevention of preeclampsia with low-dose aspirin or calcium supplementation. Arch Iran Med. 2002;5(3):151–6.
Hauth JC, Goldenberg RL, Parker CR, Philips JB, Copper RL, DuBard MB, et al. Low-dose aspirin therapy to prevent preeclampsia. Am J Obstet Gynecol. 1993;168(4):1083–91. discussion 91‐3.
Sibai BM, Caritis SN, Thom E, Klebanoff M, McNellis D, Rocco L, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1993;329(17):1213–8.
Davies NJ, Gazvani MR, Farquharson RG, Walkinshaw SA. Low-dose aspirin in the prevention of hypertensive disorders of pregnancy in relatively low-risk nulliparous women. Hypertens Pregnancy. 1995;14(1):49–55.
Golding J. A randomised trial of low dose aspirin for primiparae in pregnancy. The Jamaica Low Dose Aspirin Study Group. Br J Obstet Gynaecol. 1998;105(3):293–9.
Rotchell YE, Cruickshank JK, Gay MP, Griffiths J, Stewart A, Farrell B, et al. Barbados Low Dose aspirin study in pregnancy (BLASP): a randomised trial for the prevention of pre-eclampsia and its complications. Br J Obstet Gynaecol. 1998;105(3):286–92.
Subtil D, Goeusse P, Puech F, Lequien P, Biausque S, Breart G, et al. Aspirin (100 mg) used for prevention of pre-eclampsia in nulliparous women: the Essai Régional Aspirine Mère-Enfant study (part 1). BJOG. 2003;110(5):475–84.
Villar J, Papageorghiou AT, Knight HE, Gravett MG, Iams J, Waller SA, et al. The preterm birth syndrome: a prototype phenotypic classification. Am J Obstet Gynecol. 2012;206(2):119–23.
Kramer MS, Papageorghiou A, Culhane J, Bhutta Z, Goldenberg RL, Gravett M, et al. Challenges in defining and classifying the preterm birth syndrome. Am J Obstet Gynecol. 2012;206(2):108–12.
Goldenberg RL, Gravett MG, Iams J, Papageorghiou AT, Waller SA, Kramer M, et al. The preterm birth syndrome: issues to consider in creating a classification system. Am J Obstet Gynecol. 2012;206(2):113–8.
Mone F, Mulcahy C, McParland P, McAuliffe FM. Should we recommend universal aspirin for all pregnant women? Am J Obstet Gynecol. 2017;216(2):141.e1-e5.
Mallampati D, Grobman W, Rouse DJ, Werner EF. Strategies for prescribing aspirin to prevent Preeclampsia: a cost-effectiveness analysis. Obstet Gynecol. 2019;134(3):537–44.
Lewkowitz AK, Rouse DJ. Miscommunication about low-dose aspirin for Preeclampsia Prevention-further support for Universal Prophylaxis. JAMA Netw Open. 2021;4(10):e2130960.
Mone F, O’Mahony JF, Tyrrell E, Mulcahy C, McParland P, Breathnach F, et al. Preeclampsia Prevention using Routine Versus Screening Test-indicated aspirin in low-risk women. Hypertension. 2018;72(6):1391–6.
Mather AR, Dom AM, Thorburg LL. Low-dose aspirin in pregnancy: who? When? How much? And why? Curr Opin Obstet Gynecol. 2021;33(2):65–71.
Man R, Hodgetts Morton V, Devani P, Morris RK. Aspirin for preventing adverse outcomes in low risk nulliparous women with singleton pregnancies: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;262:105–12.
Hastie R, Tong S, Wikström AK, Sandström A, Hesselman S, Bergman L. Aspirin use during pregnancy and the risk of bleeding complications: a Swedish population-based cohort study. Am J Obstet Gynecol. 2021;224(1):95.e1-e12.
Eubanks AA, Nobles CJ, Mumford SL, Kim K, Hill MJ, Decherney AH, et al. The safety of low-dose aspirin on the Mode of Delivery: secondary analysis of the Effect of Aspirin in Gestation and Reproduction Randomized Controlled Trial. Am J Perinatol. 2022;39(6):658–65.
Sutton EF, Hauspurg A, Caritis SN, Powers RW, Catov JM. Maternal outcomes Associated with Lower Range Stage 1 hypertension. Obstet Gynecol. 2018;132(4):843–9.
Hodgetts Morton V, Stock SJ. Low-dose aspirin for the prevention of preterm birth: more questions than answers. PLoS Med. 2022;19(2):e1003908.
Acknowledgements
We extend our deepest gratitude to Nature Research Editing Service for their invaluable assistance in enhancing the language and presentation quality of our manuscript.
Funding
National Natural Science Foundation of China (82171671) supported study disign, data collection, and statistical analysis. Beijing Hospitals Authority’ Ascent Plan (DFL20191402) and Scientific Research Common Program of Beijing Municipal Commission of Education (KM202110025007) supported writing of the report and the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
XY (Xin Yan) and JW independently screened the title, abstract, and the full text of all potentially eligible studies to assess for inclusion eligibility. XY (Xin Yan) and JW extracted relevant data independently. Any disagreements during study selection and data extraction were resolved by discussion of all authors [XY (Xin Yan), JW, XY (Xianxian Yuan), WZ, GL]. WZ and GL checked for accuracy. XY (Xin Yan) and WZ assessed the risks of bias in each included study independently. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yan, X., Zheng, W., Wang, J. et al. Low-dose aspirin for the prevention of preterm birth in nulliparous women: systematic review and meta-analysis. BMC Pregnancy Childbirth 24, 260 (2024). https://doi.org/10.1186/s12884-024-06413-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-024-06413-2